Abstract
Objective
The connection between triglycerides to high-density lipoprotein cholesterol (TG/HDL-C) ratio and stroke risk is controversial. Our goal was to explore this relationship in individuals aged 45 and older enrolled in the China Health and Retirement Longitudinal Study (CHARLS).
Methods
Our analysis encompassed 10,164 participants from the CHARLS cohorts. We applied the Cox proportional-hazards regression model to evaluate the potential correlation between the TG/HDL-C ratio and stroke incidence. Using a cubic spline function and smooth curve fitting within the Cox model allowed us to unearth a possible non-linear pattern in this relationship. We also conducted thorough sensitivity and subgroup analyses to deepen our understanding of the TG/HDL-C ratio’s impact on stroke risk.
Results
Adjusting for various risk factors, we observed a significant link between the TG/HDL-C ratio and increased stroke risk in individuals aged 45 and above (HR: 1.03, 95% CI 1.00–1.05, P = 0.0426). The relationship appeared non-linear, with an inflection at a TG/HDL-C ratio of 1.85. Ratios below this threshold indicated a heightened stroke risk (HR: 1.28, 95% CI 1.06–1.54, P = 0.0089), while ratios above it did not show a significant risk increase (HR: 1.01, 95% CI 0.98–1.04, P = 0.6738). Sensitivity analysis confirmed the robustness of these findings. Notably, non-smokers exhibited a stronger correlation between the TG/HDL-C ratio and stroke risk compared to past and current smokers.
Conclusion
Our investigation revealed a significant, yet non-linear, association between the TG/HDL-C ratio and the incidence of stroke among individuals aged 45 and above. Specifically, we found that stroke risk increased in correlation with TG/HDL-C ratio below the threshold of 1.85. These insights may guide healthcare providers in advising and developing more effective strategies for stroke prevention in this demographic.
Similar content being viewed by others
Background
As a common cause of disability and death globally, stroke carried a significant financial burden for treatment and post-stroke healthcare [1]. According to the epidemiological statistics on the worldwide burden of illness, stroke caused 6.55 million deaths and 143 million disability-adjusted life years in 2019 [2]. The elevated prevalence of stroke remained a substantial financial and healthcare burden. Previous research has elucidated a significant association between the prevalence of chronic conditions such as diabetes mellitus, hypertension, cardiovascular diseases, dyslipidemia, and chronic kidney disease and an increased risk of stroke among individuals in the middle-aged and elderly cohorts [3, 4]. Nevertheless, it is important to note that conventional risk factors alone are insufficient in comprehensively elucidating all the potential risks associated with stroke [5, 6].
Dyslipidemia, characterized by abnormal levels of lipids in the blood, is often evidenced by diminished high-density lipoprotein cholesterol (HDL-C) and increased low-density lipoprotein cholesterol (LDL-C) and triglycerides (TG). Recognized as a key risk factor in stroke development [7], recent studies have shifted focus to less traditional lipid indices, such as the ratio of triglycerides to high-density lipoprotein cholesterol (TG/HDL-C) ratio. This ratio has gained recognition as a significant marker in assessing the risk for cardiovascular diseases and metabolic syndrome [8, 9]. Although a high TG/HDL-C ratio has been linked to a greater risk of stroke [10, 11], there have been studies with conflicting results [12, 13]. This appearance may be due to differences in the TG/HDL-C ratio range, the sample sizes, and adjustment variables. Moreover, nonlinear relationships may alter linear regression study conclusions, resulting in changes in the fitted linear relationship. Therefore, we analyzed the data using the China Health and Retirement Longitudinal Study (CHARLS) to observe the relationship between the TG/HDL-C ratio and stroke risk.
Methods
Data source
In this longitudinal research, the dataset was derived from the CHARLS, covering a period from 2011 to 2018. The study’s sample was drawn from a broad cross-section of the population, encompassing 450 communities within 150 counties across 28 provinces. Utilizing a systematic methodology, CHARLS gathered biennial data encompassing demographic characteristics, nutritional habits, and overall health statuses of residents, family units, and larger community settings. In the baseline survey in 2011–2012, we included 17,708 participants (Wave 1). Data on exposure variables and all covariates were measured in 2011–2012. Participants received three follow-up visits in 2013–2014 (Wave 2), 2015–2016 (Wave 3), and 2017–2018 (Wave 4). Our study included participants if they attended any of the Wave 2, Wave 3, and Wave 4 follow-ups.
Study population
Authorization for conducting the CHARLS was officially obtained from the Biomedical Ethics Review Board of Peking University, with all subjects providing written consent in accordance with ethical standards [14]. Our study was conducted in strict alignment with the ethical guidelines set forth by the Declaration of Helsinki, ensuring compliance with all relevant regulations and legal requirements during the research process.
