Abstract
Introduction
Regarding a potential relationship between diabetes and the prognostic significance of hyperglycemia in patients presenting with acute myocardial infarction (AMI), there is still debate. Therefore, we aimed in this study to demonstrate the effect of hyperglycemia on different outcomes in AMI patients, whether they are diabetic or not.
Methods
We searched PubMed, Web of Science, and Scopus using the following search strategy: “Diabetes” or “Diabetic” AND “Acute myocardial infarction” OR “AMI” AND “hyperglycemia” OR “glucose level” to find eligible articles that needed to go through the screening process for inclusion in our study. We conducted a meta-analysis of 19 included studies from Japan, Germany, China, the United Kingdom, and others using Review Manager version 5.4 software, pooling the mean difference in continuous variables, the number and total of dichotomous variables to measure the odds ratio (OR), and the generic inverse variance of OR or hazard ratio (HR) as reported in the included studies.
Results
The mean age of the participants ranged from 56.3 to 72.3 years old. The difference in blood glucose levels between diabetes and non-diabetes patients was found to be statistically significant, with an SMD of 1.39 (95%CI: 1.12, 1.66, p < 0.00001). In diabetic patients, hyperglycemia was statistically significantly associated with mortality, with a HR of 1.92 (95% CI: 1.45, 2.55, p < 0.00001) and an OR of 1.76 (95% CI: 1.15, 2.7, p = 0.01). In non-diabetic patients admitted with AMI, hyperglycemia was statistically significantly associated with mortality, with a HR of 1.56 (95% CI: 1.31, 1.86, p < 0.00001) and an OR of 2.89 (95% CI: 2.47, 3.39, p < 0.00001). AMI patients who were diabetic were statistically more likely to have a major adverse cardiovascular event (MACE) (HR = 1.9; 95% CI: 1.19–3.03; p = 0.007). AMI patients who were not diabetic were also statistically more likely to have a MACE (HR = 1.6; 95% CI: 1.15–2.23, p = 0.006).
Conclusion
Hyperglycemia in AMI patients is a predictor of worse outcomes, including MACE and mortality, regardless of whether these patients are diabetic or not. In these patients, some factors act as predictors of mortality, including older age, higher glucose levels on admission, and a high Killip class.
Similar content being viewed by others
Introduction
Globally, acute coronary syndromes (ACS) constitute a major cause of mortality, with acute myocardial infarction (AMI) being particularly concerning due to its high short- and long-term death rates [1]. The World Health Organization (WHO) predicts that by 2030, there will be over 23.6 million cardiovascular deaths worldwide, marking a significant increase from previous decades [2]. Even in the absence of preexisting diabetes, hyperglycemia can emerge during an AMI due to stress-induced increases in catecholamines, steroids, and glucagon levels, along with a decrease in insulin levels [3].
According to previous studies, 20 to 50% of patients with ST-segment elevation myocardial infarction (STEMI) experience stress hyperglycemia upon admission [4, 5]. The American Heart Association and the Endocrine Society Clinical Guidelines define stress hyperglycemia as a random plasma glucose level above 140 mg/dL in both diabetic and non-diabetic hospitalized patients [6]. A study has highlighted that hyperglycemia, whether in diabetic or non-diabetic patients, adversely affects AMI outcomes [7].
Research has shown that type 2 diabetes mellitus (T2DM) is a common comorbidity among patients with cardiovascular diseases, particularly AMI, and is detected in more than 20% of patients admitted for suspected AMI [6]. T2DM is associated with double the risk of in-hospital mortality and increases the likelihood of major adverse cardiovascular events (MACE) during follow-up [6]. Additionally, 10–20% of non-diabetic AMI patients exhibit significant hyperglycemia, which is linked to a higher risk of MACE [7]. Admission hyperglycemia is recognized as an independent predictor of poor short- and long-term outcomes in AMI patients [8].
A study examining the prognostic significance of the stress hyperglycemia ratio (SHR) and admission blood glucose (ABG) levels in AMI patients found that elevated SHR and ABG levels are associated with increased 30-day and 1-year mortality, especially in diabetic patients [9]. For instance, Meshref [10] noted that hyperglycemia correlates with larger infarct sizes, greater summation of ST-segment elevation (sum STE), maximum ST-segment elevation (max STE), higher echocardiographic wall motion score index (WMSI), and a lower segmental ejection fraction (EF).
Hyperglycemia generally increases the incidence of MACE, including re-hospitalization for heart failure, stroke, and coronary disease, in addition to raising mortality rates [11]. Regardless of whether thrombolysis or primary percutaneous coronary intervention (pPCI) is used as reperfusion therapy, hyperglycemia at admission is a significant predictor of adverse outcomes in AMI patients [12, 13]. Therefore, this study aims to demonstrate the effect of hyperglycemia on different outcomes in AMI patients, whether they are diabetic or not.
Methods
Adhering to the Cochrane Handbook of Systematic Reviews of Interventions at each step [14] and following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement’s guidelines, we conducted this systematic review and meta-analysis [15].
