Abstract
Background
Thrombotic thrombocytopenic purpura (TTP) is a rare thrombotic microangiopathy with a rapid progression and high mortality rate. We aimed to explore early risk factors for mortality in patients with TTP.
Methods
We conducted a retrospective analysis of 42 TTP patients that were admitted to our hospital between 2000 and 2021, with a median age of 49 (29–63) years. Risk factors for mortality were evaluated using multivariate logistic regression. Receiver operating characteristic curve analysis was used to determine the cut-off value of glucose for predicting mortality in patients, which was validated by comparison to a similar cohort in the published literature.
Results
Elevated glucose level and reduced red blood cells (RBC) counts were risk factors for mortality in patients with TTP (glucose, odds ratio and 95% confidence interval: 2.476 [1.368–4.484]; RBC, odds ratio and 95% confidence interval: 0.095 [0.011–0.799]). The area under the curve of glucose was 0.827, and the cut-off value was 9.2 mmol/L, with a sensitivity of 75.0% and specificity of 95.8%. A total of 26 cases from the validation cohort had a sensitivity of 71.0% and a specificity of 84.0%. The change trends of the TTP-related laboratory indices differed during hospitalization.
Conclusion
Hyperglycemia at admission and unstable blood glucose levels during hospitalization may be potential predictors of mortality for TTP patients. The improved prognosis was associated with the recovery of platelet counts and a significant decrease in serum lactate dehydrogenase after five days of treatment.
Similar content being viewed by others
Introduction
Thrombotic thrombocytopenic purpura (TTP) is a rare thrombotic microangiopathy with a global annual incidence of approximately 1.5–6 per million people and a female predominance [1]. It has a rapid progression with a mortality rate of untreated TTP as high as 90%; this can be reduced to 10 − 20% by early identification, plasma exchange, and immunosuppressive therapy [2, 3]. TTP is characterized by microangiopathic hemolytic anemia (MAHA), thrombocytopenia, and neurologic deficits (the triad of TTP symptoms); fever and renal involvement encompass the common pentad of symptoms [4, 5]. Unfortunately, TTP is often misdiagnosed or diagnosis is delayed.
TTP results from a severe deficiency of the specific von Willebrand factor (VWF) - cleaving protease, ADAMTS13 (a disintegrin - like and metalloprotease with thrombospondin type 1 motif, member 13) [4, 5]. There are two general forms of ADAMTS13 deficiency, congenital TTP (cTTP) and immune TTP (iTTP). In all TTP patients, the activity of ADAMTS13 in the plasma decreases significantly (< 10%); and patients with iTTP often show the presence of ADAMTS13 inhibitors [5, 6].
Studies have shown that hyperglycemia on admission is associated with a poor prognosis in patients with TTP [7]. A temporary increase in blood glucose can be observed when a relatively severe disease occurs, and stress hyperglycemia has also been found to be associated with increased mortality [8]. In this study, we presented the hospitalization process of 42 patients with TTP in our hospital between 2000 and 2021 and provided a new laboratory basis for clinicians to formulate timely and accurate treatment plans to reduce mortality.
Materials and methods
Patient data
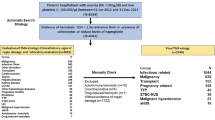
The research related to human use has been complied with all the relevant national regulations, institutional policies and in accordance the tenets of the Helsinki Declaration, and has been approved by the authors’ institutional review board or equivalent committee. We enrolled 42 patients who were first diagnosed with TTP in our hospital between 2000 and 2021. The patients were divided into two groups: survivors (25, 60%) and non-survivors (17, 40%), depending on the 30-day post-admission outcome, and then created an external validation cohort with comparable data with the study group to verify the impact of blood glucose level at admission on the mortality of TTP patients. We searched case reports in a Chinese medical full-text and PubMed databases using the search term “thrombotic thrombocytopenic purpura” between 2000 and 2021. A total of 26 patients with TTP with blood glucose results at admission were included (19 survivors [73%] and 7 non-survivors [27%]) (Fig. 1, Supplementary Table S1). In our medical center, the treatment for all TTP patients is according to guidelines and expert consensus, including plasma exchange, glucocorticoid therapy, plasma infusion, red blood cell infusion strategies [9,10,11], and management of stress hyperglycemia [12].