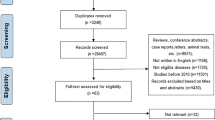
Criteria for exclusion from the participant pool included individuals with less than 2 years of follow-up, a pre-existing stroke condition, incomplete stroke data, being treated or having been treated for stroke at baseline, those under the age of 45, missing baseline data for HDL-C or TG, and TG/HDL-C ratios that were outliers (beyond three standard deviations from the mean). After applying these criteria, the cohort for our final analysis was comprised of 10,164 subjects. The methodology and flow of participants through the study are depicted in Fig. 1.
TG/HDL-C ratio
Serum TG level (mg/dL) divided by HDL-C level (mg/dL) was used to compute the TG/HDL-C ratio.
Diagnosis of stroke
In our study, the outcome of interest in this study was the occurrence of stroke during the follow-up period. We identified incident cases as participants who, being devoid of stroke at the commencement of the study, subsequently reported experiencing a stroke during the follow-up assessments. Data pertaining to the occurrence of stroke were meticulously collected through a structured self-reported questionnaire [14, 15], designed to elicit comprehensive information on three critical aspects: (1) Were you informed of a stroke diagnosis by a medical professional? (2) When did you initially receive or become aware of the diagnosis? (3) Do you have any therapy for your stroke at this time? Affirmative responses during follow-up were classified as first-time stroke diagnoses, with the reported date marking the onset. The interval between the stroke onset and baseline assessment was calculated to establish the timing of the stroke. For those without reported strokes during follow-up, we determined follow-up duration by the interval between the baseline assessment and their final survey date.
Data collection
Healthcare professionals and skilled surveyors collected a range of data from participants, including demographic details, body measurements, and health indicators. Systolic blood pressure (SBP), age, height, drinking habits, sex, diastolic blood pressure (DBP), smoking habits, weight and physical activity were all recorded. Physical activity was defined as engaging in either moderate-intensity exercise for at least 2.5 h weekly, vigorous exercise for a minimum of 1.25 h weekly, or a combination that equates to 600 or more metabolic equivalent minutes each week [16]. Smoking status was categorized into three distinct groups according to individuals’ smoking behavior: current smokers, individuals who have smoked in the past, and individuals who have never smoked. Similarly, drinking status was classified into three categories based on individuals’ drinking behavior: current drinkers, individuals who have previously consumed alcohol, and individuals who have never consumed alcohol. Weight/Height2 (kg/m2) was used to compute BMI. Hypertension was defined as SBP ≥ 140 mmHg, DBP ≥ 90 mmHg (average of 3 measurements), or hypertension history. Blood samples were taken by the medical staff from the Chinese Center for Disease Control and Prevention after an overnight fast. These samples were analyzed in a central laboratory to measure various biomarkers, including total cholesterol (TC), glycosylated hemoglobin (HbA1c), C-reactive protein (CRP), LDL-C, serum creatinine (Scr), HDL-C, serum cystatin C, TG, fasting plasma glucose (FPG), and uric acid (UA).
Missing data processing
The dataset used in the study exhibited missing values for several clinical variables, constituting a small fraction of the overall dataset. Specifically, missing values were observed for gender in 8 individuals (0.08%), BMI in 1503 individuals (14.62%), DBP in 1463 individuals (14.24%), hypertension in 55 individuals (0.54%), drinking status in 11 individuals (0.11%), SBP in 1463 individuals (14.24%), physical activity in 6011 individuals (58.49%), smoking status in 169 individuals (1.64%), FPG in 14 individuals (0.14%), TC in 3 individuals (0.03%), CRP in 1 individual (0.01%), LDL-C in 21 individuals (0.20%), Scr in 18 individuals (0.18%), HbA1c in 80 individuals (0.78%), Cystatin C in 2472 individuals (24.05%), and UA in 1 individual (0.01%). To address the missing clinical variables, multiple imputations via chained equations were employed for modeling purposes. The imputation model incorporated the following variables: smoking status, DBP, SBP, gender, physical activity, drinking status, age, hypertension, drugs for cardiovascular prevention, BMI, FPG, TC, Scr, CRP, LDL-C, Cystatin C HbA1c, and UA. The analysis of missing data followed the assumption of missing-at-random to ensure the validity of the imputation process [17].
Statistical analysis
Statistical computations were performed utilizing the R software environment along with Empower Stats. The initial categorization of baseline characteristics segmented the dataset into four groups based on quartiles of the TG/HDL-C ratio. We presented categorical variables using frequency counts and percentages, whereas median and interquartile ranges (25th–75th percentile) or mean values with standard deviations (SD) were used for continuous variables. To examine differences among the four groups, we utilized the Kruskal–Wallis H test for data with a skewed distribution, the One-Way ANOVA test for normally distributed data, or the χ2 test for categorical data.