Database searching and screening
Using the following search strategy: “Diabetes” or “Diabetic” AND “Acute myocardial infarction” OR “AMI” AND “hyperglycemia” OR “glucose level,” we searched PubMed, Web of Science, and Scopus for eligible articles that should undergo the screening process to determine their ability to be included in our study. After searching the database, we removed the duplicates from the resulting articles using EndNote version 7 [16]. software, and then we uploaded the remaining articles on Rayyan software [17] to conduct the screening process. First, four authors who worked independently conducted the screening by title and abstract to see the eligibility for inclusion. Then they conducted full-text screening of the included articles from the previous step. Any conflicts were referred to a senior author to resolve.
Inclusion and exclusion criteria
The predetermined inclusion and exclusion criteria used for screening were any observational (cohort, cross-sectional, or case-control) and randomized controlled trials (RCT) investigating the effects of hyperglycemia in diabetic or non-diabetic AMI patients on short- or long-term outcomes such as mortality and the occurrence of MACE. We excluded studies that didn’t measure the effect of hyperglycemia, populations other than AMI, case reports, case series, and reviews.
Quality assessment
For the included observational cohort studies, we used the New Castle Ottawa scale tool provided by Cochrane for the assessment of quality. It is composed of eight questions with a maximum of one star for each, except for the comparability question, which can get two stars. Therefore, the highest score is nine, while the lowest is zero. Studies scoring from 0 to 3 were considered of low quality, 4–6 were of moderate quality, and 7–9 were of high quality [18].
Data extraction
Using Microsoft Excel sheets, four independent authors conducted the process of data extraction to extract the baseline data (study design, country, sample size, groups, age, and gender) in addition to the outcomes (blood glucose on admission, odds ratio [OR], hazard ratio [HR] of mortality and MACE, mortality rate, factors affecting mortality including age, admission glucose levels, and Killip class) of the included studies. Any differences or conflicts were resolved by a senior author.
Statistical analysis and sensitivity analysis
Using Review Manager version 5.4 software, we conducted the meta-analysis of the included studies by pooling the mean difference in continuous variables, the number and total of dichotomous variables to measure the OR, and the generic inverse variance of OR or HR as they were reported in the included studies. The results were considered statistically significant at a p-value of less than or equal to 0.05. The confidence interval (CI) was 95%, and the I2 was used to test the heterogeneity with the p-value for significance. Using OpenMetaAnalyst software, we conducted a sensitivity analysis using the leave-one-out method to remove the studies that caused heterogeneity in the heterogeneous outcomes.
Results
Database searching and screening
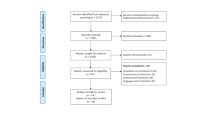
The database searching process yielded a total of 2157 articles with 761 duplicates, so a total of 1396 articles entered the title and abstract screening. A total of 1369 articles were excluded, and then 27 articles were screened in full text to yield a total of 19 articles (6,19–36) for the meta-analysis (Fig. 1).
Quality assessment
In terms of ascertainment of exposure, all studies employed reliable methods to measure hyperglycemia, typically using hospital records or standardized glucose tests. For instance, two studies ensured accurate hyperglycemia measurement through validated laboratory tests. The studies demonstrated that the outcome of interest (e.g., mortality or MACE) was not present at the start of the study. This was evidenced by baseline assessments ensuring participants were free of these outcomes upon enrollment. Comparability of cohorts was achieved in most studies by controlling for key confounders such as age, gender, and baseline health conditions. Some studies employed multivariate analyses to adjust for these variables, enhancing the reliability of their findings. Regarding the assessment of outcomes, all studies used robust methods to track mortality and MACE, often through follow-up visits and hospital records. The follow-up durations were generally adequate, with most studies ensuring sufficient time to observe significant outcomes. The adequacy of follow-up was also well-maintained in most studies, with low drop-out rates and thorough accounting for missing data. This thorough follow-up contributed to the high-quality ratings for studies. The overall high quality of the included studies reinforces the reliability of our findings.
The risk of bias assessment results for all studies are shown in (Table 1); out of the 19 studies, 13 studies were considered of high quality (scores 7–9), while only six studies were considered of moderate quality (scores 4–6). High-quality studies provided robust data with minimal bias, ensuring reliability in our meta-analysis results. Moderate-quality studies, although slightly limited in some aspects, still contributed valuable data.