Patient diagnosis and treatment flow. Between 2000 and 2021, 42 patients with TTP in our hospital were retrospectively enrolled, they were defined as survivors (25 cases) and non-survivors (17 cases) according to their survival status. A total of 26 TTP cases with serum glucose results on admission from published literature were collected as the external validation cohort, including 19 cases (73%) of survivors and 7 cases (27%) of non-survivors
The diagnostic criteria for TTP were: (a) clinical manifestations: MAHA and thrombocytopenia; (b) blood and biochemical changes: anemia, significantly decreased platelet count, red cell fragmentation > 1% in a peripheral blood smear and significantly elevated lactate dehydrogenase (LDH) serum levels; (c) a pathology consistent with TTP and response to plasma exchange therapy and (d) After 2013, when ADAMTS13 activity measurement was available, ADAMTS13 activity < 10% was also included as one diagnostic criterion [13]. We excluded patients with other thrombotic microangiopathies, such as atypical hemolytic uremic syndrome or transplant-related microangiopathy [9, 10, 14].
We collected data on the following: infections, hypertension, diabetes, connective tissue diseases, and cardiovascular diseases. We also collected laboratory data, including complete blood counts, serum biochemistry, and immunity. In addition, results of ADAMTS13 activity and inhibitor tests available for admitted patients after 2013 were collected. Among the 42 TTP patients, 23 patients received medication before admission, including glucocorticoids (9 cases), antibiotics (8 cases), omeprazole (7 cases), hemostatic drugs (5 cases), traditional Chinese medicine (3 cases), antipyretics (2 cases), antidiarrheals (1 case), and none of them received plasma exchange.
Each patient provided the first fasting blood sample after admission to the hospital. Samples of 2 ml were mixed with EDTA-K2 and an anticoagulant for a complete blood count analysis by a Sysmex routine blood analyzer XE2100 (Sysmex, Japan). Samples of 3 ml were centrifuged at 3500 rpm for 5 min to obtain serum samples for biochemical indicators by an AU5800 automatic biochemical analyzer (Beckman, USA) and antinuclear antibody levels were determined by indirect immunofluorescence. Samples of 2.7 ml were mixed with sodium citrate anticoagulant and centrifuged at 3500 rpm for 5 min to obtain plasma samples for ADAMTS13 activity assay, which was performed using residual collagen-binding assays (R-CBA). Samples for antinuclear antibody (stored at 2–8℃) and ADAMTS13 activity and its inhibitor (stored at -30℃ and analyzed by the third-party company) were examined within 48 h, and the rest samples were detected within 2–4 h after sampling. The patient’s glucose level was determined using the glucose oxidase method by serum, which was separated from samples that were centrifuged within half an hour after receipt.
Statistical analysis
Statistical analysis was performed using SPSS 26.0, and graphs were plotted using GraphPad Prism8 software and R (version 4.1.3). G*Power (version 3.1.9.7) was used for sample size and power estimation. Continuous variables are presented as median (P25 - P75), and the Mann - Whitney U test was used for comparisons between the two groups. Categorical variables are expressed as numbers (percentages), and the chi-square test was used to compare the groups. Multivariate logistic regression analysis was used to screen for risk factors. The ROC curve was used to determine the cut-off value of laboratory indicators to predict mortality. The correlation coefficient was obtained by spearman correlation analysis. Locally weighted scatterplot smoothing (LOWESS) was used to compare the dynamic changes in laboratory indices during hospitalization. Statistical significance was set at P < 0.05.
Results
Patient characteristics
The study cohort of patients with TTP included 25 survivors (18 females and 7 males) and 17 non-survivors (9 females and 8 males), aged 47 (26–65) years and 55 (33–62) years, respectively. All patients’ longitudinal clinical information, diagnosis, treatment, disease progression, and prognosis are comprehensively presented (Fig. 2). We calculated the mortality rates of TTP patients by years (Supplementary Table S2).
Clinical information, diagnosis and treatment data of patients. The x-axis shows the length of stay, and each row shows information for each patient. The length of stay of the patients is shown with blue bars. The first plasma exchange and sampling time are shown on the blue bar. Comorbidities and other treatments (plasma infusion, glucocorticoid, etc.) are displayed beside the blue bar. Pos ANA: positive antinuclear antibody test
Comparison of clinical and laboratory data
There is a statistical difference in blood glucose on admission between survivors and non-survivors in the whole cohort study with TTP patients (P = 0.000) and in the cohort between 2013 and 2021 with available ADAMTS13 data cases (P = 0.027). Besides, compared with survivors, in the whole cohort, non-survivors had a shorter length of stay (LOS), lower red blood cells (RBC) counts, higher aspartate aminotransferase (AST) levels and Lactate dehydrogenase (LDH) levels. In the cohort between 2013 and 2021, platelet counts (PLT) of non-survivors were lower than survivors (Table 1).
