Abstract
Background
Poultry remains one of the most important reservoir for zoonotic multidrug resistant pathogens. The global rise of antimicrobial resistance in Gram-negative bacteria is of reasonable concern and demands intensified surveillance.
Methods
In 2016, 576 cloacal swabs were collected from 48 broiler farms located in five governorates in northern Egypt. Isolates of Enterobacteriaceae could be cultivated on different media and were identified by MALDI-TOF MS and PCR. Escherichia coli isolates were genotyped by DNA-microarray-based assays. The antimicrobial susceptibility to 14 antibiotics was determined and resistance-associated genes were detected. The VITEK-2 system was applied for phenotypical confirmation of extended-spectrum β-lactamase-producing isolates. The determination of colistin resistance was carried out phenotypically using E-test and genotypically using PCR for detection of the mcr-1 gene.
Results
Out of 576 samples, 72 representatives of Enterobacteriaceae were isolated and identified as 63 E. coli (87.5%), 5 Enterobacter cloacae (6.9%), 2 Klebsiella pneumoniae (2.8%) and 2 Citrobacter spp. (2.8%). Seven out of 56 cultivated E. coli (12.5%) were confirmed as ESBL-producing E. coli and one isolate (1.8%) as ESBL/carbapenemase-producing E. coli. Five out of 63 E. coli isolates (7.9%) recovered from different poultry flocks were phenotypically resistant to colistin and harboured mcr-1 gene.
Conclusions
This is the first study reporting colistin resistance and emergence of multidrug resistance in Enterobacteriaceae isolated from healthy broilers in the Nile Delta region, Egypt. Colistin-resistant E. coli in poultry is of public health significance. The global rise of ESBL- and carbapenemase-producing Gram-negative bacteria demands intensified surveillance. ESBL-producing E. coli in poultry farms in Egypt are of major concern that emphasizes the possibility of spread of such strains to humans. The results also reinforce the need to develop strategies and to implement specific control procedures to reduce the use of antibiotics.
Similar content being viewed by others
Background
Poultry and their products are considered the main vehicle for pathogenic bacteria such as Salmonella (S.) serovars, Escherichia (E.) coli and Klebsiella (K.) spp. that cause foodborne infections in humans [1,2,3].
The prevalence of highly antibiotic-resistant E. coli was recorded in poultry meat more frequently than in all other kinds of meat [4, 5].
Extended-spectrum β-lactamases (ESBLs) are plasmid-encoded enzymes found in Gram-negative bacteria especially in Enterobacteriaceae conferring resistance to first, second and third generation cephalosporins while they are inhibited by clavulanic acid [6,7,8,9,10].
ESBL-producing Enterobacteriaceae have emerged as pathogens in both poultry and humans [7, 11]. Many ESBL-producers are additionally multiresistant to non-β-lactam antibiotics, including fluoroquinolones, aminoglycosides, trimethoprim, tetracyclines, sulfonamides and chloramphenicols [12, 13]. Resistance to cephalosporins is mediated by ampicillin class C β-lactamases (AmpC β-lactamase) and encoded by blaCMY genes [14, 15]. Carbapenems are still the drugs of choice to treat infections with ESBL-producing Enterobacteriaceae in humans [16] and their increasing use reinforces the probability of resistance development to carbapenems among Enterobacteriaceae [17,18,19]. The coexistence of multiple ESBL and carbapenemase genes as well as other antibiotic resistance determinants on mobile elements is of a major concern that might lead to the emergence of organisms with resistance to all antibiotics [6, 20, 21].
Most ESBLs encoding genes in bacteria of clinical interest are located on plasmids [22]. These plasmids may also carry genes encoding resistance to other drug classes including aminoglycosides and fluoroquinolones [23]. Transmission of ESBLs genes can occur either by emerging bacterial clones or by horizontal gene transfer. In the latter case, plasmids containing resistance genes, spread between bacteria [22]. Colistin recently gained attention as a last-resort antibiotic for treatment of infections caused by multidrug resistant Gram-negative bacteria. In veterinary practice, colistin is a drug of choice for the treatment of frequent digestive tract infections caused by E. coli in food-producing animals [24]. The irrational use of colistin in veterinary practice may be the main cause of the increasing rate of colistin resistance. Recently, the emergence of colistin resistance has caused great concern [25, 26] and resistance mediated by the plasmid-borne mcr-1 gene has been detected worldwide in Enterobacteriaceae [27].
In some countries, antimicrobials are used in the poultry industry for treatment of diseased animals, prevention of diseases and promotion of growth [28,29,30]. In Egypt, E. coli infections are considered as one of the most serious diseases leading to economic losses in poultry production [31].
Unfortunately, there are no legislations in Egypt regulating the use of antibiotics. Some of them such as tetracycline, quinolones and beta-lactams are still used for non-therapeutic uses [32]. This improper use of antimicrobials leads to rapid selection of multiresistant strains of Enterobacteriaceae in poultry and plays a key role in the spread of antibiotic-resistant bacteria along the food chain to humans [33,34,35].
In recent years, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) has been applied as a wide-range technique for bacterial identification [36]. Microarray systems are well-established tools for rapid genotypic characterization of bacteria and identification of resistance and virulence-associated determinants [37]. The data can be obtained in a single experiment with the benefit of saving time and money [38,39,40]. The broth microdilution method proved to be an easy and reliable method for determination of the minimum inhibitory concentration (MIC) of antibiotics and can be used as an alternative technique to agar diffusion test [41,42,43].
The use of a rapid molecular assay as an alternative to phenotypic detection was proved to be a useful option for detection of antibiotic resistance to frequently applied antimicrobial agents in poultry production [43].
The objective of this study was to gain insight into the antimicrobial susceptibility of Enterobacteriaceae, especially E. coli originating from healthy broilers from different districts in northern Egypt and to understand its public health significance. In addition, the prevalence of ESBL/carbapenemase-producing E. coli and colistin resistance were investigated.
