Abstract
Aim
Cannabinoid receptors are components of the endocannabinoid system that affect various physiological functions. We aim to investigate the effect of cannabinoid receptor modulation on kidney disease.
Methods
PubMed, Web of Science databases, and EMBASE were searched. Articles selection, data extraction and quality assessment were independently performed by two investigators. The SYRCLE’s RoB tool was used to assess the risk of study bias, and pooled SMD using a random-effect model and 95% CIs were calculated. Subgroup analyses were conducted in preselected subgroups, and publication bias was evaluated. We compared the effects of CB1 and CB2 antagonists and/or knockout and agonists and/or genetic regulation on renal function, blood glucose levels, body weight, and pathological damage-related indicators in different models of chronic and acute kidney injury.
Results
The blockade or knockout of CB1 could significantly reduce blood urea nitrogen [SMD,− 1.67 (95% CI − 2.27 to − 1.07)], serum creatinine [SMD, − 1.88 (95% CI − 2.91 to − 0.85)], and albuminuria [SMD, − 1.60 (95% CI − 2.16 to − 1.04)] in renal dysfunction animals compared with the control group. The activation of CB2 group could significantly reduce serum creatinine [SMD, − 0.97 (95% CI − 1.83 to − 0.11)] and albuminuria [SMD, − 2.43 (95% CI − 4.63 to − 0.23)] in renal dysfunction animals compared with the control group.
Conclusions
The results suggest that targeting cannabinoid receptors, particularly CB1 antagonists and CB2 agonists, can improve kidney function and reduce inflammatory responses, exerting a renal protective effect and maintaining therapeutic potential in various types of kidney disease.
Similar content being viewed by others
Introduction
Kidney diseases including chronic kidney disease (CKD) and acute kidney injury (AKI) [1, 2], are a significant global health problem, affecting millions of people worldwide [3, 4]. CKD is a common endpoint disease of kidney disease of multiple etiologies, including diabetic nephropathy, obesity-related nephropathy, chronic tubulointerstitial injury, and recurrent AKI [5,6,7]. Renal fibrosis and mild inflammation are the basis of CKD progressing to end-stage renal disease [8, 9]. Despite advances in treatment, the incidence and prevalence of kidney disease continue to rise, and new therapies are urgently needed to prevent or slow disease progression [10]. Recent studies have suggested that targeting the endocannabinoid system, particularly the cannabinoid receptor 1 (CB1) and cannabinoid receptor 2 (CB2), may have therapeutic potential in various kidney diseases [11, 12]. While a growing body of evidence suggests that cannabinoid receptors play a role in the regulation of renal function and in the pathogenesis of kidney diseases, the intervention of cannabinoid receptors in renal diseases is a relatively uncharted territory, with potential implications for both therapeutic and adverse effects. A comprehensive understanding of these interactions, specifically in the setting of kidney disease models, is critical to harnessing their potential therapeutic benefits while mitigating risks.
Meta-analysis is a powerful tool for synthesizing and analyzing data from multiple studies, providing a comprehensive overview of the available evidence and increasing statistical power, even in animal study [13, 14]. In the present study, we aim to perform a systematic review and meta-analysis of preclinical animal studies investigating the effects of cannabinoid receptor modulation on kidney disease. Specifically, we will analyze the effects of cannabinoid receptor agonists and antagonists on kidney function, histological changes, inflammation, and other relevant outcomes in animal models of kidney disease. By synthesizing and analyzing data from multiple studies, we hope to provide a more comprehensive understanding of the role of cannabinoid receptors in kidney disease, and to identify potential therapeutic targets for future studies in this field.
Materials and methods
Search strategy
PubMed, Web of Science databases, and EMBASE were searched for publications using the following search terms: “kidney disease”, “renal function”, “cannabinoid receptor”, “cannabinoids” and “animal”, with the last search performed on 20 August 2021. Detailed search strategies are shown in the Additional file 1. No language restriction filter was applied. Furthermore, we screened the reference lists of the papers identified through database search for other potentially appropriate studies. When necessary, we asked the contact author of individual studies for more information by email.
Selection criteria
All articles acquired by the search were reviewed, and irrelevant publications were removed by scanning the title and abstract. The selected articles were further assessed by full-text reading. Two investigators independently (Z. Z. and J.D.) achieved the study selection, with any differences determined by common discussion and judgment of a third reviewer (D.L.), if consensus was not reached. Studies that conformed to the following criteria were considered possibly qualified: (1) Animal models of acute and chronic kidney disease; (2) modulators of cannabinoid receptors (including agonists and antagonists) or genetic modifications of CB1 or CB2 were used as interventions, with corresponding control groups set up; (3) The primary outcomes were albuminuria, blood urea nitrogen (BUN), serum creatinine (Scr), kidney weight/body weight ratio (KW/BW) and pathology of renal tissue, and (4) the secondary outcomes were blood glucose, body weight (BW), mechanistic pathway indicators of cannabinoid receptor system involved in kidney tissue injury. Human studies, in vertebrate animal and in vitro, ex vivo experimental studies will be excluded. The protocol of this meta-analysis was registered in the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42021272950).
Quality assessment
Data extraction and quality assessment were independently performed by two investigators (Z.Z. and D.L.). All eligible study reports were used for the quality assessment using the Systematic Review Center for Laboratory Animal Experimentation (SYRCLE)’s risk of bias tool for animal studies [15] and for data collection. Any discrepancies were resolved through discussion to reach a consensus. Results are visualized using the “robvis” package in R.
Data extraction
Study quality, study characteristics such as first author, year of publication, study design and sample size, animal characteristics such as species and sex, disease model and methods of establishment, target receptor, intervention protocol including dose administration and duration, and primary and secondary outcomes index were collected into standardized extraction forms. In the case of primary and secondary outcomes, when there were multiple measurements taken at various intervals (e.g., bi-weekly readings of blood glucose, weight, urine protein, etc.), we solely considered the data from the final time point, which corresponds to the post-intervention period. When interventions with different dose subgroups existed, we extracted each results and combined them into one treatment group using a formula provided by Cochrane [16] via the web tool StatsToDo (https://www.statstodo.com/). Using WebPlotDigitizer (https://automeris.io/WebPlotDigitizer) to extract data displayed only as graph curves. For the meta-analysis, studies were required to report the number of animals per group, the mean, and a measure of variance. Standard error of mean (SEM) was converted to standard deviation (SD) value according to the method recommended by Cochrane Handbook [16], i.e., SD = SE × \(\sqrt{n}\).
Statistical analysis
This meta-analysis was performed using R 4.1.2 software (https://www.r-project.org). The standardized mean difference (SMD) have been used to assess the effects of treatment among the different continuous scales of measurement. The random-effects model was used to calculate the pooled effect by using “meta” and “metafor” package. The I2 and Q test was used to quantify the degree of heterogeneity among studies [17]. I2 > 50% considered to reveal higher heterogeneity among the included studies.
Subgroup and sensitivity analyses
Subgroup analyses were conducted stratified by the species, the intervention is pharmacological or genetic, year of study published, disease models for chronic or acute, and method of model establishment. Sensitivity analysis was used to explore the impact of a single study on the overall risk estimate, and was carried out by sequentially omitting one study in each iteration with the “metainf” package in R software.
Publication bias
Publication bias was examined by funnel plot and the Egger's test [18]. To evaluate the possibility of publication bias, we visually examined the funnel plots for asymmetry. Additionally, we utilized trim and fill analysis to address any observed funnel plot asymmetry. This involved estimating and including the potentially missing studies on the left-hand side of the plot, which allowed us to recalculate the overall effect size.
Results
Characteristics and quality of the retrieved studies
We identified 829 potentially relevant references from database searches (Fig. 1). Then, 321 duplicate studies were removed leaving a total of 508 potentially relevant citations identified from the initial stage of the literature search. Excluded 430 studies after screening the titles and abstracts and read the full text of the remaining 78 studies. We subsequently excluded 6 reviews, 8 abstracts only, 25 studies that contained unrelated disease models and/or interventions, 3 studies with no relevant outcomes and one study only contained in vitro experiment. Finally, a total of 35 studies were included in the analysis [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53]. The detailed characteristics of the included studies are shown in Table 1 and Additional file 1: Table S1.
Figure 1 All studies were published between 2007 and 2021. Of the studies included, 33 rodent models were male [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33, 35,36,37,38,39,40,41,42,43,44,45,46,47,48,49, 51,52,53], and 2 did not mention sex [34, 50]. Ten of the animal models studied were rats [19, 23, 25, 30, 33, 36, 39, 46, 50, 51], 23 were mice [20,21,22, 24, 26,27,28,29, 34, 35, 37, 38, 40,41,42,43,44,45, 47,48,49, 52, 53], and 2 included both rat and mouse models [31, 32]. Disease models include chronic kidney injury models that include streptozotocin (STZ) or genetically induced diabetes [20, 24, 27, 29,30,31, 39, 41,42,43, 49, 50], diet or genetically induced obesity [19, 23, 25, 28, 32, 35, 38, 40, 48], chronic intermittent hypoxia [51], bile duct ligation induced hepatorenal syndrome [47] and acute kidney injury models that include unilateral ureteral obstruction, ischemia–reperfusion, partial nephrectomy, and cisplatin induction [21, 22, 26, 34, 35, 37, 44,45,46, 52, 53]. The target receptors for the intervention included CB1 antagonist and knockout [19,20,21, 23, 25, 27, 28, 30, 31, 33,34,35, 39,40,41,42,43, 48,49,50,51], CB2 agonist [22, 24, 26, 36,37,38, 44, 46, 47, 53], CB2 antagonist and knockout [22, 26, 29, 37, 45, 52], and CB1 agonist [32] (the summary as shown in Table 2). The study quality was assessed according to the SYRCLE. Eighteen studies reported random assignment of animals, but no study described a random component in the specific sequence generation process. No studies have specifically reported the method of allocation concealment and randomly placed animals in animal housing. Baseline characteristics (selection bias), blinding (detection bias), incomplete outcome data (attrition bias), selective outcome reporting (reporting bias) and other sources of bias were mostly well performed. The risk-of-bias assessment of the included trials was summarized in Fig. 2 and Additional file 1: Fig. S1.