TTP risk factor analysis
In the whole cohort study, after adjusting age and gender, serum glucose levels and RBC were independent predictors of mortality in patients with TTP (glucose: odds ratio [OR], 2.476 [95% confidence interval (CI) 1.368–4.484]; RBC: OR, 0.095 [95% CI 0.011–0.799]). Glucose was significantly correlated with serum LDH (R = 0.52, P < 0.001), however, RBC was not. There was also no significant difference in blood glucose between patients who received glucocorticoid treatment or not before admission (Supplementary Figure S). The blood glucose levels of survivors during hospitalization are more stable than non-survivors. (Table 2; Fig. 3).
TTP risk factors and blood test dynamics during hospitalization. (a) Boxplots of blood glucose according to time distribution. (b) LOWESS chart of blood glucose levels during hospitalization. (c) Scatter plots depicting the relationship between glucose and LDH. (d) Boxplots of RBC according to time distribution. (e) LOWESS chart of RBC during hospitalization. (f) Scatter plots depicting the relationship between RBC and LDH. RBC: Red blood cells; LDH: lactate dehydrogenase. The correlation coefficient was obtained by spearman correlation analysis. * and ** indicate the P values of < 0.05, < 0.01, respectively; NS stands for no statistical difference
Glucose and RBC ROC curve mortality predictions
The area under the curve (AUC) of glucose for predicting mortality in patients with TTP was 0.827 (cut-off: 9.2 mmol/L, sensitivity, 75.0%; and specificity, 95.8%). The AUC of RBC was 0.696 (cut-off: 2.1 × 1012/L, sensitivity: 52.9%, and specificity: 84.0%) (Fig. 4). The external validation group showed 71% and 84% sensitivity and specificity, respectively (cut-off: 9.2 mmol/L).
Blood test changes during hospitalization
There were significant differences in PLT and LDH levels between survivors and non-survivors by the fifth day of treatment. The prognosis would be better if the patients’ platelet counts could rise to 100 × 109/L (P = 0.02) or the serum LDH level fell below 375 U/L (1.5-fold of the normal range of LDH) (P < 0.001) after 5 days of treatment (Fig. 5).
PLT and LDH level changes between survivors and non-survivors. (a) Boxplots of LDH according to time. (b) LOWESS chart of LDH during hospitalization. (c) Histograms of LDH levels on the 5th day of admission. (d) Boxplots of PLT according to time distribution. (e) LOWESS chart of PLT during hospitalization. (f) Histograms of platelet counts on the 5th day of admission. LDH: lactate dehydrogenase; PLT: platelet counts. * and ** indicate the P values of < 0.05, < 0.01, respectively; NS stands for no statistical difference
Discussion
TTP is a rare but potentially life-threatening multisystemic disorder [15]. The research process in this study is challenging and very meaningful, for we comprehensively showed the whole course of diagnosis and treatment of all patients, and the correlations between the dynamic changes of laboratory indicators and outcomes were also analyzed. Decades ago, the diagnosis of TTP mainly depended on the clinical manifestations of patients. From 2001 to 2005, the diagnosis of TTP mainly relied on the clinical manifestations of patients [9], resulting in a high mortality rate. With improved diagnostic awareness, ADAMTS13 activity and its inhibitor testing, and the use of rituximab for TTP patients, from 2006 to 2021, the mortality rate was lower than that of 2001 to 2005, but no downward trend was observed year by year, which means there is no obvious “time effect” on mortality in this cohort. Therefore, it is necessary to seek new laboratory biomarkers to identify high-risk patients to reduce TTP patients’ mortality rate.