Methods
Isolation and characterization of bacterial strains
During 2016, 576 cloacal swabs were randomly collected from apparently healthy broilers housed in 48 farms located in five governorates, namely: Dakahlia, Kafr El-Sheikh, Damietta, Gharbiya and Sharkiya, in the Nile Delta region, Egypt. An overview about investigated poultry farms, the number of birds and the number of collected samples are given in Table 1. Sampling was carried out using sterile cotton swabs. The collected samples were transported at 4 °C to the laboratory for microbiological examination. The samples were enriched in Buffered Peptone Water at 37 °C for 24 h and then streaked on MacConkey Agar and Eosin Methylene Blue (EMB) Agar (Oxoid, Manchester, UK), followed by further incubation at 37 °C for 24 h. For identification of ESBL-producing Enterobacteriaceae, the enriched bacterial cultures were cultivated on Brilliance™ ESBL agar (Oxoid GmbH, Wesel, Germany) at 37 °C for 24 h.
Identification by MALDI-TOF MS
Isolates were identified using MALDI-TOF MS [44]. Interpretation of results was performed according to the manufacturer’s recommendation: score of ≥ 2.3 represented reliable species level identification; score 2.0–2.29, probable species level identification; score 1.7–1.9, probable genus level identification, and score ≤ 1.7 was considered an unreliable identification [45].
DNA extraction and purification
Genomic DNA was extracted from bacterial cultures using High Pure PCR Template Purification Kit (Roche Diagnostics, Mannheim, Germany) according to the instructions of the manufacturer.
Identification of E. coli isolates using PCR
The identified E. coli isolates were confirmed at species level using a specific PCR assay targeting 16S rRNA genes with primers ECO-1 (5′-GAC CTC GGT TTA GTT CAC AGA-3′) and ECO-2 (5′-CAC ACG CTG ACG CTG ACC A-3′) which geared from previous study by Seidavi et al. [46]. The PCR reaction was carried out with the following amplification conditions: An initial denaturation step at 96 °C for 60 s was followed by 35 cycles of denaturation (96 °C for 15 s), annealing (58 °C for 60 s) and extension (72 °C at 45 s) with a final extension at 72 °C for 60 s. PCR resulted in 585 bp amplicons. PCR products were analyzed on a 1.5% agarose gel, stained with ethidium bromide and visualized under UV light.
Genoserotyping of E. coli isolates using microarray assay
The serotypes of E. coli isolates were determined using the E. coli SeroGenoTyping AS-1 Kit (Alere Technologies GmbH, Jena, Germany). Five microliters of extracted RNA-free DNA (with a concentration of at least 100 ng/μl) were biotin-labeled by a primer extension amplification using E. coli SeroGenoTyping AS-1 Kit according to manufacturer’s instructions. The procedures for multiplex labelling, hybridization and data analysis were carried out as described in a previous study [47].
Phenotypic antibiotic susceptibility testing
The antibiotic susceptibility testing of all isolates was performed with the MICRONAUT system using commercial 96-well microtiter plates (Merlin, Bornheim, Germany) as recommended by the manufacturer. This system allowed the determination of minimum inhibitory concentrations (MICs) of 14 antimicrobial agents (Tables 2, 3) in serial dilutions of the antibiotics. Over-night grown bacteria were suspended in NaCl solution (0.9%) to obtain a turbidity corresponding to a McFarland standard of 0.5 (Dr. Lange, CADAS photometer 30, Berlin, Germany). One hundred microliters of the suspension were diluted with 10 ml of Mueller–Hinton broth (Oxoid GmbH) resulting in a concentration of approximately 106–107 colony forming units (cfu)/ml. In total, 100 µl of the inoculum were given in each well of the plate. After sealing the plates, they were incubated for 18 h to 24 h at 37 °C. Reading of plates was done with a photometer (Merlin) at a wavelength of 620 nm. An optical density of > 0.1 was interpreted as an indication of growth. MICs were interpreted with the advanced expert system MCN-6 (Merlin) using the guidelines of the German Institute for Standardization (Deutsches Institut für Normung, Berlin, Germany). E. coli ATCC 25922, E. coli ATCC 35218 and K. pneumoniae ATCC 700603 were used as quality controls.
Vitek-2 system
All suspected ESBL isolates were subsequently confirmed using an automated microdilution system (VITEK-2, bioMérieux Deutschland GmbH, Nürtingen, Germany) according to the instructions of the manufacturer. For this study, the test card AST-N289 was used that included the following antibiotics: piperacillin (PIP), piperacillin/tazobactam (TZP), cefotaxime (CTX), ceftazidime (CAZ), cefepime (FEB), aztreonam (ATM), imipenem (IMP), meropenem (MEM), amikacin (AMK), gentamicin (GEN), tobramycin (TOP), ciprofloxacin (CIP), moxifloxacin (MXF), tigecycline (TGC), fosfomycin (FOS), colistin (CT) and trimethoprim/sulfamethoxazole (T/S).
Detection of antibiotic resistance and virulence-associated genes of E. coli isolates by microarray analysis
Antimicrobial resistance (AMR) genotypes and other resistance genes were ascertained using the CarbDetect AS-2 Kit and E. coli PanType AS-2 Kit, respectively (Alere Technologies GmbH). The data were automatically summarized by the “result collector”, a software tool provided by Alere Technologies GmbH. An antibiotic resistance genotype was assigned to be a carrier of a group of genes which have been described to confer resistance to a family of antibiotics (e.g., the genotype “blaCTX-M1/15, blaTEM” conferring resistance to 3rd generation cephalosporins).
The detection of virulence-associated genes was performed using E. coli PanType AS-2 Kit (Alere Technologies GmbH). Twenty-eight different combinations of genes encoding virulence factors associated with adhesion, fimbriae production, secretion systems, SPATE (serine protease auto-transporters), toxins and miscellaneous genes were detected. The genes were detected and analysed by the “result collector”, a software tool provided by Alere Technologies GmbH.