Baseline characteristics
All 19 included articles were cohort studies conducted across various countries, including China, Italy, Sweden, Germany, Denmark, Japan, Portugal, Tunisia, and the United Kingdom, with sample sizes ranging from as few as 60 to as many as 10,094 participants, totaling 29,659 participants in this meta-analysis. Most studies compared hyperglycemic AMI patients with diabetes to those without diabetes, while others compared either of these groups against patients with no hyperglycemia and no diabetes. The mean age of participants ranged from 56.3 to 72.3 years, with a predominance of male participants in most studies, ranging from 48 to 82%. The largest cohort was observed in a Swedish study with 10,094 participants, whereas the smallest cohort, from China, involved 60 participants. Notably, one study also analyzed participants with Myocardial Infarction with Obstructive Coronary Arteries (MIOCA) and Myocardial Infarction with Non-Obstructive Coronary Arteries (MINOCA), providing a comprehensive overview of the impact of hyperglycemia across different myocardial infarction subtypes. The detailed baseline characteristics of the participants and the specific variables analyzed in each study, can be viewed in Table 2.
Meta-analysis
The difference between diabetes and non-diabetes patients regarding blood glucose level was found to be statistically significant with SMD of 1.39 (95%CI: 1.12, 1.66, p < 0.00001) with heterogeneity (I2 = 98%, p < 0.00001) (Fig. 2).
Hyperglycemia in diabetes patients was found to be more statistically significantly associated with mortality compared to hyperglycemia in non-diabetic patients with OR of 1.47 (95%CI: 1.08, 1.99, p = 0.01) and heterogeneity measured by I2 = 73%, p = 0.005 (Fig. 3).
Hyperglycemia in diabetic patients was statistically significantly associated with mortality with HR of 1.92 (95%CI: 1.45, 2.55, p < 0.00001) with heterogeneity (I2 = 81%, p < 0.0001). (Fig. 4) and OR of 1.76 (95%CI: 1.15, 2.7, p = 0.01) with heterogeneity (I2 = 78%, p = 0.01) (Fig. 5).
In non-diabetic patients admitted with AMI, hyperglycemia was statistically significantly associated with mortality with HR of 1.56 (95%CI: 1.31, 1.86, p < 0.00001), heterogeneity (I2 = 72%, p = 0.002) and OR of 2.89 (95%CI: 2.47, 3.39, p < 0.00001) and no heterogeneity (I2 = 0%). (Figs. 6, and 7)
Moreover, hyperglycemia in diabetic patients admitted with AMI was statistically significantly associated with the occurrence of MACE with HR of 1.9 (95%CI: 1.19, 3.03, p = 0.007) and heterogeneity (I2 = 83%, p = 0.003) (Fig. 8). In addition, hyperglycemia in non-diabetic AMI patients was statistically significantly associated with the occurrence of MACE with HR of 1.6 (95%CI: 1.15, 2.23, p = 0.006) and heterogeneity (I2 = 89%, p < 0.00001) (Fig. 9).
Age was among the factors that predicted mortality after hyperglycemia in diabetic and non-diabetic AMI patients, with HR of 1.05 (1.04, 1.07, p < 0.00001) and no heterogeneity (I2 = 0%) in diabetic patients and HR of 1.07 (95%CI: 1.02, 1.12, p = 0.01) and heterogeneity (I2 = 84%, p = 0.01) in non-diabetic patients (Fig. 10). It was observed that increased glucose levels on admission are statistically significant predictors of mortality in diabetic and non-diabetic patients with OR of 4.7 (95%CI: 1.48, 14.91, p = 0.009) and no heterogeneity (I2 = 0%) and OR of 1.88 (95%CI: 1.52, 2.33, p < 0.00001) and no heterogeneity (I2 = 0%), respectively (Fig. 11). Killip class ≥ 2 was statistically significantly associated with mortality in non-diabetic AMI patients admitted with hyperglycemia with HR of 1.9 (95%CI: 1.4, 2.57, p < 0.0001) and non-significant heterogeneity (I2 = 42%, p = 0.18), while no significant association was observed between Killip class and mortality in diabetic patients with HR of 0.94 (95%CI: 0.62, 1.42, p = 0.76) (Fig. 12).
Sensitivity analysis
After conducting a leave-one-out analysis for blood glucose levels comparison among the diabetic and non-diabetic patients, it was found that Cui et al. (2022) [34] and Kojima et al. (2019) [33] were the main sources of heterogeneity. (Supplementary Fig. 1)
For the comparison between the diabetic and non-diabetic patients regarding mortality, it was observed that Schmitz et al. (2022) [23] was the main source of heterogeneity. (Supplementary Fig. 2)
For the mortality outcome in diabetic patients using HR, Cui et al. (2021) [21] and Kojima et al. (2019) [33] were considered the main reasons for heterogeneity. (Supplementary Fig. 3) While using OR, Cui et al. (2023) [19] was the main source of heterogeneity. (Supplementary Fig. 4)
For the mortality outcome in non-diabetic patients using HR, Cui et al. (2021) [21] was considered the main source for heterogeneity. (Supplementary Fig. 5) Regarding the occurrence of MACE in diabetic patients, Cui et al. (2022) [34] caused heterogeneity in the outcome (Supplementary Fig. 6), while Ristinger et al. (2021) [36] caused heterogeneity in the MACE outcome of non-diabetics. (Supplementary Fig. 7) Yuan et al. (2022) [20] was observed to be the main cause of heterogeneity in the association of age with mortality using OR. (Supplementary Fig. 8).