Effect of CB1 antagonist and knockout on renal function
Primary outcomes
BUN
Thirteen studies [19, 21, 25, 28, 30, 35, 39, 40, 42, 43, 48,49,50]reported BUN found that the blockade or knockout of CB1 group could significantly reduce BUN in renal dysfunction animals compared with the control group (Fig. 3A, 16 items, n = 352; SMD, − 1.67; 95% confidence interval (CI), − 2.27 to − 1.07; P < 0.0001; I2 = 78%).
Meta-analysis and forest plot of effects of CB1 antagonist and knockout on primary outcomes including blood urea nitrogen (A), serum creatinine (B), albuminuria (C), glomerular damage score (D), tubular damage score (E), and kidney weight/body weight ratio (F). N1 denotes the number of animals in the treatment group and N2 denotes the number of animals in the control group. The size of the square indicates the weight (%) of each study, the horizontal line shows the 95% confidence interval (95%-CI) of the individual standardized mean difference (SMD), and the black diamond represents the combined SMD and 95%-CI
Scr
A total of 12 included studies reported Scr after intervention with CB1 knockout or drug blockade [19, 21, 25, 27, 28, 30, 33, 35, 39, 40, 49, 50]. In the random-effect model, the blockade or knockout of CB1 had a significantly effect on Scr (Fig. 3B, 13 items, n = 295; SMD, − 1.88; 95% CI − 2.91 to − 0.85; P < 0.0001; I2 = 84%) compared to control.
Albuminuria
A total of 12 included studies [19, 20, 23, 27, 30, 39,40,41,42,43, 48, 50] reported albuminuria after intervention with CB1 knockout or drug blockade, of which 9 were expressed as albumin creatinine ratio (ACR) or albumin excretion rate (AER) and 3 reported as 24-h proteinuria or AER/Cr. In the random-effect model, the interventions had a significantly effect on albuminuria (Fig. 3C, 14 items, n = 321; SMD, − 1.60; 95% CI − 2.16 to − 1.04; P < 0.0001; I2 = 75%) compared to control.
Pathological changes in the kidney histology
Three studies [19, 23, 49] reported glomerular damage score found that the blockade or knockout of CB1 group could significantly reduce glomerular damage in renal dysfunction animals compared with the control group (Fig. 3D, 3 items, n = 87; SMD, − 1.28; 95% CI − 1.79 to − 0.77; P < 0.0001; I2 = 0%).
Three studies [21, 49, 51] reported tubular damage score found that the blockade or knockout of CB1 group could significantly reduce tubular damage in renal dysfunction animals compared with the control group (Fig. 3E, 4 items, n = 74; SMD, − 2.29; 95% CI − 3.56 to − 1.01; P = 0.0005; I2 = 68%).
Kidney weight/body weight ratio
A total of 8 included studies [20, 27, 30, 33, 41,42,43, 48] reported KW/ BW ratio after intervention with CB1 knockout or drug blockade. In the random-effect model, the blockade or knockout of CB1 had an uncertain effect on KW/ BW (Fig. 3F, 10 items, n = 186; SMD, − 0.06; 95% CI − 0.77 to 0.66; P = 0.88; I2 = 73%) compared to control.
Second outcomes
Blood glucose
A total of 12 included studies reported blood glucose level after intervention with CB1 genetic or drug blockade [19, 20, 27, 28, 30, 31, 39, 40, 42, 43, 49, 50]. In the random-effect model, the blockade or knockout of CB1 had an uncertain effect on blood glucose (Fig. 4A, 15 items, n = 351; SMD, − 0.17; 95% CI − 0.52 to 0.17; P = 0.33; I2 = 58%) compared to control.
Meta-analysis and forest plot of effects of CB1 antagonist and knockout on second outcomes including blood glucose (A), body weight (B), metabolic and inflammatory related index (MCP-1, IL1β, IL18, TNF-α and TGF-β mRNA level, respectively) (C–G). N1 denotes the number of animals in the treatment group and N2 denotes the number of animals in the control group. The size of the square indicates the weight (%) of each study, the horizontal line shows the 95% confidence interval (95%-CI) of the individual standardized mean difference (SMD), and the black diamond represents the combined SMD and 95%-CI
Body weight
Eleven studies [19, 20, 27, 28, 30, 33, 35, 39,40,41, 43] reported tubular damage score found that the blockade or knockout of CB1 group could no significantly change body weight in animals compared with the control group (Fig. 4B, 11 items, n = 267; SMD, − 0.11; 95% CI − 1.23 to 1.02; P = 0.85; I2 = 85%).
Metabolic and inflammatory related index
Five studies [34, 40,41,42,43] reported MCP-1 mRNA level in renal found that the blockade or knockout of CB1 group could significantly reduce MCP-1 mRNA in renal tissue compared with the control group (Fig. 4C, 5 items, n = 89; SMD, − 1.08; 95% CI − 1.66 to − 0.50; P = 0.0003; I2 = 32%).
Three studies [21, 31, 43] reported IL1β mRNA level in renal found that the blockade or knockout of CB1 group could significantly reduce IL1β mRNA in renal tissue compared with the control group (Fig. 4D, 5 items, n = 79; SMD, − 1.30; 95% CI − 2.20 to − 0.40; P = 0.0047; I2 = 63%).
Three studies [40, 42, 43] reported IL18 mRNA level in renal found that the blockade or knockout of CB1 group could significantly reduce IL18 mRNA in renal tissue compared with the control group (Fig. 4E, 5 items, n = 63; SMD, − 1.41; 95% CI − 2.21 to − 0.60; P = 0.0006; I2 = 48%).
Six studies [21, 31, 40,41,42,43] reported TNF-α mRNA level in renal found that the blockade or knockout of CB1 group could significantly reduce TNF-α mRNA in renal tissue compared with the control group (Fig. 4F, 8 items, n = 131; SMD, − 2.35; 95% CI − 3.22 to − 1.48; P < 0.0001; I2 = 63%).
Five studies [20, 31, 34, 42, 43] reported TGF-β mRNA level in renal found that the blockade or knockout of CB1 group could significantly reduce TGF-β mRNA in renal tissue compared with the control group (Fig. 4G, 7 items, n = 119; SMD, − 1.11; 95% CI − 2.04 to − 0.18; P = 0.019; I2 = 78%).
Effect of CB2 agonist on renal function
Primary outcomes
BUN
Six studies [22, 26, 37, 46, 47, 53] reported BUN found that the activation of CB2 group could no significantly change BUN in renal dysfunction animals compared with the control group (Fig. 5A, 6 items, n = 104; SMD,− 1.09; 95% CI − 2.36 to 0.17; P = 0.09; I2 = 77%).
Meta-analysis and forest plot of effects of CB2 agonist on primary outcomes including blood urea nitrogen (A), serum creatinine (B), albuminuria (C), tubular damage score (D), and kidney weight/body weight ratio (E). N1 denotes the number of animals in the treatment group and N2 denotes the number of animals in the control group. The size of the square indicates the weight (%) of each study, the horizontal line shows the 95% confidence interval (95%-CI) of the individual standardized mean difference (SMD), and the black diamond represents the combined SMD and 95%-CI
Scr
Eight studies [22, 26, 36, 37, 44, 46, 47, 53] reported Scr found that the activation of CB2 group could significantly reduce Scr in renal dysfunction animals compared with the control group (Fig. 5B, 8 items, n = 136; SMD, − 0.97; 95% CI − 1.83 to − 0.11; P = 0.03; I2 = 70%).
Albuminuria
Two studies [24, 38] reported 24 h urine protein found that the activation of CB2 group could significantly reduce albuminuria in renal dysfunction animals compared with the control group (Fig. 5C, 2 items, n = 29; SMD, − 2.43; 95% CI − 4.63 to − 0.23; P = 0.03; I2 = 75%).
Pathological changes in the kidney histology
Two studies [22, 26] reported tubular damage score found that the agonist of CB2 group could significantly reduce tubular damage in renal tissue compared with the control group (Fig. 5D, 2 items, n = 30; SMD, − 4.00; 95% CI − 7.42 to − 0.57; P = 0.02; I2 = 82%).
Kidney weight / body weight ratio
Two studies [24, 36] reported KW/BW ratio found that the activation of CB2 group could no significantly change KW/BW compared with the control group (Fig. 5E, 2 items, n = 41; SMD, 0.11; 95% CI − 0.88 to 1.09; P = 0.83; I2 = 58%).