Determination of colistin resistance
All identified E. coli isolates were tested for presence of plasmid-mediated mcr-1 gene using PCR [27]. Briefly, a PCR with 25 µL reaction mixture using CLR5-F (5′-CGG TCA GTC CGT TTG TTC-3′) and CLR5-R (5′-CTT GGT CGG TCT GTA GGG-3′) was performed with the following amplification conditions: initial denaturing at 96 °C for 60 s was followed by 35 cycles of denaturing at 96 °C for 15 s, annealing at 55 °C for 60 s and extension at 72 °C for 30 s. PCR was finished by final extension at 72 °C for 60 s. Amplicons (308 bp) were analyzed on 1.5% agarose gel, stained with ethidium bromide and visualized under UV light.
For isolates possessing mcr-1 gene, MICs were determined with RUO E-test colistin CO 256 according to the manufacturer’s guidelines (bioMérieux Deutschland GmbH). Briefly, an overnight bacterial suspension in Mueller–Hinton broth was adjusted to a density of McFarland 0.5 evenly streaked on Mueller–Hinton agar plates to ensure uniform growth. Once the agar surface was dry, an E-test® colistin strip (concentration range from 0.016 to 256 μg/ml) was applied to the plate with sterile forceps. The MIC was determined after aerobic incubation for 20 h at 37 °C as the point, where inhibition of bacterial growth intersected the E-test strip. According to clinical breakpoints given by EUCAST, an isolate was defined as resistant to colistin when the MIC value was > 2 µg/ml [48].
Results
Isolation and identification of Enterobacteriaceae
Out of 576 samples, 72 Enterobacteriaceae isolates were identified by MALDI-TOF MS. The isolates were classified as 63 E. coli (87.5%), 5 Enterobacter cloacae (6.9%), 2 K. pneumoniae (2.8%) and 2 Citrobacter spp. (2.8%).
Seven out of 63 E. coli isolates could not be re-cultivated for testing of antibiotic resistance after applying MALDI-TOF MS (11.1%), while DNA was extracted from preserving solution for further identification.
Antimicrobial susceptibility testing
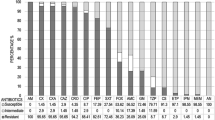
The results of phenotypic antibiotic susceptibility testing of 56 re-cultivated E. coli isolates were given in Table 2. E. coli isolates showed high resistance rates to penicillin, erythromycin and rifampicin with 98.2, 96.4 and 96.4%, respectively. Resistance rates to other tested antibiotics were between 10.7% for amikacin and 64.3% for trimethoprim/sulfamethoxazole. Only one E. coli isolate (1.8%) was resistant to imipenem (Tables 2 and 6). Fifty-five out of 56 E. coli isolates (98.2%) were resistant to antibiotics of at least three different classes of antimicrobial agents and thus they were defined as multidrug resistant isolates (Table 2).
The antimicrobial susceptibility profiles for other species of Enterobacteriaceae were presented in Table 3. All 5 Enterobacter cloacae isolates were resistant to penicillin, erythromycin and rifampicin. Two Citrobacter spp. isolates were resistant to penicillin, rifampicin, streptomycin and ceftazidime. Two K. pneumoniae strains were sensitive to amikacin and imipenem but resistant to the rest of the antibiotics tested.
Genoserotyping of E. coli isolates using microarray analysis
Three out of 63 E. coli isolates (4.8%) were determined as O91 and O15; in all other cases O type determination failed. H antigen types were identified for all isolates. Seventeen different types of H antigens (H1, H2, H4, H5, H6, H7, H8, H10, H11, H16, H19, H21, H26, H28, H32, H34 and H51) were detected. H21 (14 isolates), H28 (10 isolates) and H51 (8 isolates) are being the most common types.
Detection of antibiotic resistance determinants in E. coli by microarray analysis
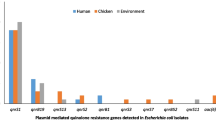
Several resistance genes were identified in 15 phenotypically resistant E. coli using microarray analysis (Table 4). The isolates were originated from four districts located in four provinces, namely Dakahliya (n = 7), Damietta (n = 3), Gharbiya (n = 3) and Kafr El-Sheikh (n = 4). Frequently identified resistance genes were aadA1 associated with resistance to aminoglycosides (n = 12), sul2 responsible for sulphonamide resistance (n = 10) and floR connected with resistance to chloramphenicol (n = 10).
In this study, five E. coli isolates harboured qnrS gene while one isolate possessed qnrB gene associated with quinolone resistance. In two phenotypically ciprofloxacin-resistant E. coli, qnrS gene was detected (Table 4).
The sul and dfrA genes associated with sulphonamides/trimethoprim resistance were detected in 16 and 13 E. coli isolates, respectively (Table 4). Meanwhile, sul3 gene corresponding to sulphonamide resistance was amplified in two susceptible E. coli to sulphonamide/trimethoprim.
Eleven E. coli phenotypically resistant to tetracycline were harboured one or more tet genes (tetA, tetB or tetC) (Table 4). Chloramphenicol resistance-associated genes catA1, catB3, cmlA1 and floR were detected in 13 E. coli isolates. Out of 13 chloramphenicol resistant isolates, 10 isolates harboured one or more resistance genes. The cmlA1 gene was detected once in an E. coli strain that was phenotypically susceptible to chloramphenicol.
Ten different genes (aac6, aac6Ib, aadA1, aadA2, aadA4, aadB, ant2, aphA, strA and strB) associated with aminoglycoside resistance were detected in 14 out of phenotypically tested E. coli isolates (Table 4). All isolates harbouring at least one of described genes were phenotypically resistant to streptomycin, but four of them were susceptible to gentamicin (Tables 3, 4). All isolates with aminoglycoside resistance-associated genes were susceptible to amikacin.
Genes associated with macrolide resistance (ereA, mphA, mrx) were identified in 9 phenotypically resistant E. coli to erythromycin. The rifampicin resistance gene arr was identified in only 2 phenotypically rifampicin resistant isolates.
Fifteen out of 63 E. coli isolates (23.8%) harboured one or more ESBL, narrow-spectrum β-lactamase (NSBL) or AmpC β-lactamase genes. The gene blaTEM was found in 13 DNAs of E. coli isolates (20.6%), blaCMY and blaOXA-7 were detected in 2 samples each (3.2%) and blaSHV, blaOXA-1, blaCTX-M1/15 and blaDHA-1 were found in one isolate (1.6%).