However, at least one of the ten TTP patients may still cause fatal consequences [3]. Indicators for early prediction of hospitalization outcomes can guide clinical practice to improve the prognosis of patients through specific interventions, thus reducing mortality. In this study, we collected clinical information and laboratory results of all patients with TTP. We found that glucose levels and RBC counts were independent predictors of mortality in patients with TTP, and there were also significant differences in blood glucose levels between survivors and non-survivors in cohorts with available ADAMTS13 data. However, hemoglobin had no significant difference between the survival and non-survival groups. We speculate that TTP patients exhibit microangiopathic hemolytic anemia, characterized by mechanical damage to RBC by microthrombus [10]. Therefore, the decrease in RBC may be earlier than that of hemoglobin. Additionally, glucose concentration was significantly correlated with LDH. Simultaneously, we also collected published studies with the same period of the study cohort as the external validation cohort to prove that the blood glucose level at admission can predict the mortality of TTP patients.
TTP is associated with infections, diabetes, hypertension, obesity, metabolic syndrome, and cardiovascular disease [6]; the incidence of cardiovascular complications in patients hospitalized for TTP is approximately 25% and is associated with higher mortality [16]. However, other reports have indicated that there was no difference between the survivors and non-survivors with diabetes, hypertension, and obesity [14]. In this cohort study, infection and connective tissue disease were the two most common trigger factors for TTP, with an incidence rate of 24% and 12%, respectively, which were similar to other studies for the same diseases [14, 17]. However, there were no differences between the survivors and non-survivors.
TTP mortality-related laboratory indicators, including anti-ADAMTS13 antibody [18], high LDH [18] and troponin I [19], low serum total protein or albumin, and extended activated partial thromboplastin time have been reported [20]. Hai-Xu Wang et al. [7] reported that high blood glucose level was associated with a poor prognosis in patients with TTP. Our study is one of the first to discuss the cut-off value of blood glucose for mortality in detail.
We found that blood glucose levels between survivors and non-survivors differed on admission and in their dynamic changes during hospitalization. Blood glucose levels in non-survivors were significantly higher than those in survivors on admission, with a wider range of fluctuations during hospitalization. The survivors were close to the upper limit of the reference range at admission and showed a slow upward trend. After day 8, the survivors showed a downward trend in glucose levels and remained near the upper limit of the reference range, which was relatively stable. We show that high blood glucose levels at admission and blood glucose fluctuation widely during hospitalization are risk mortality-related factor in TTP patients. Previous studies have also shown that a high variation in blood glucose levels is an independent predictor of mortality in critically ill patients [21].
Elevated glucose may be caused by poor control of chronic hyperglycemia disease or the stress state of the disease. And stress hyperglycemia usually occurs in relatively serious diseases [22, 23]. The American Diabetes Association and the American Association of Clinical Endocrinologists define stress hyperglycemia or hospital-related hyperglycemia as any blood glucose concentration > 140 mg/dL (> 7.8 mmol/L) in patients without a prior history of diabetes [12]. The non-survivors in this study cohort had no history of diabetes. Some scholars found that in nondiabetic patients, the existence of acute hyperglycemia can lead to poor long-term prognosis and an increase in in-hospital mortality [24]. The cut-off value of blood glucose in this study was 9.2 mmol/L, which is higher than 7.8 mmol/L.
Several studies [25, 26] have shown that metabolic syndrome and obesity cause different degrees of damage to the endocrine system and affect survival because of their connection with elevated blood glucose levels. Among the 42 patients, the BMI index of 35 patients was lower than 30 kg/m2, and the other 7 patients did not have their height and weight records because of their critical status at admission, but no one was diagnosed as obese. We have recorded the data of each patient in our study, and found a patient with hypertension and diabetes, and her BMI index was 27.24 kg/m2, but her waist circumference was not measured, so the patient is suspicious of metabolic syndrome. Among the other patients, there was one patient with diabetes, four with hypertension, and one with fatty liver, but no patient had more than three characteristics in the diagnostic criteria of metabolic syndrome [27] at the same time.
Impairment of blood glucose homeostasis is common after injury and severe disease; the body releases abundant cortisol hormones such as epinephrine and catecholamines [28]. Stress-related blood glucose elevation can protect the body to a certain extent, but excess blood glucose can be converted into a large amount of free fatty acids, which can exacerbate oxidative stress and endothelial injury and have toxic effects on the myocardium through disruption of calcium homeostasis, myocardial reperfusion injury, and intracellular acidosis [29]. Furthermore, in animal studies, high concentrations of free fatty acids during myocardial ischemia increased the myocardial oxygen demand and decreased myocardial contractility [30]. Many studies have shown that cardiovascular disease is the leading cause of mortality in patients with TTP [14, 16]. Therefore, it can be inferred that hyperglycemia caused by stress may be a mortality-related factor in these patients.