Second outcomes
Blood glucose
A total of 2 included studies reported blood glucose level after intervention with CB2 agonist [24, 38]. In the random-effect model, the CB2 agonist had no effect on blood glucose (Fig. 6A, 2 items, n = 29; SMD, 0.17; 95% CI − 0.61 to 0.94; P = 0.67; I2 = 0%) compared to control.
Meta-analysis and forest plot of effects of CB2 agonist on second outcomes including blood glucose (A), body weight (B), metabolic and inflammatory related index (MCP-1, TNF-α and TGF-β mRNA level, respectively) (C–E). N1 denotes the number of animals in the treatment group and N2 denotes the number of animals in the control group. The size of the square indicates the weight (%) of each study, the horizontal line shows the 95% confidence interval (95%-CI) of the individual standardized mean difference (SMD), and the black diamond represents the combined SMD and 95%-CI
Body weight
A total of 3 included studies reported body weight after intervention with CB2 agonist [24, 36, 38]. In the random-effect model, the CB2 agonist had no effect on body weight (Fig. 6B, 2 items, n = 49; SMD, 0.01; 95% CI − 0.64 to 0.66; P = 0.97; I2 = 29%) compared to control.
Metabolic and inflammatory related index
Four studies [22, 24, 26, 47] reported MCP-1 mRNA level in renal found that the activation of CB2 group could significantly reduce MCP-1 mRNA in renal tissue compared with the control group (Fig. 6C, 4 items, n = 58; SMD, − 1.26; 95% CI − 1.84 to − 0.68; P < 0.0001; I2 = 0%).
Four studies [22, 24, 37, 47] reported TNF-α mRNA level in renal found that the activation of CB2 group could significantly reduce TNF-α mRNA in renal tissue compared with the control group (Fig. 6D, 4 items, n = 58; SMD, − 1.26; 95% CI − 1.84 to − 0.68; P < 0.0001; I2 = 0%).
Two studies [24, 47] reported TGF-β mRNA level in renal found that the activation of CB2 group could no change TGF-β mRNA in renal tissue compared with the control group (Fig. 6E, 2 items, n = 37; SMD, − 0.64; 95% CI − 2.14 to 0.87; P = 0.41; I2 = 77%).
Effect of CB2 antagonist and knockout on renal function
Primary outcomes
BUN
Four studies [22, 26, 37, 52] reported BUN found that the knockout or blockade of CB2 group could little or no change BUN in renal dysfunction animals compared with the control group (Fig. 7A, 4 items, n = 54; SMD, 0.99; 95% CI 0.08 to 1.90; P = 0.03; I2 = 56%).
Meta-analysis and forest plot of effects of CB2 antagonist and knockout on primary outcomes including blood urea nitrogen (A), serum creatinine (B), albuminuria (C), tubular damage score (D) and kidney weight / body weight ratio (E). N1 denotes the number of animals in the treatment group and N2 denotes the number of animals in the control group. The size of the square indicates the weight (%) of each study, the horizontal line shows the 95% confidence interval (95%-CI) of the individual standardized mean difference (SMD), and the black diamond represents the combined SMD and 95%-CI
Scr
Seven studies [22, 26, 29, 36, 45, 52] reported Scr found that the knockout or blockade of CB2 group had no effect on Scr in renal dysfunction animals compared with the control group (Fig. 7B, 7 items, n = 102; SMD, 0.18; 95% CI − 0.85 to 1.20; P = 0.73; I2 = 77%).
Albuminuria
Two studies [29, 45] reported ACR found that the blockade of CB2 group could no significantly change albuminuria in renal dysfunction animals compared with the control group (Fig. 7C, 2 items, n = 28; SMD, − 0.99; 95% CI − 6.82 to 4.83; P = 0.74; I2 = 95%).
Pathological changes in the kidney histology
Two studies [22, 26] reported tubular damage score found that the knockout of CB2 group could significantly increase tubular damage in renal tissue compared with the control group (Fig. 7D, 2 items, n = 30; SMD, 2.47; 95% CI 1.39 to 3.56; P < 0.0001; I2 = 9%).
Kidney weight / body weight ratio
Two studies [29, 36] reported KW/BW ratio found that the blockade or knockout of CB2 group could no significantly change KW/BW compared with the control group (Fig. 7E, 2 items, n = 36; SMD, 0.78; 95% CI − 0.65 to 2.21; P = 0.28; I2 = 75%).
Second outcomes
Body weight
A total of 2 included studies reported body weight after intervention with CB2 antagonist or knockout [29, 36]. In the random-effect model, the CB2 antagonist or knockout had no effect on body weight (Fig. 8A, 2 items, n = 36; SMD, 0.26; 95% CI − 0.50 to 1.02; P = 0.50; I2 = 22%) compared to control.
Meta-analysis and forest plot of effects of CB2 antagonist and knockout on second outcomes including body weight (A), and metabolic and inflammatory related index, MCP-1 (B) and TNF-α (C) mRNA level. N1 denotes the number of animals in the treatment group and N2 denotes the number of animals in the control group. The size of the square indicates the weight (%) of each study, the horizontal line shows the 95% confidence interval (95%-CI) of the individual standardized mean difference (SMD), and the black diamond represents the combined SMD and 95%-CI
Metabolic and inflammatory related index
Three studies [22, 29, 45] reported MCP-1 mRNA level in renal found that the blockade or knockout of CB2 group could no change MCP-1 mRNA in renal tissue compared with the control group (Fig. 8B, 3 items, n = 42; SMD, − 0.09; 95% CI − 1.56 to 1.37; P = 0.90; I2 = 77%).
Two studies [22, 45] reported TNF-α mRNA level in renal found that the blockade or knockout of CB2 group could no change TNF-α mRNA in renal tissue compared with the control group (Fig. 8C, 2 items, n = 26; SMD, − 0.04; 95% CI − 2.69 to 2.61; P = 0.98; I2 = 89%).
Effect of CB1 agonist on renal function
One study [32] reported that CB1 transgenes increased ACR (n = 20; SMD, 1.30; 95% CI 0.32 to 2.28; P = 0.0096) in mice, with no change blood glucose level (n = 20; SMD, − 0.44; 95% CI − 1.33 to 0.45; P = 0.33); as well as CB1 agonists increased ACR (n = 20; SMD, 1.06; 95% CI 0.11 to 2.01; P = 0.03) in rats, with no change blood glucose level (n = 20; SMD, 0.46; 95% CI − 0.43 to 1.35; P = 0.31).
Another study [31] reported that in mice CB1 transgenes increased IL1β mRNA expression (n = 12; SMD, 2.49; 95% CI 0.84 to 4.15; P = 0.0031) in kidney tissue.
Publication bias
Funnel plots and Egger’s test were constructed to evaluate the publication bias of the primary outcomes. Except for inhibitors of CB1 that could be included, other types were limited by the small number of studies (less than 10 studies). Bias was assessed by the trim and fill method. As funnel plots shown in Fig. 9 and Egger’ test in Table 3). Despite publication bias, BUN and ACR results were consistent after trimming and filling.
Sensitivity analysis and subgroup analysis
Sensitivity analysis was used to explore the impact of individual studies on the overall risk estimation, and the result shown in Additional file 1: Figs. S2, 3. Subgroup analyses were performed based on predefined classifications to explore possible sources of heterogeneity, and detailed results are shown in Additional file 1: Figs. S4–10.
CB1 antagonist and knockout primary outcomes
Sensitivity analysis shown that the pooled BUN, Scr and ACR results were stable when sequentially omitting one study in each iteration (Additional file 1: Fig. S2).
In the analysis of subgroups, CB1 antagonist and knockout significantly reduced BUN, Scr and ACR, except in method of model subgroup (Fig. 10). Different approaches to disease modeling could be sources of heterogeneity.
CB2 agonist primary outcomes
Sensitivity shown that the combined BUN and Scr results were statistically significant after excluding the one study by Shan Z in 2021 (BUN, − 1.47 [− 2.19, − 0.76]; Scr, − 1.23 [− 1.78, − 0.68], Additional file 1: Fig. S3A, B).
In the analysis of subgroups, CB2 agonist could significantly reduce BUN and Scr in the CI-AKI subgroup (Fig. 11).
CB2 antagonist and knockout primary outcomes
Sensitivity shown that the pooled BUN and Scr results were stable when sequentially omitting one study in each iteration (Additional file 1: Figure S3C, D).
In the analysis of subgroups, CB2 antagonist could significantly reduce BUN and Scr in the CI-AKI subgroup (Fig. 12). Different approaches to disease modeling could be sources of heterogeneity.
Discussion
Summary of evidence and possible mechanisms
The present systematic review and meta-analysis provides a comprehensive synthesis of preclinical animal studies investigating the effects of cannabinoid receptor modulation on kidney disease. Our results indicate that both CB1 inhibition and CB2 receptor activation have reno-protective effects in animal models of kidney disease.