The correlation between the genotypic and phenotypic antimicrobial resistance of E. coli was demonstrated in Table 4.
Thirteen out of 15 isolates harbouring bla genes were analyzed using the VITEK-2 (Table 5). Two samples could not be tested, as they could not be re-cultivated. All isolates possessing beta-lactam resistance genes were resistant to piperacillin, while one isolate was susceptible to moxifloxacin. All isolates were susceptible to fosfomycin. The narrow-spectrum beta-lactamase gene blaOXA-1 was detected once in one ESBL isolates originated from poultry farm in Damietta.
Genotypic and phenotypic identification of resistance to colistin
Plasmid-mediated colistin resistance gene mcr-1 was detected in 5 out of 63 E. coli (8.0%) using a PCR assay. All of them were phenotypically confirmed as resistant to colistin using E-test (Table 6). All colistin resistant E. coli isolates were phenotypically resistant to rifampicin, penicillin and erythromycin but were susceptible to carbapenems. The colistin-resistant isolates originated from different poultry flocks in Dakahliya, Kafr El-Sheikh, Damietta and Gharbiya (Table 6).
Microarray analysis concerning virulence-associated genes
The virulence genes detected by microarray were differently distributed all over the isolated E. coli. One isolate 16CS0740 isolated from poultry farm in Dakahliya harboured 7 genes of virulence-associated secretion system: cif, espA, espF_O103H2, espJ, nleA, nleB O157:H7 and tccP.
eae and iha genes, involved in adhesion, were identified in 16CS0740 and 16CS0752, repectively. Two isolates harboured serine protease autotransporter genes. 16CS0774 carried tsh while 16CS0775 had pic and vat genes.
Several toxin genes were detected in 13 E. coli isolates including astA, cma, hlyE, mchF, sat and senB. Each of these isolates carried only one toxin gene except 16CS0775 which harboured mchF, hlyE and cma. Nineteen out of 63 E. coli isolates (30.2%) harboured lpfA and 3 others carried prfB fimbrae virulence gene. Miscellaneous genes encoding virulence factors as hemL, intI1, ireA, iroN, iss and tir genes were identified in 45 (71.4%), 10 (15.9%), 3 (4.8%), 9 (14.3%), 36 (57.1%) and 1 (1.6%) of 63 isolates, respectively.
The distribution of virulence-associated genes in E. coli isolates possessed antimicrobial resistance-associated genes was demonstrated in Table 4.
Discussion
Escherichia coli is a commensal pathogen of the intestinal tract of young and adult poultry [49]. Among healthy chickens, 10 to 15% of intestinal coliform bacteria may belong to potentially pathogenic serotypes of E. coli [50].
The identification of bacterial foodborne pathogens of zoonotic significance using rapid, accurate and reliable tools such as MALDI-TOF MS is mandatory for public health surveillance [44, 51].
In 2016, 576 cloacal swabs were collected from 48 poultry farms located in 5 governorates in northern Egypt. The samples were screened for multidrug resistant bacteria and investigated for the antimicrobial resistance of E. coli. Seven out of 56 E. coli isolates (12.5%) were producing ESBLs. To analyze the underlying molecular antimicrobial resistance mechanism, all E. coli isolates were genotyped using the multiplex microarray technique.
The results of this study were in accordance with previous reports which demonstrated a high prevalence of E. coli in poultry farms and their environment in Egypt [34, 52,53,54].
In previous studies on broiler chickens in Egypt, high phenotypic resistance rates were found to penicillin, rifampicin, erythromycin, trimethoprim/sulphamethoxazole, streptomycin and tetracycline [53]. Antimicrobial resistance rates in this study for amoxicillin (26.8%), gentamicin (19.6%) and imipenem (1.8%) were lower than those of E. coli isolates from poultry reported in Egypt [53], in China [55], in United States [56], in Korea [57], in United Kingdom [58], in Australia [59] and in Portugal [60]. In the present investigation, the most striking finding was that E. coli isolates showed a low resistance rate to fluoroquinolones (ciprofloxacin (21.4%) and levofloxacin (14.3%)). Cephalosporins are the first-line antimicrobials for treating human bacterial infections [61]. In addition, a considerable resistance to ceftazidime was detected among E. coli isolates from healthy broilers (41.1%).
In this study, one carbapenem-resistant isolate (1.8%) was found within all E. coli isolates. A higher rate was determined with retail chicken meat, 11.3% carbapenemase-producing Enterobacteriaceae including E. coli in Egypt in a previous study [62].
Few reports discussed prevalence of ESBL-producing E. coli isolated from healthy birds in Egypt. Here, ESBL and/or AmpC β-lactamase-producing isolates were detected in seven out of tested 56 E. coli (12.5%) isolated from healthy broilers. Two ESBL-producing strains were isolated from one farm in Gharbiya governorate (Farm 4) while five other isolates could be recovered from different farms in Dakahliya and Kafr El-Sheikh governorates. Two of the ESBL-producing isolates 16CS0740 and 16CS0747 from Dakahliya and Gharbiya, respectively were additionally carrying blaOXA-7 gene characteristic for β-lactamase-producing bacteria (Table 4).
In a previous study in 2017, only 6% ESBL-producing E. coli were detected in colibacillosis diseased poultry in four different Egyptian governorates [63]. In contrast, ESBL/AmpC β-lactamase-producing E. coli were found in all 50 investigated Dutch broiler farms [64]. In Sweden 34.0% of broilers carried ESBL/AmpC β-lactamase-producing E. coli in their guts [65]. In Malaysia 48.8% of isolates which were recovered from retail poultry meat markets were ESBL-AmpC positive [66].
The prevalence of ESBLs has been found to be variable worldwide with Asian countries having the highest rates [67].
In this study, the most prevalent resistance gene was blaTEM, which was identified in 85.7% of ESBL and AmpC β-lactamase-producing isolates. blaCMY-2 was found in 2 of ESBL and AmpC β-lactamase-producing isolates. blaOXA-7 was found in 2 of ESBL producing isolates. While blaCTX-M9, blaCTX-M1-15, blaOXA-1, blaDHA-1, blaLAP-1 and blaSHV were identified only in one ESBL-producing E. coli isolate.