Discussion
The meta-analysis suggests a statistically significant difference in blood glucose levels between diabetes and non-diabetes patients. The observed standardized mean difference (SMD) of 1.39 in blood glucose levels between diabetes and non-diabetes patients is consistent with findings from previous studies [37, 38]. This reinforces the notion that diabetes patients tend to exhibit significantly higher blood glucose levels compared to their non-diabetic counterparts. However, it is crucial to note that our meta-analysis revealed high heterogeneity, indicating substantial variability across the included studies. This starkly contrasts with the comparatively lower heterogeneity reported in the studies by Redondo et al. (2020) [39]. While our findings align with prior research, the substantial heterogeneity underscores the need for a nuanced interpretation and calls for further investigation into factors contributing to the observed differences among studies. Addressing these variations may enhance the reliability and generalizability of conclusions drawn from future meta-analyses in this domain.
The analysis indicates a higher risk of mortality among diabetes patients with hyperglycemia compared to non-diabetic individuals. The odds ratio (OR) for mortality in diabetes patients with hyperglycemia is 1.47. Despite moderate heterogeneity, the statistical significance of the odds ratio suggests a noteworthy association. This finding implies that hyperglycemia in diabetes patients may be linked to an increased risk of mortality [40, 41]. The presence of moderate heterogeneity emphasizes the need for caution in interpretation, prompting further exploration into potential sources of variability among the included studies.
The hazard ratio (HR) of 1.92, indicating a substantial increase in mortality risk associated with hyperglycemia in diabetic patients, aligns with findings from prior research [42, 43]. However, the observed substantial heterogeneity suggests a notable variability among the included studies, emphasizing the importance of carefully considering potential sources of this heterogeneity for a more nuanced interpretation. Similarly, the odds ratio (OR) of 1.76 underscores an increased risk of mortality in diabetic patients with hyperglycemia, consistent with the results of studies [44, 45]. Nevertheless, the considerable heterogeneity warrants caution in interpreting these results, urging researchers to explore the underlying causes of this variability for more robust conclusions.
In non-diabetic patients admitted with acute myocardial infarction (AMI), hyperglycemia is associated with a statistically significant increase in mortality, as evidenced by a hazard ratio (HR) of 1.56. The presence of heterogeneity emphasizes the importance of carefully considering potential contributing factors to this variability. This association aligns with research by Sachdeva et al. (2020), which reported similar trends in non-diabetic AMI patients [46]. Furthermore, the odds ratio (OR) for mortality in this context is notably higher at 2.89, and interestingly, no heterogeneity is observed. The absence of heterogeneity in the odds ratio contrasts with the variability seen in the hazard ratio, indicating a more consistent pattern of increased mortality risk associated with hyperglycemia in this specific context.
In diabetic patients admitted with acute myocardial infarction (AMI), hyperglycemia is consistently associated with a higher risk of major adverse cardiac events (MACE), in line with findings from previous studies [47, 48]. However, the substantial heterogeneity observed in this subgroup suggests caution in interpreting results and prompts further investigation into potential contributing factors. Similarly, in non-diabetic AMI patients, hyperglycemia is significantly linked to an increased occurrence of MACE, aligning with research by Jensen et al. (2021) [49]. The observed high heterogeneity in this subgroup underscores the need for careful consideration of variability among studies. The presence of heterogeneity emphasizes the importance of understanding and addressing variations in study characteristics for more accurate clinical implications and interventions. Future research should focus on revealing the sources of heterogeneity to enhance the precision and applicability of these findings in diverse patient populations.
In both diabetic and non-diabetic acute myocardial infarction (AMI) patients, age stands out as a significant predictor of mortality following hyperglycemia. The small but significant hazard ratio (HR) of 1.05 in diabetic patients indicates a slight increase in mortality risk per unit increase in age, with no observed heterogeneity, providing confidence in this association. Conversely, in non-diabetic AMI patients, the HR of 1.07 suggests a slightly stronger impact of age on mortality. However, the substantial heterogeneity underscores the importance of considering potential variations in study characteristics. The association between age and mortality in diabetic AMI patients aligns with studies by Tachkov et al. (2020) [50]. In non-diabetic AMI patients, similar trends have been reported by Jansson et al. (2010) [51]. Notably, the presence of heterogeneity in the non-diabetic subgroup emphasizes the need for cautious interpretation and further exploration into potential sources of variability. While the association is more straightforward in diabetic patients, addressing heterogeneity in non-diabetic patients is crucial for refining our understanding of the age-related mortality risk and informing tailored clinical approaches.
Elevated glucose levels on admission were identified as significant predictors of mortality in both diabetic and non-diabetic patients. In diabetic patients, the odds ratio (OR) of 4.7 and in non-diabetic patients, the OR of 1.88 highlight the substantial impact of hyperglycemia on mortality risk. These findings align with studies by Kattel et al. (2017), emphasizing the consistent association between elevated glucose levels and increased mortality in diverse patient populations [52].