We also compared the changes in other TTP-related blood test results between non-survivors and survivors. LDH levels and PLT counts can be essential indicators for judging the efficacy of TTP treatment and monitoring recurrence [11]. The LDH of the survivors in this study decreased significantly five days after admission and remained close to the upper limit of the reference range, which was consistent with the results reported by Staley et al. [20]. The PLT counts had a significant upward trend after admission, and there was a considerable difference on the 5th day.
This study had some limitations. First, it was a single-center retrospective study with a relatively small sample size due to the low incidence rate of TTP; the power of this study was 0.74. Second, because the outcome of patients with TTP in this study was examined 30 days after admission, there may be bias for some patients with a longer disease course. Third, because the non-survivors were all patients with no history of diabetes, stress-induced hyperglycemia in patients with diabetes was not evaluated, there may be some unknown and unpredictable potential factors in this study. Fourth, ADAMTS13 data was not available for TTP patients before 2013.
Conclusion
This study found that hyperglycemia at admission and unstable blood glucose levels during hospitalization may be the mortality-related factors in patients with TTP. This provides a new laboratory basis for monitoring the treatment of patients with TTP, improving prognosis, and reducing mortality. In addition, we found that the recovery of platelets and the significant decrease in serum LDH within five days of treatment are related to a good prognosis in TTP patients. We suggest that these two concepts can be used as an early warning index for the mortality stratification of TTP patients.
Data availability
The datasets are available from the corresponding author upon reasonable request.
References
Sukumar S, Lammle B, Cataland SR. Thrombotic Thrombocytopenic Purpura: Pathophysiology, Diagnosis, and Management. J Clin Med (2021) 10(3). Epub 2021/02/06. https://doi.org/10.3390/jcm10030536. IF: 3.9 Q2 B3 IF: 3.9 Q2 B3 IF: 3.9 Q2 B3 IF: 3.9 Q2 B3 IF: 3.9 Q2 B3 IF: 3.9 Q2 B3 IF: 3.9 Q2 B3.
George JN. Clinical practice. Thrombotic Thrombocytopenic Purpura. N Engl J Med. 2006;354(18):1927–35. https://doi.org/10.1056/NEJMcp053024. IF: 158.5 Q1 B1 IF: 158.5 Q1 B1 IF: 158.5 Q1 B1 IF: 158.5 Q1 B1 IF: 158.5 Q1 B1 IF: 158.5 Q1 B1 IF: 158.5 Q1 B1. Epub 2006/05/05.
Joly BS, Coppo P, Veyradier A. Thrombotic Thrombocytopenic Purpura. Blood. 2017;129(21):2836–46. https://doi.org/10.1182/blood-2016-10-709857. IF: 20.3 Q1 B1 IF: 20.3 Q1 B1 IF: 20.3 Q1 B1 IF: 20.3 Q1 B1 IF: 20.3 Q1 B1 IF: 20.3 Q1 B1 IF: 20.3 Q1 B1. Epub 2017/04/19.
Sadler JE. Pathophysiology of Thrombotic Thrombocytopenic Purpura. Blood. 2017;130(10):1181–8. https://doi.org/10.1182/blood-2017-04-636431. IF: 20.3 Q1 B1 IF: 20.3 Q1 B1 IF: 20.3 Q1 B1 IF: 20.3 Q1 B1 IF: 20.3 Q1 B1 IF: 20.3 Q1 B1 IF: 20.3 Q1 B1. Epub 2017/08/05.
Kremer Hovinga JA, Coppo P, Lammle B, Moake JL, Miyata T, Vanhoorelbeke K. Thrombotic Thrombocytopenic Purpura. Nat Rev Dis Primers (2017) 3:17020. Epub 2017/04/07. https://doi.org/10.1038/nrdp.2017.20. IF: 81.5 Q1 B1 IF: 81.5 Q1 B1 IF: 81.5 Q1 B1 IF: 81.5 Q1 B1 IF: 81.5 Q1 B1 IF: 81.5 Q1 B1 IF: 81.5 Q1 B1.