The resistance-associated genes blaTEM, blaSHV, and blaCMY were previously reported in Enterobacteriaceae isolated from septicaemic broilers [68] and from humans [69, 70] in Egypt.
In this study, blaTEM resistance gene was detected in 20.6% of E. coli isolates. This result was in accordance with previous reports in China [71, 72]. blaCMY was detected in 3.5% of 56 E. coli isolates while the prevalence of blaCMY-2 amongst E. coli isolates from broilers in Japan was 69.5% [73]. In Belgium, 49.0% of ceftiofur-resistant E. coli isolates derived from five broiler farms carried blaCMY-2 [74]. Moreover, 12.1% of avian pathogenic E. coli strains and 9.5% of strains recovered from meat were found positive as carriers of blaCTX-M in Palestine [75].
qnrB and qnrS genes associated with quinolone resistance were detected in one and five isolates, respectively, which is lower than described previously in E. coli isolated from chickens in China [72, 76]. On the other hand, qnrA, qnrB, and qnrS genes were detected in 0.75, 3.9 and 5.1%, respectively of E. coli from chicken samples in China [77].
Many studies found similarities between virulence-associated genes in human and avian E. coli isolates including iss, fliC, iha and ireA genes [78].
In a previous study, the virulence genes iroN, ompT, iss, iutA, and hlyF were detected in 80.2% of isolated E. coli [68]. In this study, only 9 (16.7%) of the 56 E. coli isolates carried 2 genes (iroN, iss) together characteristic for avian pathogenic E. coli.
The mcr-1 gene is now reported all over the world in Enterobacteriaceae from animals, food and humans [79]. In 2015, first time mcr-1 gene was detected in livestock and raw meat samples in addition to human beings in China [27]. In this study, five E. coli isolates (8.9%) were phenotypically resistant to colistin and harboured mcr-1 gene associated with colistin resistance. This result was higher than reported in E. coli isolates from pigs, poultry and turkey in France with 0.5, 1.8 and 5.9%, respectively [80] and 5.6% of E. coli isolates from broilers in Germany [81], while it was lower than in E. coli isolates from poultry in China [27].
In previous studies conducted in China and Austria, the majority of phenotypically colistin-resistant E. coli isolates carried the mcr-1 gene [82, 83].
Conclusion
To the best of our knowledge, this study is the first report discussing the antibiotic susceptibility profiles of Enterobacteriaceae and ESBL-producing E. coli isolated from healthy broilers in the Nile Delta in Egypt. The emergence of colistin-resistant E. coli isolates in poultry is of public health significance and considered as potential source of transmission of plasmid-mediated mcr-1 to humans. It was shown that molecular biological methods such as microarray investigation are reliable and fast tools for detection of geno-serotypes, resistance- and virulence-associated determinants.
The results reinforce the need to develop surveillance strategies and to implement specific control procedures to reduce the use of antibiotics and subsequently the development of antimicrobial resistance by over-/misuse of antibiotic agents.
References
Apata DF. Antibiotic resistance in poultry. Int J Poult Sci. 2009;8:404–8.
Helmy YA, El-Adawy H, Abdelwhab EM. A comprehensive review of common bacterial, parasitic and viral zoonoses at the human-animal interface in Egypt. Pathogens. 2017;6:33.
Hafez HM, Hauck R. Zoonoses with public health relevance in poultry. Zoonoses—infections affecting humans and animals. Netherland: Springer; 2015.
NARMS. Retail meat report of national antimicrobial resistance monitoring system. NARMS; 2012. p. 60–75.
Rasheed MU, Thajuddin N, Ahamed P, Teklemariam Z, Jamil K. Antimicrobial drug resistance in strains of Escherichia coli isolated from food sources. Rev Inst Med Trop São Paulo. 2014;56:341–6.
Bush K, Jacoby GA. Updated functional classification of beta-lactamases. Antimicrob Agents Chemother. 2010;54:969–76.
Bradford PA. Extended-spectrum beta-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clin Microbiol Rev. 2001;14:933–51.
Giamarellou H. Multidrug resistance in Gram-negative bacteria that produce extended-spectrum beta-lactamases (ESBLs). Clin Microbiol Infect. 2005;4:1–16.
Rawat D, Nair D. Extended-spectrum beta-lactamases in Gram negative bacteria. J Glob Infect Dis. 2010;2:263–74.
Shaikh S, Fatima J, Shakil S, Rizvi SM, Kamal MA. Antibiotic resistance and extended spectrum beta-lactamases: types, epidemiology and treatment. Saudi J Biol Sci. 2015;22:90–101.
Paterson DL. Resistance in gram-negative bacteria: Enterobacteriaceae. Am J Med. 2006;119:520–58.
Livermore DM, Canton R, Gniadkowski M, Nordmann P, Rossolini GM, Arlet G, et al. CTX-M: changing the face of ESBLs in Europe. J Antimicrob Chemother. 2007;59:165–74.
Karisik E, Ellington MJ, Pike R, Warren RE, Livermore DM, Woodford N. Molecular characterization of plasmids encoding CTX-M-15 beta-lactamases from Escherichia coli strains in the United Kingdom. J Antimicrob Chemother. 2006;58:665–8.
Folster JP, Pecic G, Bolcen S, Theobald L, Hise K, Carattoli A, et al. Characterization of extended-spectrum cephalosporin-resistant Salmonella enterica serovar Heidelberg isolated from humans in the United States. Foodborne Pathog Dis. 2010;7:181–7.
Folster JP, Grass JE, Bicknese A, Taylor J, Friedman CR, Whichard JM. Characterization of resistance genes and plasmids from outbreaks and illness clusters caused by Salmonella resistant to ceftriaxone in the United States, 2011–2012. Microb Drug Resist. 2017;23:188–93.