Firstly, the pooled analysis revealed potential reno-protective benefits of CB1 antagonists in animal models of kidney diseases, particularly in cases of diabetic nephropathy. In mouse models, CB1 antagonists or gene knockout significantly reduced urea nitrogen, creatinine, proteinuria, and pathological injury without affecting blood glucose levels, body weight or kidney/body weight ratio. Both sensitivity and subgroup analyses supported the consistent outcomes, although the heterogeneity could stem from the different disease modeling methods. Notably, CB1 antagonists and knockout effectively reduced BUN, Scr, and albuminuria, especially in diabetes cases. Furthermore, the decreased mRNA expression levels of inflammatory cytokines (including MCP-1, IL1β, IL18, TNF-α, and TGF-β) following CB1 antagonists treatment suggest potential anti-inflammatory effects. Inflammation plays a crucial role in the pathogenesis and progression of kidney disease by harming renal tissue, decreasing kidney function, and accelerating fibrosis. Conversely, kidney disease can lead to inflammation by activating immune cells and releasing pro-inflammatory cytokines [54,55,56]. Reducing inflammation can thus retard or halt the progression of kidney disease [55]. TGF-β, in particular, is the key mediator of renal inflammation and fibrosis [57, 58]. In response to injury or inflammation, TGF-β stimulates cells (including fibroblasts) in the kidney to produce extracellular matrix proteins, leading to scar tissue accumulation and fibrosis development [59, 60]. Additionally, TGF-β participates in immune cells' regulation and promotes pro-inflammatory cytokines and chemokines' production. Targeting TGF-β thus offers an attractive therapeutic intervention for renal inflammation and fibrosis [61,62,63,64]. By inhibiting TGF-β production, CB1 antagonists can prevent renal inflammation and fibrosis progression. These findings support previous studies demonstrating CB1 receptors' involvement in kidney disease's development and progression. Rimonabant, a representative CB1 antagonist [65,66,67], was approved by the European Medicines Agency in 2006 to reduce appetite via CB1 receptor antagonism in the brain, as an adjunct to diet and exercise in the treatment of obesity [68, 69]. Nonetheless, it was later withdrawn from the market due to higher risks of mental side effects (such as depression, anxiety, and suicidal thoughts), as clinical trials showed [70, 71].Recent years have seen the development of various new peripheral restricted CB1 inhibitors [72,73,74] (such as SLV319, JD5037, and AM6545, inclusive in this study), selectively inhibiting CB1 receptor activity in peripheral tissues to minimize brain exposure, while preserving mammalian receptor affinity and selectivity, potentially offering benefits in managing complications [75]. Notably, the researchers from the literature included in this study rarely added side effects such as mental status and cardiovascular events in evaluating cannabinoid receptors related to renal function in animals.
On the other hand, our analysis showed that CB2 agonists decreased creatinine and proteinuria in mice, while having no significant effect on urea nitrogen, blood glucose, body weight, and kidney to body weight ratio. Additionally, the use of CB2 agonists resulted in a reduction of renal pathological damage and mRNA expression levels of MCP-1 and TNF-α, but not TGF-β in this present meta-analysis. However, our sensitivity analysis revealed that the study by Shan Zhou et al. in [52] affected the statistical significance of the combined urea and creatinine results. They established an IRI mouse model by initial clipping of the left pedicle and unilateral nephrectomy one day before sacrifice and found that using the CB2 agonist AM1241 activated β-actin, increased urea nitrogen, and creatinine in UIRI mice. This contradicts other previous studies, including three models of cisplatin-induced acute kidney injury [22, 26, 37], a model of hepatorenal syndrome [47], and a rat model of IRI [46], which demonstrated the reduction of blood urea nitrogen and serum creatinine by CB2 agonists. Additionally, another study using SMM-295 [44], a novel CB2 agonist, significantly reduced serum creatinine in mice with acute kidney injury. In contrast, AM1241 has been shown to reduce proteinuria in STZ-induced diabetic mice [24]. Four of these studies reported a decrease in MCP-1 and TNF-α mRNA in renal tissue, collectively suggesting a protective effect of CB2 agonists. Differences in test time points, drugs used, and animal models of renal injury may be the main reasons for the inconsistent results. Subgroup analyses of the primary outcome with CB1 agonists showed that the modeling method may be a potential source of heterogeneity, with a statistically significant reduction in both SCR and BUN observed only in the CI-AKI subgroup.
Our analysis also showed that the combined results of CB2 receptor antagonists were not statistically significant, except for a slight alteration of urea nitrogen. CB2 receptor antagonist or gene knockout did not significantly affect creatinine, proteinuria, kidney weight/body weight ratio, and the expression levels of inflammation-related MCP-1 and TNF-α mRNA in the kidney of animals with renal injury, except for a possible slight increase in urea nitrogen from the results of four studies combined, and a significant increase in renal tubular pathological injury score from the results of two studies combined. However, caution must be exercised when interpreting the results due to the bias associated with a small number of studies and the variable diversity of interventions. Moreover, genetic approaches are harder to evaluate compared to small molecule drug interventions. In contrast, the effects of CB1 agonists were rarely reported in the included studies, with only one study reporting that the overexpression of CB1 gene increased proteinuria [32] and another study reporting that it increased the level of IL1β mRNA in the kidney [31]. Such limited results might have been due to our inclusion and exclusion criteria and concerns about cannabinoid abuse [76, 77]. Δ9-tetrahydrocannabinol (THC), a representative agonist of CB1, was the first to be chemically characterized as cannabinoids [78, 79]. Cannabidiol was found to be non-psychotic, while THC is responsible for the psychoactive effects of cannabis. CB1 is probably the most abundant and widespread G protein-coupled receptor in the mammalian brain, which makes therapeutic use of THC in pathological conditions very difficult [80].
Systematic reviews have investigated the effects of the cannabinoid system on pain [81, 82], but to our best knowledge, no other studies have been published with a meta-analysis in kidney disease. The endocannabinoid system comprises cannabinoid receptors (CB1 and CB2), endocannabinoids (eCB), and enzymes that regulate eCB biosynthesis and degradation. Dysregulation of this system has been linked to various pathological conditions, including pain disorders, neurodegenerative diseases, and metabolic disorders [83]. eCB is a lipid molecule synthesized on demand in response to various stimuli and acts as a retrograde messenger to regulate neurotransmitter release, predominantly anandamide and 2-arachidonoylglycerol. The CB1 receptor was initially identified as responsible for the mental effects of THC, or marijuana, to which anandamide binds with higher affinity [84, 85]. Subsequent research has shown that its high expression in peripheral and central nervous system cells can cause neuropathic and inflammatory pain, whereas CB2 is more expressed in immune cells, playing an anti-inflammatory role [86,87,88]. A large number of preclinical studies have recently revealed that peripheral CB1 can promote energy storage, affect lipid metabolism and insulin sensitivity, and significantly contribute to the pathogenesis of obesity, metabolic syndrome, and diabetes [89,90,91]. In contrast, the pathological state of CB2 is mainly present in inflammatory cells, where their anti-inflammatory effect includes inhibition of cytokine release [92,93,94,95]. In humans, most newly published structural biology studies have revealed different crystal structures of human CB1 and CB2, and small molecule drugs that affect CB1 and CB2 in different binding modes [96,97,98,99]. Cryo-electron microscopy has revealed the possible existence of this opposite activation spectrum of CB2 antagonism/CB1 agonism, representing a yin/yang functional relationship of CB2/CB1 [99, 100]. Particularly, CB1 and CB2 have been found to be expressed in a variety of cells in human normal kidney samples [11, 101], and increased expression of CB1 in renal biopsy specimens has been demonstrated in many renal diseases, including IgA nephropathy, acute interstitial nephritis, diabetic nephropathy, obesity-related glomerulopathy, and focal segmental glomerulosclerosis [34, 48]. In contrast, CB2 is expressed in decreased levels in diabetic nephropathy; however, it is thought to be greatly enhanced in lupus nephritis, membranous nephropathy, amyloid nephropathy, and immunoglobulin A nephropathy compared to a weak signal in healthy kidneys [24, 53]. Several reviews have been conducted on the pharmacological effects and research progress of CB1 and CB2 [80, 102, 103], especially in renal diseases [12, 104,105,106]. In general, the endocannabinoid system is complex, and the development of synthetic multi-target drugs from the endocannabinoidome is a promising direction. However, there are few studies exploring the interaction between CB1 and CB2 subtypes in renal diseases. Previous studies suggest that CB1 blockers primarily act on metabolism, as evidenced by parallel improvements in body weight, blood pressure, lipids, and insulin resistance [11]. In our meta-analysis, animal model groups treated with CB1 antagonist or knockout did not show statistically significant changes in KW/BW, blood glucose, or body weight compared to control groups. However, preclinical studies reported in the included literature suggest that CB1, affects renin-angiotensin system activity [30], influences the dynamic translocation of glucose transporter 2 in proximal tubular cells and glucose reabsorption [42], regulates the liver kinase B1/AMP-activated protein kinase signaling pathway [40], and affects cytoskeleton, extracellular matrix, apoptosis, and inflammatory cytokine secretion in vivo and in vitro [27, 34, 43]. The conflicting results on the renal protective effects of CB2 agonists are the most intriguing aspect of our study, and further research is needed to confirm and explore the underlying causes and mechanisms.