We constructed multivariate Cox proportional hazards regression models in a three-tiered approach to test the correlation between the TG/HDL-C ratio and stroke: (1) Model I: this Model did not incorporate any covariates; (2) Model II: this Model adjusted for socio-demographic factors, including smoking status, gender, physical activity, drinking status, age, and BMI; (3) Model III: this Model adjusted for all factors, including smoking status, gender, physical activity, drinking status, age, BMI, hypertension, drugs for cardiovascular prevention, FPG, TC, Scr, CRP, LDL-C, Cystatin C HbA1c, and UA. We reported both adjusted and unadjusted hazard ratios (HR) with their 95% confidence intervals (CI).
The sensitivity analyses were conducted to check the validity of our findings. We categorized the TG/HDL-C ratio into groups based on its quartile distribution. We then determined the P-value for the trend to evaluate the significance of the TG/HDL-C ratio when considered a continuous variable and to examine its potential non-linear relationship with stroke risk. Because hypertension and drugs for cardiovascular prevention may influence the relationship between TG/HDL-C and stroke, we performed additional sensitivity analyses by excluding individuals with hypertension or drugs for cardiovascular prevention to investigate the connection between the TG/HDL-C ratio and stroke in subgroups.
To evaluate the non-linear relationship between the TG/HDL-C ratio and the occurrence of stroke, we utilized the Cox proportional hazards regression model, incorporating cubic spline functions and smooth curve fitting techniques. Upon detection of a non-linear correlation, we pinpointed the inflection point through recursive techniques. Subsequently, we applied a two-piecewise Cox proportional hazards regression model to each segment divided by the inflection point. The determination of the most suitable model to clarify the link between the TG/HDL-C ratio and stroke risk relied on the outcomes of a log-likelihood ratio test.
The subgroup analysis was performed through the utilization of the Cox proportional hazard model. The following variables were converted into categorical variables: BMI (< 25 kg/m2, ≥ 25 kg/m2) and age (< 65 years, ≥ 65 years) according to clinical cutoffs. With the exception of the stratification variable, each stratification was given a fully adjusted analysis. The likelihood ratio test was performed to validate the interactions between subgroups. All results follow the STROBE statement [18]. Statistical significance was established using a two-tailed test with a threshold of P < 0.05.
Results
Characteristics of individuals
This investigation included a cohort of 10,164 individuals without any history of stroke at baseline. The average age was 59.18 ± 9.35 years. 1,191 (11.72%) individuals developed stroke during follow-up.
The population’s baseline characteristics are shown in Table 1. Based on quartiles of the TG/HDL-C ratio (Q1 ≤ 1.32; 1.32 < Q2 ≤ 2.11; 2.11 < Q3 ≤ 3.56; Q4 > 3.56), all subjects were classified into four groups. Participants had higher levels of DBP, FPG, TG, BMI, SBP, TC, CRP, HbA1c, Scr, and UA in the Q4 group. There were higher rates of hypertension, drugs for cardiovascular prevention, ever drinkers and ever smokers in the Q4 group. A lower level of HDL-C and LDL-C was observed in the Q1 group. Participants had higher rates of physical activity and males in the Q1 group.
The incidence rate of stroke
Table 2 presents the stroke incidence rates observed in a cohort of 10,164 individuals during the follow-up period. The overall population’s cumulative incidence rate of stroke was 11.72% (11.09–12.34%). The cumulative incidence rates of four TG/HDL-C ratio groups were 8.63% (7.53–9.72%), 10.78% (9.57–11.99%), 12.63% (11.34–13.92%) and 14.84% (13.45–16.22%), respectively. The total incidence rate of all people, Q1 groups, Q2 groups, Q3 groups, and Q4 groups were 19.21, 13.94, 17.64, 20.72, and 24.69 per 1000 person-years, respectively. Subjects in the Q4 group exhibited a significantly higher stroke incidence rate compared to those in the Q1 group (P < 0.001 for trend).
The results of the correlation between the TG/HDL-C ratio and stroke
As the TG/HDL-C ratio met the proportional hazards assumption, the association between the TG/HDL-C ratio and stroke risk was evaluated by the Cox proportional hazards regression model. Table 3 shows the correlation between TG/HDL-C ratio and stroke based on Cox proportional hazards regression models. In Model I, the TG/HDL-C ratio exhibited a positive association with stroke (HR: 1.06, 95% CI 1.04–1.08, P < 0.0001). The HR in the Model II and Model III were 1.04 (1.02–1.06) and 1.03 (1.00–1.05), respectively. After accounting for all relevant factors, the results demonstrated that each additional unit of the TG/HDL-C ratio was found to be associated with only a 3% increase in stroke risk.
Sensitivity analyses
To solidify the credibility of our research outcomes, multiple sensitivity checks were performed. The TG/HDL-C metric was recategorized for inclusion in our model. The analysis revealed inconsistent effect size trends across categories, hinting at a possible non-linear link with stroke events. In our research, we also ran sensitivity checks on participants without hypertension. After adjusting for various confounders, the data still showed a significant link between the TG/HDL-C ratio and the risk of stroke (HR = 1.05, 95% CI 1.01–1.09, P = 0.0170) as indicated in Table 4. Moreover, we carried out additional sensitivity analyses on individuals without drugs for cardiovascular prevention. These analyses also supported the positive relationship between the TG/HDL-C ratio and stroke risk, even after adjustments for confounders (HR = 1.03, 95% CI 1.00–1.06, P = 0.0263), as shown in Table 4.