Zhanel GG, Wiebe R, Dilay L, Thomson K, Rubinstein E, Hoban DJ, et al. Comparative review of the carbapenems. Drugs. 2007;67:1027–52.
Canton R, Akova M, Carmeli Y, Giske CG, Glupczynski Y, Gniadkowski M, et al. Rapid evolution and spread of carbapenemases among Enterobacteriaceae in Europe. Clin Microbiol Infect. 2012;18:413–31.
Nordmann P, Dortet L, Poirel L. Carbapenem resistance in Enterobacteriaceae: here is the storm! Trends Mol Med. 2012;18:263–72.
Pitout JD. Infections with extended-spectrum beta-lactamase-producing Enterobacteriaceae: changing epidemiology and drug treatment choices. Drugs. 2010;70:313–33.
Pitout JD. Extraintestinal pathogenic Escherichia coli: an update on antimicrobial resistance, laboratory diagnosis and treatment. Expert Rev Anti Infect Ther. 2012;10:1165–76.
Hussain A, Ranjan A, Nandanwar N, Babbar A, Jadhav S, Ahmed N. Genotypic and phenotypic profiles of Escherichia coli isolates belonging to clinical sequence type 131 (ST131), clinical non-ST131, and fecal non-ST131 lineages from India. Antimicrob Agents Chemother. 2014;58:7240–9.
Brolund A. Overview of ESBL-producing Enterobacteriaceae from a Nordic perspective. Infect Ecol Epidemiol. 2014;4:24555.
Perez F, Bonomo RA. Can we really use beta-lactam/beta-lactam inhibitor combinations for the treatment of infections caused by extended-spectrum beta-lactamase-producing bacteria? Clin Infect Dis. 2012;54:175–7.
Timmerman T, Dewulf B, Feyen CB, Opsomer G, Kruif AD, Maes D. Quantification and evaluation of antimicrobial drug use in group treatments for fattening pigs in Belgium. Prev Vet Med. 2006;74:251–63.
Biswas S, Brunel JM, Dubus JC, Reynaud-Gaubert M, Rolain JM. Colistin: an update on the antibiotic of the 21st century. Expert Rev Anti Infect Ther. 2012;10:917–34.
Giamarellou H. Epidemiology of infections caused by polymyxin-resistant pathogens. Int J Antimicrob Agents. 2016;48:614–21.
Liu YY, Wang Y, Walsh TR, Yi LX, Zhang R, Spencer J, et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis. 2016;16:161–8.
Yang H, Chen S, White DG, Zhao S, McDermott P, Walker R, et al. Characterization of multiple-antimicrobial-resistant Escherichia coli isolates from diseased chickens and swine in China. J Clin Microbiol. 2004;42:3483–9.
Aidara-Kane A. Containment of antimicrobial resistance due to use of antimicrobial agents in animals intended for food: WHO perspective. Rev Sci Tech. 2012;31:277–87.
Roy Chowdhury P, McKinnon J, Wyrsch E, Hammond JM, Charles IG, Djordjevic SP. Genomic interplay in bacterial communities: implications for growth promoting practices in animal husbandry. Front Microbiol. 2014;5:394.
Tivendale KA, Logue CM, Kariyawasam S, Jordan D, Hussein A, Li G, et al. Avian-pathogenic Escherichia coli strains are similar to neonatal meningitis E. coli strains and are able to cause meningitis in the rat model of human disease. Infect Immun. 2010;78:3412–9.
WHO. Report on the consultative meeting on antimicrobial resistance for countries in the Eastern Mediterranean Region:from policies to action. In: WHO, editor. Consultative meeting on antimicrobial resistance for countries in the Eastern Mediterranean Region: from policies to action Sharm El Sheikh, Egypt: World Health Organization, Regional Office for the Eastern Mediterranean. Berlin: World Health Organization; 2014. p. 8–9.
Moawad AA, Hotzel H, Awad O, Tomaso H, Neubauer H, Hafez HM, et al. Occurrence of Salmonella enterica and Escherichia coli in raw chicken and beef meat in northern Egypt and dissemination of their antibiotic resistance markers. Gut Pathog. 2017;9:57–69.
Dahshan H, Abd-Elall AM, Megahed AM, Abd-El-Kader MA, Nabawy EE. Veterinary antibiotic resistance, residues, and ecological risks in environmental samples obtained from poultry farms. Egypt. Environ Monit Assess. 2015;187:2.
El-Sharkawy H, Tahoun A, El-Gohary AEA, El-Abasy M, El-Khayat F, Gillespie T, et al. Epidemiological, molecular characterization and antibiotic resistance of Salmonella enterica serovars isolated from chicken farms in Egypt. Gut Pathog. 2017;9:8.
Croxatto A, Prod’hom G, Greub G. Applications of MALDI-TOF mass spectrometry in clinical diagnostic microbiology. FEMS Microbiol Rev. 2012;36:380–407.
Michael Dunne WJ, Pouseele H, Monecke S, Ehricht R, van Belkum A. Epidemiology of transmissible diseases: array hybridization and next generation sequencing as universal nucleic acid-mediated typing tools. Infect Genet Evol. 2017;63:332–45.
Dally S, Lemuth K, Kaase M, Rupp S, Knabbe C, Weile J. DNA microarray for genotyping antibiotic resistance determinants in Acinetobacter baumannii clinical isolates. Antimicrob Agents Chemother. 2013;57:4761–8.
Braun SD, Ahmed MF, El-Adawy H, Hotzel H, Engelmann I, Weiß D, et al. Surveillance of extended-spectrum beta-lactamase-producing Escherichia coli in dairy cattle farms in the Nile delta, Egypt. Front Microbiol. 2016;7:1020.
El-Adawy H, Hotzel H, Tomaso H, Neubauer H, Taboada EN, Ehricht R, et al. Detection of genetic diversity in Campylobacter jejuni isolated from a commercial turkey flock using flaA typing, MLST analysis and microarray assay. PLoS ONE. 2013;8:e51582.
Luber P, Bartelt E, Genschow E, Wagner J, Hahn H. Comparison of broth microdilution, E Test, and agar dilution methods for antibiotic susceptibility testing of Campylobacter jejuni and Campylobacter coli. J Clin Microbiol. 2003;41:1062–8.