Limitations
Despite the increasing attention given to the limitations of animal welfare, methodological concerns, and reproducibility of preclinical animal research, preclinical efficacy testing through specific disease animal models remains an indispensable element in drug development [107,108,109,110,111]. In particular, various disease models for kidney disease have been summarized [112,113,114], each endeavoring to mimic the human disease model, although none of them can fully replicate it. Therefore, we included models of acute kidney disease, such as cisplatin-induced drug-induced AKI, ischemia–reperfusion AKI with vascular occlusion, unilateral ureteral obstruction AKI, and the unusual bile duct ligation hepatorenal syndrome-induced AKI. The chronic kidney disease models included diabetes, obesity-induced CKD, partial nephrectomy CKD simulation, and the infrequent chronic intermittent hypoxia-induced chronic kidney injury. Multiple disease models in mice and rats may create confounding factors that increase potential bias. Additionally, the methodological quality of studies in our systematic review faced analogous issues. Most studies lacked descriptions of allocation concealment, random housing, and blinding, which significantly elevated the risk of bias in the study. The existence of publication bias further accentuates the need to promote the prospective registration of animal experiments and greater acceptance of negative results. Another issue that cannot be overlooked is the difference in drug dosages and the timing of evaluation indicator detection, which holds great importance for future research. Thus, there are multiple limitations in our meta-analysis that we should acknowledge. The heterogeneity of animal models, dosages, and treatment regimens used across studies may have influenced the results. Furthermore, the extrapolation of findings from animal models to humans should be done with caution due to the possible species-specific differences in the effects of cannabinoid receptor modulation. Although the complexity and diversity of the mechanisms involved in the endocannabinoid system entail the potential for interventional drugs with multiple targets, many mature marketed drugs contain multiple drug targets [80], such as the recently demonstrated renal protective effect of sodium-glucose co-transporter-2 inhibitors [115], reinforcing the importance of dosage and cross-species studies.
Conclusions
In conclusion, our study offers significant insights into the potential therapeutic effects of cannabinoid receptor modulation on kidney disease (Fig. 13). In particular, our results underscore the potential for CB1 inhibition and CB2 activation to mitigate kidney damage and inflammation. Future studies should seek to investigate the mechanisms of action of cannabinoid receptor modulation in kidney disease more comprehensively, along with the optimal dosages and treatment schedules for clinical use. It is worth further clinical inquiries into potential side effects and long-term safety of cannabinoid receptor modulation in animal models and eventually in human trials. Ultimately, our research contributes to the growing body of evidence that targeting cannabinoid receptors may be effective as a therapeutic approach to treating kidney disease.
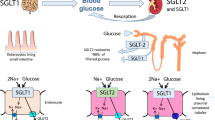
Schematic illustration of renal protection by intervention with CB1 and CB2. Pharmacological antagonism and genetic knockout of CB1, as well as pharmacological activation or genetic overexpression of CB2, may exert renal protective effects by downregulating TGF-β and α-SMA, and alleviating collagen matrix to exert anti-fibrotic effects. Additionally, by downregulating MCP-1, TNF-α, and interleukin factors, they act against inflammation. These mechanisms contribute to the reduction of markers like creatinine, urea nitrogen, and urinary protein in animal models of renal injury, without altering blood glucose levels or body weight. The regulatory effects of CB1 and CB2 on renal function can be likened to a "yin-yang" relationship
Availability of data and materials
Data available on request from the authors.
Abbreviations
- ACR:
-
Albumin Creatinine Ratio
- AER:
-
Albumin Excretion Rate
- AKI:
-
Acute Kidney Injury
- BUN:
-
Blood Urea Nitrogen
- BW:
-
Body Weight
- CB1:
-
Cannabinoid Receptor 1
- CB2:
-
Cannabinoid Receptor 2
- CI:
-
Confidence Interval
- CI-AKI:
-
Contrast-Induced Acute Kidney Injury
- CKD:
-
Chronic Kidney Disease
- eCB:
-
Endocannabinoids
- IL 18:
-
Interleukin 18
- IL 1β:
-
Interleukin-1 beta
- IRI:
-
Ischemia Reperfusion Injury
- KW:
-
Kidney Weight
- MCP-1:
-
Monocyte Chemotactic Protein 1
- Scr:
-
Serum Creatinine
- SD:
-
Standard Deviation
- SEM:
-
Standard Error of Mean
- SMD:
-
Standardized Mean Difference
- STZ:
-
Streptozotocin
- TGF-β:
-
Transforming Growth Factor-beta
- THC:
-
Δ9-Tetrahydrocannabinol
- TNF-α:
-
Tumor necrosis factor-alpha
- UIRI:
-
Unilateral Ischemia Reperfusion Injury
References
Levey AS, Coresh J. Chronic kidney disease. Lancet. 2012;379(9811):165–80.
Kellum JA, Romagnani P, Ashuntantang G, Ronco C, Zarbock A, Anders H-J. Acute kidney injury. Nat Rev Dis Primers. 2021;7(1):52.
Susantitaphong P, Cruz DN, Cerda J, Abulfaraj M, Alqahtani F, Koulouridis I, Jaber BL. World incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol. 2013;8(9):1482–93.
Bikbov B, Purcell CA, Levey AS, Smith M, Abdoli A, Abebe M, Adebayo OM, Afarideh M, Agarwal SK, Agudelo-Botero M, Ahmadian E. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395(10225):709–33.
Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, Gansevoort RT, Kasiske BL, Eckardt KU. The definition, classification, and prognosis of chronic kidney disease: a KDIGO controversies conference report. Kidney Int. 2011;80(1):17–28.
Lameire NH, Levin A, Kellum JA, Cheung M, Jadoul M, Winkelmayer WC, Stevens PE. Harmonizing acute and chronic kidney disease definition and classification: report of a kidney disease: improving global outcomes (KDIGO) consensus conference. Kidney Int. 2021;100(3):516–26.
Chawla LS, Bellomo R, Bihorac A, Goldstein SL, Siew ED, Bagshaw SM, Bittleman D, Cruz D, Endre Z, Fitzgerald RL, et al. Acute kidney disease and renal recovery: consensus report of the acute disease quality initiative (ADQI) 16 workgroup. Nat Rev Nephrol. 2017;13(4):241–57.
Humphreys BD. Mechanisms of renal fibrosis. Annu Rev Physiol. 2018;80:309–26.
Komada T, Muruve DA. The role of inflammasomes in kidney disease. Nat Rev Nephrol. 2019;15(8):501–20.
Schaub JA, Hamidi H, Subramanian L, Kretzler M. Systems biology and kidney disease. Clin J Am Soc Nephrol. 2020;15(5):695–703.
Barutta F, Bruno G, Mastrocola R, Bellini S, Gruden G. The role of cannabinoid signaling in acute and chronic kidney diseases. Kidney Int. 2018;94(2):252–8.
Chua JT, Argueta DA, DiPatrizio NV, Kovesdy CP, Vaziri ND, Kalantar-Zadeh K, Moradi H. Endocannabinoid system and the kidneys: from renal physiology to injury and disease. Cannabis Cannab Res. 2019;4(1):10–20.
Glass GV. Meta-analysis at middle age: a personal history. Res Synth Methods. 2015;6(3):221–31.
Siddaway AP, Wood AM, Hedges LV. How to do a systematic review: a best practice guide for conducting and reporting narrative reviews, meta-analyses, and meta-syntheses. Annu Rev Psychol. 2019;70:747–70.
Vries RD, Hooijmans CR, Langendam MW, Luijk JV, Leenaars M, Ritskes-Hoitinga M, Wever KE. A protocol format for the preparation, registration and publication of systematic reviews of animal intervention studies. Evid Based Preclin Med. 2015;2(1):1–9.
Higgins JPT TJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors): Cochrane Handbook for Systematic Reviews of Interventions. In.: Chichester (UK): John Wiley & Sons. 2019.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
Janiak P, Poirier B, Bidouard JP, Cadrouvele C, Pierre F, Gouraud L, Barbosa I, Dedio J, Maffrand JP, Le Fur G, et al. Blockade of cannabinoid CB1 receptors improves renal function, metabolic profile, and increased survival of obese Zucker rats. Kidney Int. 2007;72(11):1345–57.
Barutta F, Corbelli A, Mastrocola R, Gambino R, Marzo VD, Pinach S, Rastaldi MP, Perin PC, Gruden G. Cannabinoid receptor 1 blockade ameliorates albuminuria in experimental diabetic nephropathy. Diabetes. 2010;59(4):1046–54.
Mukhopadhyay P, Pan H, Rajesh M, Batkai S, Patel V, Harvey-White J, Mukhopadhyay B, Hasko G, Gao B, Mackie K, et al. CB1 cannabinoid receptors promote oxidative/nitrosative stress, inflammation and cell death in a murine nephropathy model. Br J Pharmacol. 2010;160(3):657–68.
Mukhopadhyay P, Rajesh M, Pan H, Patel V, Mukhopadhyay B, Bátkai S, Gao B, Haskó G, Pacher P. Cannabinoid-2 receptor limits inflammation, oxidative/nitrosative stress, and cell death in nephropathy. Free Radical Biol Med. 2010;48(3):457–67.
Russell JC, Kelly SE, Diane A, Wang Y, Mangat R, Novak S, Vine DF. Proctor SD Rimonabant-mediated changes in intestinal lipid metabolism and improved renal vascular dysfunction in the JCR:LA-cp rat model of prediabetic metabolic syndrome. Am J Physiol Gastroint Liver Physiol. 2010;299(2):507–16.