The analysis of the nonlinear relationship
Figure 2 illustrates the non-linear correlation between the TG/HDL-C ratio and stroke incidence. A non-linear association was confirmed after adjusting for a comprehensive set of variables (Table 5). The analysis identified an inflection point in the TG/HDL-C ratio at 1.85 using a two-piecewise Cox proportional hazards regression model. Below this inflection point, an increased likelihood of stroke was associated with the TG/HDL-C ratio (HR: 1.28, 95% CI 1.06–1.54, P = 0.0089). Above this ratio, however, the association did not reach statistical significance (HR: 1.01, 95% CI 0.98–1.04, P = 0.6738).
The nonlinear relationship between TG/HDL-C ratio and incident stroke in Chinese individuals aged 45 years or older. A nonlinear relationship between the TG/HDL-C ratio and stroke was detected after adjustment for BMI, gender, age, drinking status, physical activity, smoking status, drugs for cardiovascular prevention, hypertension, FPG, TC, CRP, LDL-C, Scr, HbA1c, Cystatin C, and UA
Subgroup analysis
As shown in Table 6, the potential confounding variables that might have affected the correlation between the TG/HDL-C ratio and stroke were found in subgroup analysis. Stratification was conducted based on pertinent variables: smoking status, gender, physical activity, drinking status, age, and BMI. With the exception of smoking status, none of the confounding variables had an effect on the correlation between the TG/HDL-C ratio and stroke (P = 0.0331). The relationship was more significant in individuals with never smokers.
Discussion
Our study supported that the TG/HDL-C ratio was positively associated with stroke incidence. An inflection point was also identified, and different connections between the TG/HDL-C ratio and stroke risk were observed on either side of this point. The correlation between the TG/HDL-C ratio and stroke was more significant in individuals with never smokers.
Non-traditional lipid profiles, such as non-high-density lipoprotein cholesterol, LDL-C to HDL-C ratio, TC to HDL-C ratio, and TG/HDL-C ratio, have recently drawn increasing attention. The development of the metabolic syndrome is closely correlated with non-traditional lipid parameters, particularly the TG/HDL-C ratio. TG/HDL-C ratio was regarded as a prognostic marker of insulin resistance that may increase the risk of metabolic syndrome more quickly than single lipid measurements [19]. However, the question of whether the TG/HDL-C ratio raises stroke risk is still up for debate. In a prospective study involving 96,542 Chinese individuals, the high TG/HDL-C ratio group was an independent risk factor for stroke compared with the low TG/HDL-C ratio group after controlling for confounding variables (HR:1.11, 95% CI 1.03–1.18) [10]. Another study from China involving 5099 hypertensive patients showed that the TG/HDL-C ratio was strongly associated with future stroke after adjusting for ethnicity, current smoking, sex, age, BMI, diabetes, DBP, heavy drinking, SBP, and anti-hypertension drug treatment (HR: 1.58, 95% CI 1.21–2.06) [7]. Existing literature has also substantiated that the TG/HDL-C ratio serves as a more discerning biomarker for the identification of insulin resistance and the prognostication of diabetes, gestational diabetes, and cardiovascular incidents when compared to the unconventional lipid parameters, such as LDL-C to HDL-C ratio, and TC to HDL-C ratio [20,21,22,23,24]. In addition, previous studies have demonstrated that Lifestyle interventions and pharmacotherapy that target the reduction of triglycerides and the elevation of HDL-C have been shown to improve the TG/HDL-C ratio, thereby enhancing insulin sensitivity and reducing the risk of atherosclerosis, cardiovascular disease, and stroke risk [25,26,27]. However, a prospective cohort study from Japan, including 11,699 individuals, demonstrated that the TG/HDL-C ratio was not associated with stroke risk in the overall population (HR: 1.28, 95% CI 0.94–1.75) after controlling for age, hypertension, BMI, smoking status, diabetes mellitus, TC, and alcohol consumption [13]. In another prospective cohort study that included 2940 residents who did not have a stroke in the northern Manhattan, New York, the results showed that the TG/HDL-C ratio was not linked to the risk of stroke after adjusting for confounders (HR: 0.98, 95% CI 0.92–1.04) [12]. Our results supported the idea that the high TG/HDL-C ratio increases new-onset stroke risk. This may be due to differences in the TG/HDL-C ratio range, the sample sizes, and different adjustment variables. Moreover, nonlinear relationships may alter linear regression study conclusions, resulting in changes in the fitted linear relationship. Hence, the TG/HDL-C ratio may be non-linearly associated with incident stroke, explaining the disparity in these studies’ results. On the other hand, the sensitivity analysis discovered that a positive association still exists among those without hypertension or drugs for cardiovascular prevention. These findings serve as a reference point for improving therapies that aim to reduce the individuals aged 45 years or older risk of developing stroke.