Scully M, Cataland S, Coppo P, de la Rubia J, Friedman KD, Kremer Hovinga J, et al. Consensus on the standardization of terminology in thrombotic Thrombocytopenic Purpura and related thrombotic microangiopathies. J Thromb Haemost. 2017;15(2):312–22. https://doi.org/10.1111/jth.13571. IF: 10.4 Q1 B2 IF: 10.4 Q1 B2 IF: 10.4 Q1 B2 IF: 10.4 Q1 B2 IF: 10.4 Q1 B2 IF: 10.4 Q1 B2 IF: 10.4 Q1 B2. Epub 2016/11/22.
Wang HX, Han B, Zhao YY, Kou L, Guo LL, Sun TW, et al. Serum D-Dimer as a potential New Biomarker for Prognosis in patients with Thrombotic Thrombocytopenic Purpura. Med (Baltim). 2020;99(13):e19563. https://doi.org/10.1097/MD.0000000000019563. IF: 1.6 Q3 B4 IF: 1.6 Q3 B4 IF: 1.6 Q3 B4 IF: 1.6 Q3 B4 IF: 1.6 Q3 B4 IF: 1.6 Q3 B4 IF: 1.6 Q3 B4. Epub 2020/03/30.
Ali Abdelhamid Y, Kar P, Finnis ME, Phillips LK, Plummer MP, Shaw JE, et al. Stress hyperglycaemia in critically ill patients and the subsequent risk of diabetes: a systematic review and Meta-analysis. Crit Care. 2016;20(1):301. https://doi.org/10.1186/s13054-016-1471-6. IF: 15.1 Q1 B1 IF: 15.1 Q1 B1 IF: 15.1 Q1 B1 IF: 15.1 Q1 B1 IF: 15.1 Q1 B1 IF: 15.1 Q1 B1 IF: 15.1 Q1 B1. Epub 2016/09/30.
Allford SL, Hunt BJ, Rose P, Machin SJ, Haemostasis, Thrombosis Task Force BCfSiH. Guidelines on the diagnosis and management of the thrombotic Microangiopathic Haemolytic Anaemias. Br J Haematol. 2003;120(4):556–73. https://doi.org/10.1046/j.1365-2141.2003.04049.x. IF: 6.5 Q1 B2 IF: 6.5 Q1 B2 IF: 6.5 Q1 B2 IF: 6.5 Q1 B2 IF: 6.5 Q1 B2 IF: 6.5 Q1 B2 IF: 6.5 Q1 B2. Epub 2003/02/18.
Matsumoto M, Fujimura Y, Wada H, Kokame K, Miyakawa Y, Ueda Y, et al. Diagnostic and Treatment guidelines for thrombotic Thrombocytopenic Purpura (ttp) 2017 in Japan. Int J Hematol. 2017;106(1):3–15. https://doi.org/10.1007/s12185-017-2264-7. IF: 2.1 Q4 B4 IF: 2.1 Q4 B4 IF: 2.1 Q4 B4 IF: 2.1 Q4 B4 IF: 2.1 Q4 B4 IF: 2.1 Q4 B4 IF: 2.1 Q4 B4. Epub 2017/05/28.
Thrombosis, Hemostasis Group CSoHCMA. [Chinese Guideline on the Diagnosis and Management of Thrombotic Thrombocytopenic Purpura. (2022)]. Zhonghua Xue Ye Xue Za Zhi (2022) 43(1):7–12. Epub 2022/03/02. https://doi.org/10.3760/cma.j.issn.0253-2727.2022.01.002. IF: NA NA NA IF: NA NA NA IF: NA NA NA IF: NA NA NA IF: NA NA NA IF: NA NA NA IF: NA NA NA.
Moghissi ES, Korytkowski MT, DiNardo M, Einhorn D, Hellman R, Hirsch IB, et al. American Association of Clinical Endocrinologists and American Diabetes Association Consensus Statement on Inpatient Glycemic Control. Diabetes Care. 2009;32(6):1119–31. https://doi.org/10.2337/dc09-9029. IF: 16.2 Q1 B1 IF: 16.2 Q1 B1 IF: 16.2 Q1 B1 IF: 16.2 Q1 B1 IF: 16.2 Q1 B1 IF: 16.2 Q1 B1 IF: 16.2 Q1 B1. Epub 2009/05/12.
Thrombosis, Hemostasis group HSCMA. [Consensus of Chinese experts on diagnosis and treatment of thrombotic Thrombocytopenic Purpura (Version 2012)]. Zhonghua Xue Ye Xue Za Zhi. 2012;33(11):983–4. Epub 2013/02/01.