In non-diabetic AMI patients with hyperglycemia, Killip class ≥ 2 is significantly associated with mortality, in agreement with research by Mamadjanov et al. (2021) [29]. Conversely, no significant association between Killip class and mortality is observed in diabetic patients (HR: 0.94, 95% CI: 0.62, 1.42, p = 0.76), consistent with the findings of Hashmi et al. (2020) [53]. These interpretations underscore the universal impact of hyperglycemia on mortality risk in acute myocardial infarction patients while also emphasizing the divergent relevance of the Killip class in predicting outcomes based on diabetic status [54]. The insights from these comparisons contribute to a more nuanced understanding of risk factors and aid in tailoring interventions for diverse patient cohorts.
The sensitivity analysis highlighted significant sources of heterogeneity in various aspects of the study. In the comparison of blood glucose levels between diabetic and non-diabetic patients, Cui et al. (2022) [34] and Kojima et al. (2019) [33] were identified as primary contributors to heterogeneity. While the sources of heterogeneity are acknowledged, In the analysis of mortality between diabetic and non-diabetic patients, Schmitz et al. (2022) [23] emerged as the primary source of heterogeneity. Understanding the specific characteristics or methodologies contributing to this heterogeneity may facilitate a more nuanced interpretation of mortality outcomes in diabetic and non-diabetic groups. Examining mortality outcomes in diabetic patients using hazard ratios (HR), Cui et al. (2021) [21] and Kojima et al. (2019) [33] were identified as significant contributors to heterogeneity.
In the analysis using odds ratios (OR) for mortality in diabetic patients, Cui et al. (2023) [19] identified the main source of heterogeneity. Understanding the distinct factors introduced by this study can aid in refining interpretations and addressing potential biases. For mortality outcomes in non-diabetic patients using HR, Cui et al. (2021) [21] were pinpointed as the primary source of heterogeneity. Exploring how this study differs from others in the analysis may offer insights into the observed variations in non-diabetic mortality outcomes. Regarding major adverse cardiac events (MACE) in diabetic patients, Cui et al. (2022) [34] were recognized as the main contributors to heterogeneity. Understanding the specific aspects introduced by this study may help contextualize variations in MACE outcomes among diabetic patients.
In non-diabetic patients, Ristinger et al. (2021) [36] identified heterogeneity as the main source of heterogeneity in MACE outcomes. Previous studies may reveal distinct characteristics contributing to variations in MACE occurrences among non-diabetic individuals [55, 56]. Yuan et al. (2022) [20] observed age as the primary cause of heterogeneity in the association of age with mortality using odds ratios (OR). Understanding the unique aspects introduced by this study can contribute to a more comprehensive interpretation of age-related mortality associations.
A significant strength of this study is the inclusion of a large and diverse population across multiple countries, which enhances the generalizability of the findings. Additionally, the inclusion of studies analyzing both diabetic and non-diabetic populations, as well as different subtypes of myocardial infarction, provides a comprehensive understanding of the impact of hyperglycemia in various contexts. The study demonstrates that hyperglycemia is a strong predictor of negative outcomes, such as mortality and major adverse cardiovascular events (MACE). This comprehensive analysis substantiates the necessity of targeted interventions to manage hyperglycemia in AMI patients, further reinforcing the reliability of the findings.
However, this study has some limitations, including the fact that all included studies are observational in design, which carries a higher risk of bias compared to randomized controlled trials (RCTs). The reliance on retrospective data from diverse studies might introduce biases, affecting the overall robustness of the meta-analysis. Furthermore, the observed associations between hyperglycemia and mortality or major adverse cardiovascular events may be influenced by confounding factors not fully accounted for in the included studies. Therefore, while the findings are significant, they should be interpreted with caution. Further research, particularly randomized controlled trials (RCTs), is needed to refine these conclusions and determine effective interventions. Despite these limitations, our study contributes valuable evidence to understanding hyperglycemia’s role in AMI outcomes.
Conclusion
Hyperglycemia in AMI patients are a predictor of worse outcomes including MACE, and mortality whether these patients are diabetic or not. Some factors act as predictors for mortality in these patients including older age, higher glucose levels on admission, and high Killip class. However, further studies are required to put a definite value for hyperglycemia and cut off for prognosis.
Data availability
All data generated or analyzed in this study are included in this publish article. For any further information or clarifications, please contact Alawaji, Reem (rzalawaji@gmail.com).
References
Steg PG, James SK, Atar D, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33(20):2569 − 619.
Ramic-Catak A, Mesihovic-Dinarevic S, Prnjavorac B, Naser N, Masic I. Public Health Dimensions of Cardiovascular Diseases (CVD) Prevention and Control–Global Perspectives and Current Situation in the Federation of Bosnia and Herzegovina. Materia Socio-Medica. 2023;35(2):88.