Luber P, Wagner J, Hahn H, Bartelt E. Antimicrobial resistance in Campylobacter jejuni and Campylobacter coli strains isolated in 1991 and 2001–2002 from poultry and humans in Berlin. Germany Antimicrob Agents Chemother. 2003;47:3825–30.
El-Adawy H, Hotzel H, Düpre S, Tomaso H, Neubauer H, Hafez HM. Determination of antimicrobial sensitivities of Campylobacter jejuni isolated from commercial turkey farms in Germany. Avian Dis. 2012;56:685–92.
Bizzini A, Greub G. Matrix-assisted laser desorption ionization time of flight mass spectrometry, a revolution in clinical microbial identification. Clin Microbiol Infect. 2010;16:1614–9.
Paauw A, Jonker D, Roeselers G, Heng JM, Mars-Groenendijk RH, Trip H, et al. Rapid and reliable discrimination between Shigella species and Escherichia coli using MALDI-TOF mass spectrometry. Int J Med Microbiol. 2015;305:446–52.
Seidavi A, Mirhosseini SZ, Shivazad M, Chamani M, Sadeghi AA, Pourseify R. Detection and investigation of Escherichia coli in contents of duodenum, jejunum, ileum and cecum of broilers at different ages by PCR. AsPac J Mol Biol Biotechnol. 2010;18:321–6.
Braun SD, Monecke S, Thurmer A, Ruppelt A, Makarewicz O, Pletz M, et al. Rapid identification of carbapenemase genes in gram-negative bacteria with an oligonucleotide microarray-based assay. PLoS ONE. 2014;9:e107079.
EUCAST. Breakpoint tables for interpretation of MICs and zone diameters. In: The European Committee on Antimicrobial Susceptibility Testing. 2017. p. 11.
Wooley RE, Brown J, Gibbs PS, Nolan LK, Turner KR. Effect of normal intestinal flora of chickens on colonization by virulent colicin V-producing, avirulent, and mutant colicin V-producing avian Escherichia coli. Avian Dis. 1994;38:141–5.
Nolan LK, Barnes HJ, Vaillancourt JP, Abdul-Aziz T, Logue CM. Colibacillosis. In: Swayne D, editor. Diseases of poultry. 13th ed. Ames: Iowa State University Press; 2013. p. 751–806.
O’Hara CM. Manual and automated instrumentation for identification of Enterobacteriaceae and other aerobic Gram-negative bacilli. Clin Microbiol Rev. 2005;18:147–62.
Yousef SA, Ammar AM, Ahmed DA. Serological and molecular typing of avian pathogenic E. coli originating from outbreaks of colibacillosis in chicken flocks. Int J Sci Res. 2015;4:2082–8.
Mohamed MA, Shehata MA, Rafeek E. Virulence genes content and antimicrobial resistance in Escherichia coli from broiler chickens. Vet Med Int. 2014;2014:195189.
Roshdy H, Abd El-Aziz S, Refai M. Incidence of E. coli in chickens and ducks in different governorates in Egypt. In: 1st Conf Anim Health Res Inst Assoc. Cairo. 2012, p. 420–6.
Yang W, Moore IF, Koteva KP, Bareich DC, Hughes DW, Wright GD. TetX is a flavin-dependent monooxygenase conferring resistance to tetracycline antibiotics. J Biol Chem. 2004;279:52346–52.
Johnson JR, Kuskowski MA, Smith K, O’Bryan TT, Tatini S. Antimicrobial-resistant and extraintestinal pathogenic Escherichia coli in retail foods. J Infect Dis. 2005;191:1040–9.
Kim J, Bae IK, Jeong SH, Chang CL, Lee CH, Lee K. Characterization of IncF plasmids carrying the bla CTX-M-14 gene in clinical isolates of Escherichia coli from Korea. J Antimicrob Chemother. 2011;66:1263–8.
Randall LP, Clouting C, Horton RA, Coldham NG, Wu G, Clifton-Hadley FA, et al. Prevalence of Escherichia coli carrying extended-spectrum beta-lactamases (CTX-M and TEM-52) from broiler chickens and turkeys in Great Britain between 2006 and 2009. J Antimicrob Chemother. 2011;66:86–95.
Obeng AS, Rickard H, Ndi O, Sexton M, Barton M. Antibiotic resistance, phylogenetic grouping and virulence potential of Escherichia coli isolated from the faeces of intensively farmed and free range poultry. Vet Microbiol. 2012;154:305–15.
Mendonca N, Figueiredo R, Mendes C, Card RM, Anjum MF, da Silva GJ. Microarray evaluation of antimicrobial resistance and virulence of Escherichia coli isolates from Portuguese poultry. Antibiotics. 2016;5:4.
Lei T, Tian W, He L, Huang XH, Sun YX, Deng YT, et al. Antimicrobial resistance in Escherichia coli isolates from food animals, animal food products and companion animals in China. Vet Microbiol. 2010;146:85–9.
Abdallah HM, Reuland EA, Wintermans BB, Al Naiemi N, Koek A, Abdelwahab AM, et al. Extended-spectrum beta-lactamases and/or carbapenemases-producing Enterobacteriaceae isolated from retail chicken meat in Zagazig, Egypt. PLoS ONE. 2015;10:e0136052.
El-Shazly DA, Nasef SA, Mahmoud FF, Jonas D. Expanded spectrum beta-lactamase producing Escherichia coli isolated from chickens with colibacillosis in Egypt. Poult Sci. 2017;96:2375–84.
Huijbers PM, Graat EA, Haenen AP, van Santen MG, van Essen-Zandbergen A, Mevius DJ, et al. Extended-spectrum and AmpC beta-lactamase-producing Escherichia coli in broilers and people living and/or working on broiler farms: prevalence, risk factors and molecular characteristics. J Antimicrob Chemother. 2014;69:2669–75.
Börjesson S, Egervärn M, Lindblad M, Englund S. Frequent occurrence of extended-spectrum beta-lactamase- and transferable AmpC beta-lactamase-producing Escherichia coli on domestic chicken meat in Sweden. Appl Environ Microbiol. 2013;79:2463–6.