This study’s subgroup analysis produced some intriguing results. In comparison to other smoking status groups, individuals with never-smokers had a more significant connection between the TG/HDL-C ratio and stroke risk. Further analysis of the study population’s baseline data, which was categorized by smoking status, revealed that never smokers had lower age, DBP, CRP, Scr, Cystatin C, UA, and a smaller percentage of current drinkers (Additional file 1: Table S1). In never smokers, the levels of these risks, as mentioned above, are lower, so the impact of the TG/HDL-C ratio on stroke is strengthened.
Uncertainty surrounds the mechanism by which the TG/HDL-C ratio is linked to stroke. Nonetheless, we surmise that arteriosclerosis plays a role in pathogenesis. The TG/HDL-C ratio is closely related to metabolic syndrome [28,29,30], which causes a high inflammatory response and increases oxidative stress [31], leading to endothelial dysfunction and consequent atherosclerosis [32, 33]. Moreover, the small dense low-density lipoprotein cholesterol (sdLDL-C), which is linked to the pathophysiology of atherosclerosis, may be connected with the TG/HDL-C ratio. The sdLDL-C’s prolonged circulation period and small particle size facilitate their penetration into the artery wall, allowing them to store lipids and cholesterol [34]. Moreover, their propensity for oxidation may contribute to an increase in their atherogenicity [35]. The sdLDL-C levels are helpful in assessing residual coronary heart disease risk [36] and are an important indicator of incident stroke [37]. TG is transferred into LDL-C particles when the liver secretes TG-rich lipoprotein, where hepatic lipases further delipidate it to produce smaller LDL particles [33]. Moreover, hepatic lipase activity is made more active in this process when HDL-C is lower [38]. These pathways may provide an explanation for why higher TG/HDL-C levels raise the risk of stroke.
Furthermore, the current investigation discovered a nonlinear connection between the TG/HDL-C ratio and stroke risk. To comprehend non-linear correlation more effectively, this study employed a two-piecewise Cox proportional hazards regression model. The correlation between the TG/HDL-C ratio and stroke among subjects aged 45 or older demonstrated a non-linear pattern, indicating a saturation effect. Following adjustment for confounders, the TG/HDL-C ratio inflection point was identified as 1.85. The study revealed that for every unit increase in the TG/HDL-C ratio below 1.85, the risk of stroke escalated by 28% (HR: 1.28, 95% CI 1.06–1.54, P = 0.0089). However, elevating the TG/HDL-C ratio above 1.85 did not exhibit an associated increase in the likelihood of stroke (HR: 1.01, 95% CI 0.98–1.04, P = 0.6738). Our findings establish a theoretical basis supporting the management of the TG/HDL-C ratio within clinical settings, particularly emphasizing the significance when the TG/HDL-C ratio decreases to less than 1.85. Furthermore, our results provide additional insights aimed at aiding subjects across varying TG/HDL-C ratios in mitigating the risk of stroke.
We listed the following strengths of our research. Primarily, we employed multiple imputations to address missing data, thus enhancing the statistical power and alleviating potential biases stemming from the absence of covariate information. Additionally, to minimize the impact of residual confounding factors on the results, we rigorously adjusted statistically. Furthermore, we conducted sensitivity analyses to ensure the robustness of our findings. These analyses involved categorizing the TG/HDL-C ratio and reexamining the association after excluding individuals with hypertension or drugs for cardiovascular prevention. Lastly, we conducted subgroup analyses to assess other potential confounding data that might influence the correlation between the TG/HDL-C ratio and stroke.
There are certain limitations to the current investigation. First, our research recruited Chinese over 45 years of age. Therefore, these results need further validation for other ethnicities and younger groups. Second, a self-reported questionnaire used in the CHARLS trial to confirm event strokes may have introduced recollection bias and misclassification mistakes. Third, the stroke diagnosis was based on self-reported questionnaires to ascertain incident strokes, and this study did not distinguish between ischaemic and hemorrhagic strokes in the diagnosis of stroke. There are some notable differences in risk factors between ischaemic and hemorrhagic stroke [39]. In the future, we will conduct our study, and we will combine stroke-related symptoms and imaging findings to differentiate between ischaemic and hemorrhagic stroke. Fourth, some variables, such as physical activity and BMI, have substantial missing data that may affect the reliability and accuracy of the estimates. In the future, we will also design our study to reduce the missing data and make our findings reliable. Fifth, possible unmeasured confounders may have affected the association between the TG/HDL-C ratio and stroke risks, such as the family history of stroke and nutrition.
Conclusion
Our study demonstrated a positive and nonlinear association between the TG/HDL-C ratio and stroke risk among people aged 45 or older. A statistically significant positive connection exists between the TG/HDL-C ratio and incident stroke when the TG/HDL-C ratio is lower than 1.85. Lowering the TG/HDL-C ratio, especially when it drops to less than 1.85, may reduce stroke risk in clinical practice.