Qaseem A, Humphrey LL, Chou R, Snow V, Shekelle P. Use of intensive insulin therapy for the management of glycemic control in hospitalized patients: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2011;154(4):260-7.
Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet. 2000;355(9206):773-8.
Wahab NN, Cowden EA, Pearce NJ, Gardner MJ, Merry H, Cox JL. Is blood glucose an independent predictor of mortality in acute myocardial infarction in the thrombolytic era? J Am Coll Cardiol. 2002;40(10):1748-54.
Paolisso P, Foà A, Bergamaschi L, et al. Impact of admission hyperglycemia on short and long-term prognosis in acute myocardial infarction: MINOCA versus MIOCA. Cardiovasc Diabetol. 2021;20(1):192.
Paolisso P, Foà A, Bergamaschi L, et al. Hyperglycemia, inflammatory response and infarct size in obstructive acute myocardial infarction and MINOCA. Cardiovasc Diabetol. 2021;20(1):33.
Marenzi G, Cosentino N, Milazzo V, De Metrio M, Cecere M, Mosca S, Rubino M, Campodonico J, Moltrasio M, Marana I, Grazi M. Prognostic value of the acute-to-chronic glycemic ratio at admission in acute myocardial infarction: a prospective study. Diabetes Care. 2018 Apr 1;41(4):847 − 53.
Liang S, Tian X, Gao F, et al. Prognostic significance of the stress hyperglycemia ratio and admission blood glucose in diabetic and nondiabetic patients with spontaneous intracerebral hemorrhage. Diabetol Metab Syndr. 2024;16:58.
Meshref TS, Ashry MA, El-Aal RFA, et al. Unique role of admission hyperglycemia on myocardial infarction size and area at risk following an acute ST-elevation myocardial infarction. Egypt J Intern Med. 2020;32(1):15.
Sardu C, Barbieri M, Balestrieri ML, Siniscalchi M, Paolisso P, Calabrò P, et al. Thrombus aspiration in hyperglycemic ST-elevation myocardial infarction (STEMI) patients: clinical outcomes at 1-year follow-up. Cardiovasc Diabetol. 2018;17(1):152.
Malmberg K, Norhammar A, Wedel H, Rydén L. Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long-term results from the Diabetes and Insulin-Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study. Circulation. 1999;99(20):2626-32.
Timmer JR, Hoekstra M, Nijsten MW, van der Horst IC, Ottervanger JP, Slingerland RJ, et al. Prognostic value of admission glycosylated hemoglobin and glucose in nondiabetic patients with ST-segment-elevation myocardial infarction treated with percutaneous coronary intervention. Circulation. 2011;124(6):704 − 11.
Cochrane Handbook for Systematic Reviews of Interventions.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336 − 41.
Eapen BR. EndNote 7.0. Indian J Dermatol Venereol Leprol. 2006;72(2):165-6.
Rayyan [Available from: https://rayyan.ai/.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603-5.
Cui K, Fu R, Yang J, Xu H, Yin D, Song W, et al. The impact of fasting stress hyperglycemia ratio, fasting plasma glucose and hemoglobin A1c on in-hospital mortality in patients with and without diabetes: findings from the China acute myocardial infarction registry. Cardiovascular Diabetology. 2023;22(1):165.
Yuan Y, Tao J, Shen X, Cheng H, Dong X, Muyesai N, et al. Elevated random glucose levels at admission are associated with all-cause mortality and cardiogenic shock during hospitalisation in patients with acute myocardial infarction and without diabetes: A retrospective cohort study. Diabetes Metab Res Rev. 2023;39(4)
Cui CY, Zhou MG, Cheng LC, Ye T, Zhang YM, Zhu F, et al. Admission hyperglycemia as an independent predictor of long-term prognosis in acute myocardial infarction patients without diabetes: A retrospective study. J Diabetes Investig. 2021;12(7):1244-51.
Upur H, Li J-L, Zou X-G, Hu Y-Y, Yang H-Y, Abudoureyimu A, et al. Short and long-term prognosis of admission hyperglycemia in patients with and without diabetes after acute myocardial infarction: a retrospective cohort study. Cardiovascular Diabetology. 2022;21(1):114.
Schmitz T, Freuer D, Harmel E, Heier M, Peters A, Linseisen J, et al. Prognostic value of stress hyperglycemia ratio on short- and long-term mortality after acute myocardial infarction. Acta Diabetol. 2022;59(8):1019-29.
Jomaa W, El Mhamdi S, Ben Ali I, Azaiez MA, El Hraiech A, Ben Hamda K, et al. Prognostic value of hyperglycemia on-admission in diabetic versus non-diabetic patients presenting with ST-elevation myocardial infarction in Tunisia. Indian Heart J. 2018;70(6):772-6.
Chattopadhyay S, George A, John J, Sathyapalan T. Newly diagnosed abnormal glucose tolerance determines post-MI prognosis in patients with hospital related hyperglycaemia but without known diabetes. J Diabetes Complications. 2020;34(4):107518.