Aliyu AB, Saleha AA, Jalila A, Zunita Z. Risk factors and spatial distribution of extended spectrum beta-lactamase-producing Escherichia coli at retail poultry meat markets in Malaysia: a cross-sectional study. BMC Public Health. 2016;16:699.
Hawkey PM. Prevalence and clonality of extended-spectrum beta-lactamases in Asia. Clin Microbiol Infect. 2008;1:159–65.
Ahmed AM, Shimamoto T, Shimamoto T. Molecular characterization of multidrug-resistant avian pathogenic Escherichia coli isolated from septicemic broilers. Int J Med Microbiol. 2013;303:475–83.
Al-Agamy MH. Phenotypic and molecular characterization of extended-spectrum beta-lactamases and AmpC beta-lactamases in Klebsiella pneumoniae. Pak J Pharm Sci. 2013;26:291–8.
Fam N, Gamal D, El Said M, El Defrawy I, El Dadei E, El Attar S, et al. Prevalence of plasmid-mediated ampC genes in clinical isolates of Enterobacteriaceae from Cairo, Egypt. Br Microbiol Res J. 2013;3:525–37.
Yuan L, Liu JH, Hu GZ, Pan YS, Liu ZM, Mo J, et al. Molecular characterization of extended-spectrum beta-lactamase-producing Escherichia coli isolates from chickens in Henan Province, China. J Med Microbiol. 2009;58:1449–53.
Li L, Wang B, Feng S, Li J, Wu C, Wang Y, et al. Prevalence and characteristics of extended-spectrum beta-lactamase and plasmid-mediated fluoroquinolone resistance genes in Escherichia coli isolated from chickens in Anhui province, China. PLoS ONE. 2014;9:e104356.
Shahada F, Chuma T, Kosugi G, Kusumoto M, Iwata T, Akiba M. Distribution of extended-spectrum cephalosporin resistance determinants in Salmonella enterica and Escherichia coli isolated from broilers in southern Japan. Poult Sci. 2013;92:1641–9.
Smet A, Martel A, Persoons D, Dewulf J, Heyndrickx M, Catry B, et al. Diversity of extended-spectrum beta-lactamases and class C beta-lactamases among cloacal Escherichia coli isolates in Belgian broiler farms. Antimicrob Agents Chemother. 2008;52:1238–43.
Qabajah M, Awwad E, Ashhab Y. Molecular characterisation of Escherichia coli from dead broiler chickens with signs of colibacillosis and ready-to-market chicken meat in the West Bank. Br Poult Sci. 2014;55:442–51.
Li Y, Chen L, Wu X, Huo S. Molecular characterization of multidrug-resistant avian pathogenic Escherichia coli isolated from septicemic broilers. Poult Sci. 2015;94:601–11.
Huang SY, Dai L, Xia LN, Du XD, Qi YH, Liu HB, et al. Increased prevalence of plasmid-mediated quinolone resistance determinants in chicken Escherichia coli isolates from 2001 to 2007. Foodborne Pathog Dis. 2009;6:1203–9.
Johnson TJ, Logue CM, Johnson JR, Kuskowski MA, Sherwood JS, Barnes HJ, et al. Associations between multidrug resistance, plasmid content, and virulence potential among extraintestinal pathogenic and commensal Escherichia coli from humans and poultry. Foodborne Pathog Dis. 2012;9:37–46.
Wang X, Liu Y, Qi X, Wang R, Jin L, Zhao M, et al. Molecular epidemiology of colistin-resistant Enterobacteriaceae in inpatient and avian isolates from China: high prevalence of mcr-negative Klebsiella pneumoniae. Int J Antimicrob Agents. 2017;50:536–41.
Perrin-Guyomard A, Bruneau M, Houee P, Deleurme K, Legrandois P, Poirier C, et al. Prevalence of mcr-1 in commensal Escherichia coli from French livestock, 2007 to 2014. Euro Surveill. 2016;21:30135.
Irrgang A, Roschanski N, Tenhagen BA, Grobbel M, Skladnikiewicz-Ziemer T, Thomas K, et al. Prevalence of mcr-1 in E. coli from livestock and food in Germany, 2010–2015. PLoS ONE. 2016;11:e0159863.
Huang X, Yu L, Chen X, Zhi C, Yao X, Liu Y, et al. High prevalence of colistin resistance and mcr-1 gene in Escherichia coli isolated from food animals in China. Front Microbiol. 2017;8:562.
Allerberger F, Weissensteiner G, Springer B, Schlagenhaufen C, Lassnig H, Ruppitsch W, et al. Plasmid-mediated colistin-resistance in Escherichia coli isolated from poultry and broiler meat in Austria in 2016. Int J Infect Dis. 2016;53:36–7.
Authors’ contributions
AAM, HH, HMH, RE, SM and HE participated in the conception and design of the study. AAM, HH and HE performed farm and laboratory work. AAM, HH, HN, HT, RE, SM, HMH and HE analyzed the data and drafted the manuscript. HN, HT, UR and HMH participated in manuscript revision. All authors read and approved the final manuscript.
Acknowledgements
The authors thank B. Hofmann and P. Methner at the Friedrich-Loeffler-Institut and I. Engelmann at Alere Technologies GmbH for their cooperation and technical assistance. The authors thank Animal Health Research Institute, Mansoura Laboratory for its great effort in sample collection and preparation.
Competing interests
None of the authors of this paper has a financial or personal relationship with other people or organisations that could inappropriately influence or bias the content of the paper. RE and SM are employees of Alere Technologies GmbH.
Availability of data
All the data supporting the results are presented in the main manuscript.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Moawad, A.A., Hotzel, H., Neubauer, H. et al. Antimicrobial resistance in Enterobacteriaceae from healthy broilers in Egypt: emergence of colistin-resistant and extended-spectrum β-lactamase-producing Escherichia coli. Gut Pathog 10, 39 (2018). https://doi.org/10.1186/s13099-018-0266-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13099-018-0266-5