Sukumar S, Brodsky M, Hussain S, Yanek L, Moliterno A, Brodsky R, et al. Cardiovascular Disease is a leading cause of mortality among ttp survivors in clinical remission. Blood Adv. 2022;6(4):1264–70. https://doi.org/10.1182/bloodadvances.2020004169. IF: 7.5 Q1 B1 IF: 7.5 Q1 B1 IF: 7.5 Q1 B1 IF: 7.5 Q1 B1 IF: 7.5 Q1 B1 IF: 7.5 Q1 B1 IF: 7.5 Q1 B1. Epub 2021/08/31.
Bommer M, Wolfle-Guter M, Bohl S, Kuchenbauer F. The Differential diagnosis and treatment of thrombotic microangiopathies. Dtsch Arztebl Int. 2018;115(19):327–34. https://doi.org/10.3238/arztebl.2018.0327. IF: 7.7 Q1 B2 IF: 7.7 Q1 B2 IF: 7.7 Q1 B2 IF: 7.7 Q1 B2 IF: 7.7 Q1 B2 IF: 7.7 Q1 B2 IF: 7.7 Q1 B2. Epub 2018/06/08.
Balasubramaniyam N, Yandrapalli S, Kolte D, Pemmasani G, Janakiram M, Frishman WH. Cardiovascular Complications and Their Association with mortality in patients with Thrombotic Thrombocytopenic Purpura. Am J Med. 2021;134(2):e89–e97. https://doi.org/10.1016/j.amjmed.2020.06.020. IF: 5.9 Q1 B3 IF: 5.9 Q1 B3 IF: 5.9 Q1 B3 IF: 5.9 Q1 B3 IF: 5.9 Q1 B3 IF: 5.9 Q1 B3 IF: 5.9 Q1 B3. Epub 2020/07/21.
Prevel R, Roubaud-Baudron C, Gourlain S, Jamme M, Peres K, Benhamou Y, et al. Immune Thrombotic Thrombocytopenic Purpura in older patients: prognosis and long-term survival. Blood. 2019;134(24):2209–17. https://doi.org/10.1182/blood.2019000748. IF: 20.3 Q1 B1 IF: 20.3 Q1 B1 IF: 20.3 Q1 B1 IF: 20.3 Q1 B1 IF: 20.3 Q1 B1 IF: 20.3 Q1 B1 IF: 20.3 Q1 B1. Epub 2019/09/19.
Alwan F, Vendramin C, Vanhoorelbeke K, Langley K, McDonald V, Austin S, et al. Presenting Adamts13 antibody and Antigen levels predict prognosis in Immune-mediated thrombotic Thrombocytopenic Purpura. Blood. 2017;130(4):466–71. https://doi.org/10.1182/blood-2016-12-758656. IF: 20.3 Q1 B1 IF: 20.3 Q1 B1 IF: 20.3 Q1 B1 IF: 20.3 Q1 B1 IF: 20.3 Q1 B1 IF: 20.3 Q1 B1 IF: 20.3 Q1 B1. Epub 2017/06/04.
Benhamou Y, Boelle PY, Baudin B, Ederhy S, Gras J, Galicier L, et al. Cardiac Troponin-I on diagnosis predicts early death and refractoriness in acquired thrombotic Thrombocytopenic Purpura. Experience of the French thrombotic microangiopathies Reference Center. J Thromb Haemost. 2015;13(2):293–302. https://doi.org/10.1111/jth.12790. IF: 10.4 Q1 B2 IF: 10.4 Q1 B2 IF: 10.4 Q1 B2 IF: 10.4 Q1 B2 IF: 10.4 Q1 B2 IF: 10.4 Q1 B2 IF: 10.4 Q1 B2. Epub 2014/11/19.
Staley EM, Cao W, Pham HP, Kim CH, Kocher NK, Zheng L, et al. Clinical factors and biomarkers predict outcome in patients with Immune-mediated thrombotic Thrombocytopenic Purpura. Haematologica. 2019;104(1):166–75. https://doi.org/10.3324/haematol.2018.198275. IF: 10.1 Q1 B1 IF: 10.1 Q1 B1 IF: 10.1 Q1 B1 IF: 10.1 Q1 B1 IF: 10.1 Q1 B1 IF: 10.1 Q1 B1 IF: 10.1 Q1 B1. Epub 2018/09/02.