Chattopadhyay S, George A, John J, Sathyapalan T. Two-hour post-challenge glucose is a better predictor of adverse outcome after myocardial infarction than fasting or admission glucose in patients without diabetes. Acta Diabetol. 2018;55(5):449 − 58.
Ferreira JA, Baptista RM, Monteiro SR, Gonçalves FM, Monteiro PF, Gonçalves LM. Admission hyperglycemia and all-cause mortality in diabetic and non-diabetic patients with acute myocardial infarction: a tertiary center analysis. Intern Emerg Med. 2021;16(8):2109-19.
Zhou J, Sheng Z, Liu C, Zhou P, Li J, Chen R, et al. Association between Admission Hyperglycemia and Culprit Lesion Characteristics in Nondiabetic Patients with Acute Myocardial Infarction: An Intravascular Optical Coherence Tomography Study. J Diabetes Res. 2020;2020:1763567.
Mamadjanov T, Volaklis K, Heier M, Freuer D, Amann U, Peters A, et al. Admission glucose level and short-term mortality in older patients with acute myocardial infarction: results from the KORA Myocardial Infarction Registry. BMJ Open. 2021;11(6)
Ritsinger V, Jensen J, Ohm D, Omerovic E, Koul S, Fröbert O, et al. Elevated admission glucose is common and associated with high short-term complication burden after acute myocardial infarction: Insights from the VALIDATE-SWEDEHEART study. Diab Vasc Dis Res. 2019;16(6):582-4.
Demarchi A, Cornara S, Somaschini A, Fortuni F, Mandurino-Mirizzi A, Crimi G, et al. Has hyperglycemia a different prognostic role in STEMI patients with or without diabetes? Nutr Metab Cardiovasc Dis. 2021;31(2):528 − 31.
Thoegersen M, Josiassen J, Helgestad OK, Berg Ravn H, Schmidt H, Holmvang L, et al. The association of diabetes and admission blood glucose with 30-day mortality in patients with acute myocardial infarction complicated by cardiogenic shock. Eur Heart J Acute Cardiovasc Care. 2020;9(6):626 − 35.
Kojima T, Hikoso S, Nakatani D, Suna S, Dohi T, Mizuno H, et al. Impact of Hyperglycemia on Long-Term Outcome in Patients With ST-Segment Elevation Myocardial Infarction. Am J Cardiol. 2020;125(6):851-9.
Cui K, Fu R, Yang J, Xu H, Yin D, Song W, et al. Admission Blood Glucose and 2-Year Mortality After Acute Myocardial Infarction in Patients With Different Glucose Metabolism Status: A Prospective, Nationwide, and Multicenter Registry. Front Endocrinol (Lausanne). 2022;13:898384.
Ding XS, Wu SS, Chen H, Zhao XQ, Li HW. High admission glucose levels predict worse short-term clinical outcome in non-diabetic patients with acute myocardial infraction: a retrospective observational study. BMC Cardiovasc Disord. 2019;19(1):163.
Ritsinger V, Hagström E, Lagerqvist B, Norhammar A. Admission Glucose Levels and Associated Risk for Heart Failure After Myocardial Infarction in Patients Without Diabetes. J Am Heart Assoc. 2021;10(22).
Patel BJ, Dave B, Dave D, Karmakar P, Shah M, Sarvaiya B. Comparison and Correlation of Glucose Levels in Serum and Saliva of Both Diabetic and Non-diabetic Patients. J Int Oral Health. 2015 Aug;7(8):70 − 6.
Frankum S, Ogden J. Estimation of blood glucose levels by people with diabetes: a cross-sectional study. Br J Gen Pract. 2005 Dec;55(521):944-8.
Redondo MJ, Geyer S, Steck AK, Sharp SA, Wentworth JM, Weedon M, et al. The clinical consequences of heterogeneity within and between different diabetes types. Diabetologia. 2020 Oct;63(10):2040-8. https://doi.org/10.1007/s00125-020-05211-7.
Morse J, Gayle A, Wood A, Man-Phillips D, Curtis E, Nazir A, et al. Hyperglycaemia increases mortality risk in non-diabetic patients with COVID-19 even more than in diabetic patients. Endocrinol Diabetes Metab. 2021 Oct;4(4). https://doi.org/10.1002/edm2.291.
Moghaddam Tabrizi F, Rasmi Y, Hosseinzadeh E, Rezaei S, Balvardi M, Kouchari MR, et al. Diabetes is associated with higher mortality and severity in hospitalized patients with COVID-19. Excli J. 2021;20:444 − 53. https://doi.org/10.17179/excli2021-3403.
Asadollahi K, Beeching N, Gill G. Hyperglycaemia and mortality. J R Soc Med. 2007 Nov;100(11):503-7. https://doi.org/10.1177/014107680710001112.