Availability of data and materials
Data are available from http://www.isss.pku.edu.cn/cfps/. Follow the prompts to register as a user and download the data once it has been reviewed and approved.
Abbreviations
- TG/HDL-C ratio:
-
Ratio of triglyceride to high-density lipoprotein cholesterol
- CHARLS:
-
China Health and Retirement Longitudinal Study
- BMI:
-
Body mass index
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- FPG:
-
Fasting plasma glucose
- TC:
-
Total cholesterol
- TG:
-
Triglyceride
- HDL-C:
-
High-density lipoprotein cholesterol
- LDL-C:
-
Low-density lipid cholesterol
- Scr:
-
Serum creatinine
- HbA1c:
-
Glycosylated hemoglobin
- CRP:
-
C-reactive protein
- UA:
-
Uric acid
- HR:
-
Hazard ratios
- CI:
-
Confidence intervals
- Ref:
-
Reference
- sdLDL-C:
-
Small dense low-density lipoprotein-cholesterol
References
Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O’Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson U, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation. 2018;137(12):e67–492. https://doi.org/10.1161/CIR.0000000000000558.
Feigin VL, Stark BA, Johnson CO, Roth GA, Bisignano C, Abady GG, Abbasifard M, Abbasi-Kangevari M, Abd-Allah F, Abedi V, Abualhasan A. Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol. 2021;20(10):795–820. https://doi.org/10.1016/S1474-4422(21)00252-0.
Qi W, Ma J, Guan T, Zhao D, Abu-Hanna A, Schut M, Chao B, Wang L, Liu Y. Risk factors for incident stroke and its subtypes in China: a prospective study. J Am Heart Assoc. 2020;9(21): e016352. https://doi.org/10.1161/JAHA.120.016352.
Kelly DM, Rothwell PM. Does chronic kidney disease predict stroke risk independent of blood pressure?: A systematic review and meta-regression. Stroke. 2019;50(11):3085–92. https://doi.org/10.1161/STROKEAHA.119.025442.
Feigin VL, Norrving B, Mensah GA. Global burden of stroke. Circ Res. 2017;120(3):439–48. https://doi.org/10.1161/CIRCRESAHA.116.308413.
Feigin VL, Roth GA, Naghavi M, Parmar P, Krishnamurthi R, Chugh S, Mensah GA, Norrving B, Shiue I, Ng M, Estep K, Cercy K, Murray C, Forouzanfar MH. Global burden of stroke and risk factors in 188 countries, during 1990–2013: a systematic analysis for the global burden of disease study 2013. Lancet Neurol. 2016;15(9):913–24. https://doi.org/10.1016/S1474-4422(16)30073-4.
Liu Y, Jin X, Fu K, Li J, Xue W, Tian L, Teng W. Non-traditional lipid profiles and the risk of stroke: a systematic review and meta-analysis. Nutr Metab Cardiovasc Dis. 2023. https://doi.org/10.1016/j.numecd.2023.01.003.
Nie G, Hou S, Zhang M, Peng W. High TG/HDL ratio suggests a higher risk of metabolic syndrome among an elderly Chinese population: a cross-sectional study. BMJ Open. 2021;11(3): e041519. https://doi.org/10.1136/bmjopen-2020-041519.
Park B, Jung DH, Lee HS, Lee YJ. Triglyceride to HDL-cholesterol ratio and the incident risk of ischemic heart disease among Koreans without diabetes: a longitudinal study using national health insurance data. Front Cardiovasc Med. 2021;8: 716698. https://doi.org/10.3389/fcvm.2021.716698.
Chen Z, Chen G, Qin H, Cai Z, Huang J, Chen H, Wu W, Chen Z, Wu S, Chen Y. Higher triglyceride to high-density lipoprotein cholesterol ratio increases cardiovascular risk: 10-year prospective study in a cohort of Chinese adults. J Diabetes Investig. 2020;11(2):475–81. https://doi.org/10.1111/jdi.13118.
Zheng J, Sun Z, Zhang X, Li Z, Guo X, Xie Y, Sun Y, Zheng L. Non-traditional lipid profiles associated with ischemic stroke not hemorrhagic stroke in hypertensive patients: results from an 8.4 years follow-up study. Lipids Health Dis. 2019;18(1):9. https://doi.org/10.1186/s12944-019-0958-y.
Willey JZ, Xu Q, Boden-Albala B, Paik MC, Moon YP, Sacco RL, Elkind MS. Lipid profile components and risk of ischemic stroke: the Northern Manhattan study (NOMAS). Arch Neurol. 2009;66(11):1400–6. https://doi.org/10.1001/archneurol.2009.210.
Sato F, Nakamura Y, Kayaba K, Ishikawa S. TG/HDL-C ratio as a predictor of stroke in the population with healthy BMI: the Jichi medical school cohort study. Nutr Metab Cardiovasc Dis. 2022;32(8):1872–9. https://doi.org/10.1016/j.numecd.2022.05.002.