Barutta F, Piscitelli F, Pinach S, Bruno G, Gambino R, Rastaldi MP, Salvidio G, Di Marzo V, Perin PC, Gruden G. Protective role of cannabinoid receptor type 2 in a mouse model of diabetic nephropathy. Diabetes. 2011;60(9):2386–96.
Alonso M, Serrano A, Vida M, Crespillo A, Hernandez-Folgado L, Jagerovic N, Goya P, Reyes-Cabello C, Perez-Valero V, Decara J, et al. Anti-obesity efficacy of LH-21, a cannabinoid CB1 receptor antagonist with poor brain penetration, in diet-induced obese rats. Br J Pharmacol. 2012;165(7):2274–91.
Horváth B, Mukhopadhyay P, Kechrid M, Patel V, Tanchian G, Wink DA, Gertsch J, Pacher P. β-caryophyllene ameliorates cisplatin-induced nephrotoxicity in a cannabinoid 2 receptor-dependent manner. Free Radical Biol Med. 2012;52(8):1325–33.
Nam DH, Lee MH, Kim JE, Song HK, Kang YS, Lee JE, Kim HW, Cha JJ, Hyun YY, Kim SH, et al. Blockade of cannabinoid receptor 1 improves insulin resistance, lipid metabolism, and diabetic nephropathy in db/db mice. Endocrinology. 2012;153(3):1387–96.
Tang Y, Ho G, Li Y, Hall MA, Hills RL, Black SC, Liang Y, Demarest KT. Beneficial metabolic effects of cb1r anti-sense oligonucleotide treatment in diet-induced obese AKR/J Mice. PLoS ONE. 2012;7(8):e42134.
Barutta F, Grimaldi S, Franco I, Bellini S, Gambino R, Pinach S, Corbelli A, Bruno G, Rastaldi MP, Aveta T, et al. Deficiency of cannabinoid receptor of type 2 worsens renal functional and structural abnormalities in streptozotocin-induced diabetic mice. Kidney Int. 2014;86(5):979–90.
Jourdan T, Szanda G, Rosenberg AZ, Tam J, Earley BJ, Godlewski G, Cinar R, Liu Z, Liu J, Ju C, et al. Overactive cannabinoid 1 receptor in podocytes drives type 2 diabetic nephropathy. Proc Natl Acad Sci USA. 2014;111(50):E5420-5428.
Lin CL, Hsu YC, Lee PH, Lei CC, Wang JY, Huang YT, Wang SY, Wang FS. Cannabinoid receptor 1 disturbance of PPARγ2 augments hyperglycemia induction of mesangial inflammation and fibrosis in renal glomeruli. J Mol Med. 2014;92(7):779–92.
Hsu Y-C, Lei C-C, Shih Y-H, Ho C, Lin C-L. Induction of proteinuria by cannabinoid receptors 1 signaling activation in CB1 transgenic mice. Am J Med Sci. 2015;349(2):162–8.
Jenkin KA, O’Keefe L, Simcocks AC, Grinfeld E, Mathai ML, McAinch AJ, Hryciw DH. Chronic administration of AM251 improves albuminuria and renal tubular structure in obese rats. J Endocrinol. 2015;225(2):113–24.
Lecru L, Desterke C, Grassin-Delyle S, Chatziantoniou C, Vandermeersch S, Devocelle A, Vernochet A, Ivanovski N, Ledent C, Ferlicot S, et al. Cannabinoid receptor 1 is a major mediator of renal fibrosis. Kidney Int. 2015;88(1):72–84.
Lin CY, Hsu YJ, Hsu SC, Chen Y, Lee HS, Lin SH, Huang SM, Tsai CS, Shih CC. CB1 cannabinoid receptor antagonist attenuates left ventricular hypertrophy and Akt-mediated cardiac fibrosis in experimental uremia. J Mol Cell Cardiol. 2015;85:249–61.
Jenkin KA, O’Keefe L, Simcocks AC, Briffa JF, Mathai ML, McAinch AJ, Hryciw DH. Renal effects of chronic pharmacological manipulation of CB2 receptors in rats with diet-induced obesity. Br J Pharmacol. 2016;173(7):1128–42.
Mukhopadhyay P, Baggelaar M, Erdelyi K, Cao Z, Cinar R, Fezza F, Ignatowska-Janlowska B, Wilkerson J, van Gils N, Hansen T, et al. The novel, orally available and peripherally restricted selective cannabinoid CB2 receptor agonist LEI-101 prevents cisplatin-induced nephrotoxicity. Br J Pharmacol. 2016;173(3):446–58.
Zoja C, Locatelli M, Corna D, Villa S, Rottoli D, Nava V, Verde R, Piscitelli F, Di Marzo V, Fingerle J, et al. Therapy with a selective cannabinoid receptor type 2 agonist limits albuminuria and renal injury in mice with type 2 diabetic nephropathy. Nephron. 2016;132(1):59–69.
Jourdan T, Szanda G, Cinar R, Godlewski G, Holovac DJ, Park JK, Nicoloro S, Shen Y, Liu J, Rosenberg AZ, et al. Developmental role of macrophage cannabinoid-1 receptor signaling in type 2 diabetes. Diabetes. 2017;66(4):994–1007.
Udi S, Hinden L, Earley B, Drori A, Reuveni N, Hadar R, Cinar R, Nemirovski A, Tam J. Proximal tubular cannabinoid-1 receptor regulates obesity-induced CKD. J Am Soc Nephrol. 2017;28(12):3518–32.
Barutta F, Bellini S, Mastrocola R, Gambino R, Piscitelli F, di Marzo V, Corbetta B, Vemuri VK, Makriyannis A, Annaratone L, et al. Reversal of albuminuria by combined AM6545 and perindopril therapy in experimental diabetic nephropathy. Br J Pharmacol. 2018;175(23):4371–85.
Hinden L, Udi S, Drori A, Gammal A, Nemirovski A, Hadar R, Baraghithy S, Permyakova A, Geron M, Cohen M, et al. Modulation of renal GLUT2 by the cannabinoid-1 receptor: implications for the treatment of diabetic nephropathy. J Am Soc Nephrol. 2018;29(2):434–48.
Jourdan T, Park JK, Varga ZV, Pálóczi J, Coffey NJ, Rosenberg AZ, Godlewski G, Cinar R, Mackie K, Pacher P, et al. Cannabinoid-1 receptor deletion in podocytes mitigates both glomerular and tubular dysfunction in a mouse model of diabetic nephropathy. Diabetes Obes Metab. 2018;20(3):698–708.
Pressly JD, Mustafa SM, Adibi AH, Alghamdi S, Pandey P, Roy KK, Doerksen RJ, Moore BM, Park F. Selective cannabinoid 2 receptor stimulation reduces tubular epithelial cell damage after renal ischemia-reperfusion injury. J Pharmacol Exp Ther. 2018;364(2):287–99.
Zhou L, Zhou S, Yang P, Tian Y, Feng Z, Xie XQ, Liu Y. Targeted inhibition of the type 2 cannabinoid receptor is a novel approach to reduce renal fibrosis. Kidney Int. 2018;94(4):756–72.
Çakır M, Tekin S, Doğanyiğit Z, Çakan P, Kaymak E. The protective effect of cannabinoid type 2 receptor activation on renal ischemia–reperfusion injury. Mol Cell Biochem. 2019;462(1–2):123–32.
Trojnar E, Erdelyi K, Matyas C, Zhao S, Paloczi J, Mukhopadhyay P, Varga ZV, Hasko G, Pacher P. Cannabinoid-2 receptor activation ameliorates hepatorenal syndrome. Free Radical Biol Med. 2020;152:540–50.
Udi S, Hinden L, Ahmad M, Drori A, Iyer MR, Cinar R, Herman-Edelstein M, Tam J. Dual inhibition of cannabinoid CB(1) receptor and inducible NOS attenuates obesity-induced chronic kidney disease. Br J Pharmacol. 2020;177(1):110–27.
Gonzalez-Mariscal I, Carmona-Hidalgo B, Winkler M, Unciti-Broceta JD, Escamilla A, Gomez-Canas M, Fernandez-Ruiz J, Fiebich BL, Romero-Zerbo S-Y, Bermudez-Silva FJ, et al. (+)-trans-Cannabidiol-2-hydroxy pentyl is a dual CB1R antagonist/CB2R agonist that prevents diabetic nephropathy in mice. Pharmacol Res. 2021;169:105492.
Medapati JR, Rapaka D, Bitra VR, Ranajit SK, Guntuku GS, Akula A. Role of endocannabinoid CB1 receptors in Streptozotocin-induced uninephrectomised Wistar rats in diabetic nephropathy. Beni-Suef Univer J Basic Appl Sci. 2021;10(1):1–2.
Zhao L, Liu T, Dou Z-J, Wang M-T, Hu Z-X, Wang B. CB1 receptor antagonist rimonabant protects against chronic intermittent hypoxia-induced renal injury in rats. BMC Nephrol. 2021;22(1):153.
Zhou S, Ling X, Meng P, Liang Y, Shen K, Wu Q, Zhang Y, Chen Q, Chen S, Liu Y, et al. Cannabinoid receptor 2 plays a central role in renal tubular mitochondrial dysfunction and kidney ageing. J Cell Mol Med. 2021;25:8957–72.