Palaiodimos L, Chamorro-Pareja N, Karamanis D, Li W, Zavras PD, Chang KM, et al. Diabetes is associated with increased risk for in-hospital mortality in patients with COVID-19: a systematic review and meta-analysis comprising 18,506 patients. Hormones. 2021 Jun;20(2):305 − 14. https://doi.org/10.1007/s42000-020-00246-2.
Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002 Mar;87(3):978 − 82.
Ding D, Cheng H, Li Y, Shao Q, Wang Z, Huang G, et al. Hyperglycemia and mortality among patients with coronary artery disease. Diabetes Care. 2014 Feb;37(2):546 − 54.
Sachdeva S, Desai R, Gupta U, Prakash A, Jain A, Aggarwal A. Admission Hyperglycemia in Non-diabetics Predicts Mortality and Disease Severity in COVID-19: a Pooled Analysis and Meta-summary of Literature. SN Compr Clin Med. 2020 Nov;2(11):2161-6. https://doi.org/10.1007/s42399-020-00575-8.
Kewcharoen J, Ali M, Trongtorsak A, Mekraksakit P, Vutthikraivit W, Kanjanauthai S. Admission hyperglycemia is associated with reperfusion failure in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention: a systematic review and meta-analysis. Am J Cardiovasc Dis. 2021;11(3):348 − 59.
Pistrosch F, Natali A, Hanefeld M. Is hyperglycemia a cardiovascular risk factor? Diabetes Care. 2011 May;34 Suppl 2. https://doi.org/10.2337/dc11-s207.
Jensen ES, Christensen MK, Ladefoged SA, Christiansen CB, Sandgaard NC, Jensen MT. Cardiovascular risk in patients with and without diabetes presenting with chronic coronary syndrome in 2004–2016. BMC Cardiovasc Disord. 2021 Dec;21(1):579. https://doi.org/10.1186/s12872-021-02312-y.
Tachkov K, Tachkov K, Manov A, Petkov R, Bozhinova S, Yordanova S. Life expectancy and survival analysis of patients with diabetes compared to the non diabetic population in Bulgaria. PLoS One. 2020 May;15(5). https://doi.org/10.1371/journal.pone.0232815.
Jansson SP, Andersson DK, Svärdsudd K. Mortality trends in subjects with and without diabetes during 33 years of follow-up. Diabetes Care. 2010 Mar;33(3):551-6. https://doi.org/10.2337/dc09-0680.
Kattel S, Li Q, Ball J, Teng T, Vakili K, Stewart S, et al. Association between elevated blood glucose level on admission and long-term mortality in patients with acute decompensated heart failure. J Cardiol. 2017 Apr;69(4):619 − 24. https://doi.org/10.1016/j.jjcc.2016.05.013.
Hashmi KA, Malik T, Adnan M, Ahmed J, Khalid FA, Jabbar S, et al. Risk Assessment of Patients After ST-Segment Elevation Myocardial Infarction by Killip Classification: An Institutional Experience. Cureus. 2020 Dec;12(12). https://doi.org/10.7759/cureus.12209.
Mello BH, Oliveira GB, Ramos RF, Lopes BB, Barros CB, Carvalho E, et al. Validation of the Killip-Kimball classification and late mortality after acute myocardial infarction. Arq Bras Cardiol. 2014 Aug;103(2):107 − 17. https://doi.org/10.5935/abc.20140091.
Sayadi M, Zibaeenezhad MJ, Safaei K, Elyaspour Z, Verdecchia P, Razeghian-Jahromi I. Impact of type II diabetes and gender on major clinical events after percutaneous coronary intervention. Prim Care Diabetes. 2021 Apr;15(2):347 − 51. https://doi.org/10.1016/j.pcd.2020.11.004.
Currie CJ, Berni ER, Jenkins-Jones S, Poole CD, Morgan CL, Evans M. Major adverse cardiovascular events in people with chronic kidney disease in relation to disease severity and diabetes status. PLoS One. 2019 Aug;14(8). https://doi.org/10.1371/journal.pone.0221044.
Funding
This research did not receive a specific grant from any funding agency in the public, commercial, or not-for-profit sectors. The authors themselves covered all expenses related to the study.
Author information
Authors and Affiliations
Contributions
All authors have contributed significantly to the study and manuscript preparation, and all authors are in agreement with the content of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study collected and analyzed data from previously published studies. No new human or animal subjects were directly involved in this research. All included studies were required to have obtained appropriate ethical approval and informed consent from participants, as indicated in their respective publications.
Consent to participate and publish
Since this research is a secondary analysis of existing data, direct consent to participate and publish was not required from individual subjects. However, the original studies included in this review and meta-analysis documented their consent procedures, ensuring compliance with ethical standards.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.








Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alawaji, R., Musslem, M., Alshalahi, E. et al. A systematic review and meta-analysis of the effect of hyperglycemia on admission for acute myocardial infarction in diabetic and non-diabetic patients. Diabetol Metab Syndr 16, 224 (2024). https://doi.org/10.1186/s13098-024-01459-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13098-024-01459-w