Zhao Y, Hu Y, Smith JP, Strauss J, Yang G. Cohort profile: the China health and retirement longitudinal study (CHARLS). Int J Epidemiol. 2014;43(1):61–8. https://doi.org/10.1093/ije/dys203.
Li H, Zheng D, Li Z, Wu Z, Feng W, Cao X, Wang J, Gao Q, Li X, Wang W, Hall BJ, Xiang YT, Guo X. Association of depressive symptoms with incident cardiovascular diseases in middle-aged and older Chinese adults. JAMA Netw Open. 2019;2(12): e1916591. https://doi.org/10.1001/jamanetworkopen.2019.16591.
Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, George SM, Olson RD. The physical activity guidelines for Americans. JAMA. 2018;320(19):2020–8. https://doi.org/10.1001/jama.2018.14854.
White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30(4):377–99. https://doi.org/10.1002/sim.4067.
von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495–9. https://doi.org/10.1016/j.ijsu.2014.07.013.
Yang T, Liu Y, Li L, Zheng Y, Wang Y, Su J, Yang R, Luo M, Yu C. Correlation between the triglyceride-to-high-density lipoprotein cholesterol ratio and other unconventional lipid parameters with the risk of prediabetes and type 2 diabetes in patients with coronary heart disease: a RCSCD-TCM study in China. Cardiovasc Diabetol. 2022;21(1):93. https://doi.org/10.1186/s12933-022-01531-7.
You Y, Hu H, Cao C, Han Y, Tang J, Zhao W. Association between the triglyceride to high-density lipoprotein cholesterol ratio and the risk of gestational diabetes mellitus: a second analysis based on data from a prospective cohort study. Front Endocrinol. 2023;14:1153072. https://doi.org/10.3389/fendo.2023.1153072.
Barat S, Ghanbarpour A, Bouzari Z, Batebi Z. Triglyceride to HDL cholesterol ratio and risk for gestational diabetes and birth of a large-for-gestational-age newborn. Caspian J Intern Med. 2018;9(4):368–75. https://doi.org/10.22088/cjim.9.4.368.
Sheng G, Kuang M, Yang R, Zhong Y, Zhang S, Zou Y. Evaluation of the value of conventional and unconventional lipid parameters for predicting the risk of diabetes in a non-diabetic population. J Transl Med. 2022;20(1):266. https://doi.org/10.1186/s12967-022-03470-z.
Ghani ZA, Qaddori H, Al-Mayah Q. Triglyceride/high-density lipoprotein ratio as a predictor for insulin resistance in a sample of healthy Iraqi adults. J Med Life. 2023;16(5):668–74. https://doi.org/10.25122/jml-2022-0239.
Hajian-Tilaki K, Heidari B, Bakhtiari A. Triglyceride to high-density lipoprotein cholesterol and low-density lipoprotein cholestrol to high-density lipoprotein cholesterol ratios are predictors of cardiovascular risk in Iranian adults: evidence from a population-based cross-sectional study. Caspian J Intern Med. 2020;11(1):53–61. https://doi.org/10.22088/cjim.11.1.53.
Ginsberg HN, Packard CJ, Chapman MJ, Boren J, Aguilar-Salinas CA, Averna M, Ference BA, Gaudet D, Hegele RA, Kersten S, Lewis GF, Lichtenstein AH, Moulin P, Nordestgaard BG, Remaley AT, Staels B, Stroes E, Taskinen MR, Tokgozoglu LS, Tybjaerg-Hansen A, Stock JK, Catapano AL. Triglyceride-rich lipoproteins and their remnants: metabolic insights, role in atherosclerotic cardiovascular disease, and emerging therapeutic strategies—a consensus statement from the European atherosclerosis society. Eur Heart J. 2021;42(47):4791–806. https://doi.org/10.1093/eurheartj/ehab551.
Mahdy AK, Wonnerth A, Huber K, Wojta J. Cardiovascular disease risk reduction by raising HDL cholesterol—current therapies and future opportunities. Br J Pharmacol. 2012;167(6):1177–94. https://doi.org/10.1111/j.1476-5381.2012.02081.x.
Marston NA, Giugliano RP, Im K, Silverman MG, O’Donoghue ML, Wiviott SD, Ference BA, Sabatine MS. Association between triglyceride lowering and reduction of cardiovascular risk across multiple lipid-lowering therapeutic classes: a systematic review and meta-regression analysis of randomized controlled trials. Circulation. 2019;140(16):1308–17. https://doi.org/10.1161/CIRCULATIONAHA.119.041998.
Liang J, Fu J, Jiang Y, Dong G, Wang X, Wu W. TriGlycerides and high-density lipoprotein cholesterol ratio compared with homeostasis model assessment insulin resistance indexes in screening for metabolic syndrome in the Chinese obese children: a cross section study. BMC Pediatr. 2015;15:138. https://doi.org/10.1186/s12887-015-0456-y.