Zhou S, Wu Q, Lin X, Ling X, Miao J, Liu X, Hu C, Zhang Y, Jia N, Hou FF, et al. Cannabinoid receptor type 2 promotes kidney fibrosis through orchestrating β-catenin signaling. Kidney Int. 2021;99(2):364–81.
Rabb H, Griffin MD, McKay DB, Swaminathan S, Pickkers P, Rosner MH, Kellum JA, Ronco C. Inflammation in AKI: current understanding, key questions, and knowledge gaps. J Am Soc Nephrol. 2016;27(2):371–9.
Lv W, Booz GW, Wang Y, Fan F, Roman RJ. Inflammation and renal fibrosis: recent developments on key signaling molecules as potential therapeutic targets. Eur J Pharmacol. 2018;820:65–76.
Zhou S, Wang P, Qiao Y, Ge Y, Wang Y, Quan S, Yao R, Zhuang S, Wang LJ, Du Y, et al. Genetic and pharmacologic targeting of glycogen synthase kinase 3β reinforces the Nrf2 antioxidant defense against podocytopathy. J Am Soc Nephrol. 2016;27(8):2289–308.
Meng X-M, Nikolic-Paterson DJ, Lan HY. TGF-β: the master regulator of fibrosis. Nat Rev Nephrol. 2016;12(6):325–38.
Sureshbabu A, Muhsin SA, Choi ME. TGF-β signaling in the kidney: profibrotic and protective effects. Am J Physiol Renal Physiol. 2016;310(7):F596–606.
Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci. 2003;116(Pt 2):217–24.
Chin BY, Mohsenin A, Li SX, Choi AM, Choi ME. Stimulation of pro-alpha(1)(I) collagen by TGF-beta(1) in mesangial cells: role of the p38 MAPK pathway. Am J Physiol Renal Physiol. 2001;280(3):F495–504.
Meng X-M, Chung ACK, Lan HY. Role of the TGF-β/BMP-7/Smad pathways in renal diseases. Clin Sci. 2013;124(4):243–54.
Lan HY. Diverse roles of TGF-β/Smads in renal fibrosis and inflammation. Int J Biol Sci. 2011;7(7):1056–67.
Gagliardini E, Benigni A. Role of anti-TGF-beta antibodies in the treatment of renal injury. Cytokine Growth Factor Rev. 2006;17(1–2):89–96.
Hong Q, Cai H, Zhang L, Li Z, Zhong F, Ni Z, Cai G, Chen X-M, He JC, Lee K. Modulation of transforming growth factor-β-induced kidney fibrosis by leucine-rich ⍺-2 glycoprotein-1. Kidney Int. 2022;101(2):299–314.
Rinaldi-Carmona M, Barth F, Héaulme M, Shire D, Calandra B, Congy C, Martinez S, Maruani J, Néliat G, Caput D. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350(2–3):240–4.
Bouaboula M, Perrachon S, Milligan L, Canat X, Rinaldi-Carmona M, Portier M, Barth F, Calandra B, Pecceu F, Lupker J, et al. A selective inverse agonist for central cannabinoid receptor inhibits mitogen-activated protein kinase activation stimulated by insulin or insulin-like growth factor 1. Evidence for a new model of receptor/ligand interactions. J Bio Chem. 1997;272(35):22330–9.
Carai MAM, Colombo G, Gessa GL. Rimonabant: the first therapeutically relevant cannabinoid antagonist. Life Sci. 2005;77(19):2339–50.
Després J-P, Golay A, Sjöström L. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med. 2005;353(20):2121–34.
Pi-Sunyer FX, Aronne LJ, Heshmati HM, Devin J, Rosenstock J. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial. JAMA. 2006;295(7):761–75.
Christensen R, Kristensen PK, Bartels EM, Bliddal H, Astrup A. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet. 2007;370(9600):1706–13.
Jones D. End of the line for cannabinoid receptor 1 as an anti-obesity target? Nat Rev Drug Discovery. 2008;7(12):961–2.
Lange JHM, Coolen HKAC, van Stuivenberg HH, Dijksman JAR, Herremans AHJ, Ronken E, Keizer HG, Tipker K, McCreary AC, Veerman W, et al. Synthesis, biological properties, and molecular modeling investigations of novel 3,4-diarylpyrazolines as potent and selective CB(1) cannabinoid receptor antagonists. J Med Chem. 2004;47(3):627–43.
Tam J, Cinar R, Liu J, Godlewski G, Wesley D, Jourdan T, Szanda G, Mukhopadhyay B, Chedester L, Liow J-S, et al. Peripheral cannabinoid-1 receptor inverse agonism reduces obesity by reversing leptin resistance. Cell Metab. 2012;16(2):167–79.
Cluny NL, Vemuri VK, Chambers AP, Limebeer CL, Bedard H, Wood JT, Lutz B, Zimmer A, Parker LA, Makriyannis A, et al. A novel peripherally restricted cannabinoid receptor antagonist, AM6545, reduces food intake and body weight, but does not cause malaise, in rodents. Br J Pharmacol. 2010;161(3):629–42.
Tam J, Hinden L, Drori A, Udi S, Azar S, Baraghithy S. The therapeutic potential of targeting the peripheral endocannabinoid/CB1 receptor system. Eur J Intern Med. 2018;49:23–9.
Rein JL. The nephrologist’s guide to cannabis and cannabinoids. Curr Opin Nephrol Hypertens. 2020;29(2):248–57.
Rein JL, Wyatt CM. Marijuana and cannabinoids in ESRD and earlier stages of CKD. Am J Kidney Dis. 2018;71(2):267–74.
Mechoulam R, Shani A, Edery H, Grunfeld Y. Chemical basis of hashish activity. Science. 1970;169(3945):611–2.
Varvel SA, Bridgen DT, Tao Q, Thomas BF, Martin BR, Lichtman AH. Delta9-tetrahydrocannbinol accounts for the antinociceptive, hypothermic, and cataleptic effects of marijuana in mice. J Pharmacol Exp Ther. 2005;314(1):329–37.
Di Marzo V. New approaches and challenges to targeting the endocannabinoid system. Nat Rev Drug Discovery. 2018;17(9):623–39.
Soliman N, Haroutounian S, Hohmann AG, Krane E, Liao J, Macleod M, Segelcke D, Sena C, Thomas J, Vollert J, et al. Systematic review and meta-analysis of cannabinoids, cannabis-based medicines, and endocannabinoid system modulators tested for antinociceptive effects in animal models of injury-related or pathological persistent pain. Pain. 2021;162(Suppl 1):S26–44.
Scuteri D, Rombolà L, Hamamura K, Sakurada T, Watanabe C, Sakurada S, Guida F, Boccella S, Maione S, Gallo Afflitto G, et al. Is there a rational basis for cannabinoids research and development in ocular pain therapy? A systematic review of preclinical evidence. Biomed Pharmacother. 2022;146: 112505.
Lu HC, Mackie K. Review of the endocannabinoid system. Bio Psy Cognit Neurosci Neuroimag. 2021;6(6):607–15.
Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346(6284):561–4.
Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365(6441):61–5.
Lötsch J, Weyer-Menkhoff I, Tegeder I. Current evidence of cannabinoid-based analgesia obtained in preclinical and human experimental settings. Eur J Pain. 2018;22(3):471–84.
Di Marzo V. Targeting the endocannabinoid system: to enhance or reduce? Nat Rev Drug Discov. 2008;7(5):438–55.
Leinwand KL, Jones AA, Huang RH, Jedlicka P, Kao DJ, de Zoeten EF, Ghosh S, Moaddel R, Wehkamp J, Ostaff MJ, et al. Cannabinoid receptor-2 ameliorates inflammation in murine model of crohn’s disease. J Crohns Colitis. 2017;11(11):1369–80.
Sharma MK, Murumkar PR, Kanhed AM, Giridhar R, Yadav MR. Prospective therapeutic agents for obesity: molecular modification approaches of centrally and peripherally acting selective cannabinoid 1 receptor antagonists. Eur J Med Chem. 2014;79:298–339.
O’Keefe L, Simcocks AC, Hryciw DH, Mathai ML, McAinch AJ. The cannabinoid receptor 1 and its role in influencing peripheral metabolism. Diabetes Obes Metab. 2014;16(4):294–304.
RuizdeAzua I, Mancini G, Srivastava RK, Rey AA, Cardinal P, Tedesco L, Zingaretti CM, Sassmann A, Quarta C, Schwitter C, et al. Adipocyte cannabinoid receptor CB1 regulates energy homeostasis and alternatively activated macrophages. J Clin Invest. 2017;127(11):4148–62.
Turcotte C, Blanchet M-R, Laviolette M, Flamand N. The CB2 receptor and its role as a regulator of inflammation. Cell Mol Life Sci. 2016;73(23):4449–70.
Julien B, Grenard P, Teixeira-Clerc F, Van Nhieu JT, Li L, Karsak M, Zimmer A, Mallat A, Lotersztajn S. Antifibrogenic role of the cannabinoid receptor CB2 in the liver. Gastroenterology. 2005;128(3):742–55.
Bátkai S, Mukhopadhyay P, Horváth B, Rajesh M, Gao RY, Mahadevan A, Amere M, Battista N, Lichtman AH, Gauson LA, et al. Δ8-Tetrahydrocannabivarin prevents hepatic ischaemia/reperfusion injury by decreasing oxidative stress and inflammatory responses through cannabinoid CB2 receptors. Br J Pharmacol. 2012;165(8):2450–61.
Steffens S, Veillard NR, Arnaud C, Pelli G, Burger F, Staub C, Karsak M, Zimmer A, Frossard J-L, Mach F. Low dose oral cannabinoid therapy reduces progression of atherosclerosis in mice. Nature. 2005;434(7034):782–6.
Hua T, Vemuri K, Pu M, Qu L, Han GW, Wu Y, Zhao S, Shui W, Li S, Korde A, et al. Crystal structure of the human cannabinoid receptor CB1. Cell. 2016;167(3):750–62.
Shao Z, Yin J, Chapman K, Grzemska M, Clark L, Wang J, Rosenbaum DM. High-resolution crystal structure of the human CB1 cannabinoid receptor. Nature. 2016;540(7634):602–6.
Hua T, Vemuri K, Nikas SP, Laprairie RB, Wu Y, Qu L, Pu M, Korde A, Jiang S, Ho J-H, et al. Crystal structures of agonist-bound human cannabinoid receptor CB1. Nature. 2017;547(7664):468–71.
Li X, Hua T, Vemuri K, Ho J-H, Wu Y, Wu L, Popov P, Benchama O, Zvonok N, Locke K, et al. Crystal structure of the human cannabinoid receptor CB2. Cell. 2019;176(3):459–67.
Hua T, Li X, Wu L, Iliopoulos-Tsoutsouvas C, Wang Y, Wu M, Shen L, Brust CA, Nikas SP, Song F, et al. Activation and signaling mechanism revealed by cannabinoid receptor-gi complex structures. Cell. 2020;180(4):655–65.
Larrinaga G, Varona A, Pérez I, Sanz B, Ugalde A, Cándenas ML, Pinto FM, Gil J, López JI. Expression of cannabinoid receptors in human kidney. Histol Histopathol. 2010;25(9):1133–8.
Cinar R, Iyer MR, Kunos G. The therapeutic potential of second and third generation CB1R antagonists. Pharmacol Ther. 2020;208: 107477.
Whiting ZM, Yin J, de la Harpe SM, Vernall AJ, Grimsey NL. Developing the Cannabinoid Receptor 2 (CB2) pharmacopoeia: past, present, and future. Trends Pharmacol Sci. 2022;43(9):754–71.
Dao M, François H. Cannabinoid receptor 1 inhibition in chronic kidney disease: a new therapeutic toolbox. Front Endocrinol. 2021;12: 720734.
Tam J. The emerging role of the endocannabinoid system in the pathogenesis and treatment of kidney diseases. J Basic Clin Physiol Pharmacol. 2016;27(3):267–76.
Park F, Potukuchi PK, Moradi H, Kovesdy CP. Cannabinoids and the kidney: effects in health and disease. Am J Physiol Renal Physiol. 2017;313(5):F1124–32.
Smith AJ, Lilley E. The role of the three rs in improving the planning and reproducibility of animal experiments. Animals. 2019;9(11):975.
Karp NA. Reproducible preclinical research-Is embracing variability the answer? PLoS Biol. 2018;16(3): e2005413.
Vogt L, Reichlin TS, Nathues C, Würbel H. Authorization of animal experiments is based on confidence rather than evidence of scientific rigor. PLoS Biol. 2016;14(12): e2000598.
Zhou X-T, Zou J-J, Ao C, Gong D-Y, Chen X, Ma Y-R. Renal protective effects of astragaloside IV, in diabetes mellitus kidney damage animal models: a systematic review, meta-analysis. Pharmacol Res. 2020;160: 105192.
Henderson VC, Kimmelman J, Fergusson D, Grimshaw JM, Hackam DG. Threats to validity in the design and conduct of preclinical efficacy studies: a systematic review of guidelines for in vivo animal experiments. PLoS Med. 2013;10(7): e1001489.
Becker GJ, Hewitson TD. Animal models of chronic kidney disease: useful but not perfect. Nephrol Dialys Transplantat. 2013;28(10):2432–8.
Azushima K, Gurley SB, Coffman TM. Modelling diabetic nephropathy in mice. Nat Rev Nephrol. 2018;14(1):48–56.
Bao Y-W, Yuan Y, Chen J-H, Lin W-Q. Kidney disease models: tools to identify mechanisms and potential therapeutic targets. Zool Res. 2018;39(2):72–86.
Tuttle KR, Brosius FC, Cavender MA, Fioretto P, Fowler KJ, Heerspink HJL, Manley T, McGuire DK, Molitch ME, Mottl AK, et al. SGLT2 Inhibition for CKD and cardiovascular disease in type 2 diabetes: report of a scientific workshop sponsored by the national kidney foundation. Am J Kidney Dis. 2021;77(1):1–6.
Acknowledgements
None.
Funding
This work was supported by the National Natural Science Foundation of China (No.81970633) and Natural Science Foundation of Henan Province (No. 222300420330).
Author information
Authors and Affiliations
Contributions
LZS and ZZH designed research studies. All members of the team jointly formulated a retrieval strategy. ZZH, YQQ, PSK, and LY were responsible for the literature search and completed the screening of the literature. ZZH, LZJ, LFX, and XJW performed data extraction and research quality evaluation. ZZH conducted data analysis. ZZH and DJY wrote the manuscript. LDW, ZSJ and LZS revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
An ethics statement is not applicable because this study is based exclusively on published literature.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Quality assessment graph of the included studies: reviewers’ judgments about each risk of bias item for eligible studies based on SYRCLE’s RoB tool for animal studies. Figure S2. Forest plot for sensitivity analysis on CB1 antagonist and knockout primary outcomes including blood urea nitrogen (A), serum creatinine (B) and albuminuria (C). Figure S3. Forest plot for sensitivity analysis on CB2 agonist primary outcomes including blood urea nitrogen (A) and serum creatinine (B); CB2 antagonist and knockout primary outcomes including blood urea nitrogen (C); and serum creatinine (D). Figure S4. Forest plots for subgroup analyses of the CB1 antagonist and knockout on blood urea nitrogen. Subgroup analyses were conducted stratified by the specie is rat or mouse (A); the intervention is antagonist or genetic (B); year of study published (C), (published = 1 means published in 2011 and earlier, published = 2 means published in 2012 and later); disease model is CKD or AKI (D); and method of model establishment is diabetes, cisplatin-induce AKI, DIO, or nephrectomy uremia (E). Figure S5. Forest plots for subgroup analyses of the CB1 antagonist and knockout on serum creatinine. Subgroup analyses were conducted stratified by the specie is rat or mouse (A); the intervention is antagonist or genetic (B); year of study published (C), (published = 1 means published in 2011 and earlier, published = 2 means published in 2012 and later); disease model is CKD or AKI (D); and method of model establishment is diabetes, cisplatin-induce AKI, DIO, or nephrectomy uremia (E). Figure S6. Forest plots for subgroup analyses of the CB1 antagonist and knockout on albuminuria. Subgroup analyses were conducted stratified by the specie is rat or mouse (A); the intervention is antagonist or genetic (B); year of study published (C), (published = 1 means published in 2011 and earlier, published = 2 means published in 2012 and later); disease model is CKD or AKI (D); and method of model establishment is diabetes or genetic obesity (E). Figure S7. Forest plots for subgroup analyses of the CB2 agonist on blood urea nitrogen. Subgroup analyses were conducted stratified by the specie is rat or mouse (A); the intervention is agonist or genetic (B); year of study published (C), (published = 1 means published in 2011 and earlier, published = 2 means published in 2012 and later); disease model is CKD or AKI (D); and method of model establishment is cisplatin-induce AKI, HRS or UUO (E). Figure S8. Forest plots for subgroup analyses of the CB2 agonist on serum creatinine. Subgroup analyses were conducted stratified by the specie is rat or mouse (A); the intervention is agonist or genetic (B); year of study published (C), (published = 1 means published in 2011 and earlier, published = 2 means published in 2012 and later); disease model is CKD or AKI (D); and method of model establishment is cisplatin-induce AKI, DIO, IRI or HRS (E). Figure S9. Forest plots for subgroup analyses of the CB2 antagonist and knockout on blood urea nitrogen. Subgroup analyses were conducted stratified by the specie is rat or mouse (A); the intervention is antagonist or gene knockout (B); year of study published (C), (published = 1 means published in 2011 and earlier, published = 2 means published in 2012 and later); disease model is CKD or AKI (D); and method of model establishment is cisplatin-induce AKI or unilateral nephrectomy and D-gal aged (E). Figure S10. Forest plots for subgroup analyses of the CB2 antagonist and knockout on serum creatinine. Subgroup analyses were conducted stratified by the specie is rat or mouse (A); the intervention is antagonist or gene knockout (B); year of study published (C), (published = 1 means published in 2011 and earlier, published = 2 means published in 2012 and later); disease model is CKD or AKI (D); and method of model establishment is cisplatin-induce AKI, DIO, IRI, or HRS (E).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhao, Z., Yan, Q., Xie, J. et al. The intervention of cannabinoid receptor in chronic and acute kidney disease animal models: a systematic review and meta-analysis. Diabetol Metab Syndr 16, 45 (2024). https://doi.org/10.1186/s13098-024-01283-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13098-024-01283-2