Chen Z, Hu H, Chen M, Luo X, Yao W, Liang Q, Yang F, Wang X. Association of triglyceride to high-density lipoprotein cholesterol ratio and incident of diabetes mellitus: a secondary retrospective analysis based on a Chinese cohort study. Lipids Health Dis. 2020;19(1):33. https://doi.org/10.1186/s12944-020-01213-x.
Sun Y, Wang Z, Huang Z, Hu H, Han Y. The association between the triglyceride-to-high-density lipoprotein cholesterol ratio and the risk of progression to diabetes from prediabetes: a 5-year cohort study in Chinese adults. Front Endocrinol. 2022;13: 947157. https://doi.org/10.3389/fendo.2022.947157.
Van Guilder GP, Hoetzer GL, Greiner JJ, Stauffer BL, Desouza CA. Influence of metabolic syndrome on biomarkers of oxidative stress and inflammation in obese adults. Obesity. 2006;14(12):2127–31. https://doi.org/10.1038/oby.2006.248.
Di Pino A, DeFronzo RA. Insulin resistance and atherosclerosis: implications for insulin-sensitizing agents. Endocr Rev. 2019;40(6):1447–67. https://doi.org/10.1210/er.2018-00141.
Bang OY. Intracranial atherosclerotic stroke: specific focus on the metabolic syndrome and inflammation. Curr Atheroscler Rep. 2006;8(4):330–6. https://doi.org/10.1007/s11883-006-0012-1.
Ivanova EA, Myasoedova VA, Melnichenko AA, Grechko AV, Orekhov AN. Small dense low-density lipoprotein as biomarker for atherosclerotic diseases. Oxid Med Cell Longev. 2017;2017:1273042. https://doi.org/10.1155/2017/1273042.
Tribble DL, Rizzo M, Chait A, Lewis DM, Blanche PJ, Krauss RM. Enhanced oxidative susceptibility and reduced antioxidant content of metabolic precursors of small, dense low-density lipoproteins. Am J Med. 2001;110(2):103–10. https://doi.org/10.1016/s0002-9343(00)00700-2.
Hoogeveen RC, Gaubatz JW, Sun W, Dodge RC, Crosby JR, Jiang J, Couper D, Virani SS, Kathiresan S, Boerwinkle E, Ballantyne CM. Small dense low-density lipoprotein-cholesterol concentrations predict risk for coronary heart disease: the atherosclerosis risk in communities (ARIC) study. Arterioscler Thromb Vasc Biol. 2014;34(5):1069–77. https://doi.org/10.1161/ATVBAHA.114.303284.
Zhao CX, Cui YH, Fan Q, Wang PH, Hui R, Cianflone K, Wang DW. Small dense low-density lipoproteins and associated risk factors in patients with stroke. Cerebrovasc Dis. 2009;27(1):99–104. https://doi.org/10.1159/000175768.
Brunzell JD, Zambon A, Deeb SS. The effect of hepatic lipase on coronary artery disease in humans is influenced by the underlying lipoprotein phenotype. Biochim Biophys Acta. 2012;1821(3):365–72. https://doi.org/10.1016/j.bbalip.2011.09.008.
Andersen KK, Olsen TS, Dehlendorff C, Kammersgaard LP. Hemorrhagic and ischemic strokes compared: stroke severity, mortality, and risk factors. Stroke. 2009;40(6):2068–72. https://doi.org/10.1161/STROKEAHA.108.540112.
Acknowledgements
The CHARLS provided data and explanations. We want to express our gratitude to the entire CHARLS study team as well as the study participants.
Funding
This study was supported by the the first batch of medical and health science and technology plan projects in Yantian District in 2024 (YTWS202401).
Author information
Authors and Affiliations
Contributions
Haofei Hu and Xiandao Zheng designed the study. Shike Zhang, Changchun Cao and Yong Han drafted the manuscript and performed the statistical analysis. Haofei Hu and Xiandao Zheng revised the manuscript. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Each participant in CHARLS cohort which was conducted under Peking University’s ethics review committee gave their informed permission before taking part (IRB00001052-11015). In addition, the Declaration of Helsinki was followed during our research. The necessary standards and legislation were followed in the execution of all procedures, including the declarations in “Declarations” section.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
The characteristics of participants in smoking status. Table S2. Relationship between TG/HDL-C ratio and the incident stroke in different sensitivity models. Table S3. Relationship between TG/HDL-C ratio and the incident stroke in different sensitivity models.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, S., Cao, C., Han, Y. et al. A nonlinear relationship between the triglycerides to high-density lipoprotein cholesterol ratio and stroke risk: an analysis based on data from the China Health and Retirement Longitudinal Study. Diabetol Metab Syndr 16, 96 (2024). https://doi.org/10.1186/s13098-024-01339-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13098-024-01339-3