Catargi B, Darier R, Montaudon M, Beauvieux MC, Coffin-Boutreux C, Coste P et al. Response to Comment on Gerbaud Et Al. Glycemic Variability Is a Powerful Independent Predictive Factor of Midterm Major Adverse Cardiac Events in Patients with Diabetes with Acute Coronary Syndrome. Diabetes Care. 2019;42:674–681. Diabetes Care (2019) 42(10):e170-e1. Epub 2019/09/22. https://doi.org/10.2337/dci19-0033. IF: 16.2 Q1 B1 IF: 16.2 Q1 B1 IF: 16.2 Q1 B1 IF: 16.2 Q1 B1 IF: 16.2 Q1 B1 IF: 16.2 Q1 B1 IF: 16.2 Q1 B1.
Mi D, Li Z, Gu H, Jiang Y, Zhao X, Wang Y, et al. Stress hyperglycemia is Associated with in-hospital mortality in patients with diabetes and Acute Ischemic Stroke. CNS Neurosci Ther. 2022;28(3):372–81. https://doi.org/10.1111/cns.13764. IF: 5.5 Q1 B1 IF: 5.5 Q1 B1 IF: 5.5 Q1 B1 IF: 5.5 Q1 B1 IF: 5.5 Q1 B1 IF: 5.5 Q1 B1 IF: 5.5 Q1 B1. Epub 2022/01/28.
Luitse MJ, Biessels GJ, Rutten GE, Kappelle LJ. Diabetes, hyperglycaemia, and Acute Ischaemic Stroke. Lancet Neurol. 2012;11(3):261–71. https://doi.org/10.1016/S1474-4422(12)70005-4. IF: 48.0 Q1 B1 IF: 48.0 Q1 B1 IF: 48.0 Q1 B1 IF: 48.0 Q1 B1 IF: 48.0 Q1 B1 IF: 48.0 Q1 B1 IF: 48.0 Q1 B1. Epub 2012/02/22.
Cui CY, Zhou MG, Cheng LC, Ye T, Zhang YM, Zhu F, et al. Admission hyperglycemia as an independent predictor of long-term prognosis in Acute myocardial infarction patients without diabetes: a retrospective study. J Diabetes Investig. 2021;12(7):1244–51. https://doi.org/10.1111/jdi.13468. IF: 3.2 Q3 B3 IF: 3.2 Q3 B3 IF: 3.2 Q3 B3 IF: 3.2 Q3 B3 IF: 3.2 Q3 B3 IF: 3.2 Q3 B3 IF: 3.2 Q3 B3. Epub 2020/11/30.
Dabke K, Hendrick G, Devkota S. The gut microbiome and metabolic syndrome. J Clin Invest. 2019;129(10):4050–7. https://doi.org/10.1172/JCI129194. IF: 15.9 Q1 B1 IF: 15.9 Q1 B1 IF: 15.9 Q1 B1 IF: 15.9 Q1 B1 IF: 15.9 Q1 B1 IF: 15.9 Q1 B1 IF: 15.9 Q1 B1. Epub 2019/10/02.
Gui J, Li Y, Liu H, Guo LL, Li J, Lei Y, et al. Obesity- and lipid-related indices as a predictor of obesity metabolic syndrome in a National Cohort Study. Front Public Health. 2023;11:1073824. https://doi.org/10.3389/fpubh.2023.1073824. Epub 2023/03/07.
Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–5. https://doi.org/10.1161/CIRCULATIONAHA.109.192644. IF: 37.8 Q1 B1 IF: 37.8 Q1 B1 IF: 37.8 Q1 B1 IF: 37.8 Q1 B1 IF: 37.8 Q1 B1 IF: 37.8 Q1 B1 IF: 37.8 Q1 B1. Epub 2009/10/07.
Ingels C, Vanhorebeek I, Van den Berghe G. Glucose homeostasis, Nutrition and infections during critical illness. Clin Microbiol Infect. 2018;24(1):10–5. https://doi.org/10.1016/j.cmi.2016.12.033. IF: 14.2 Q1 B1 IF: 14.2 Q1 B1 IF: 14.2 Q1 B1 IF: 14.2 Q1 B1 IF: 14.2 Q1 B1 IF: 14.2 Q1 B1 IF: 14.2 Q1 B1. Epub 2017/01/14.
Paolisso P, Foa A, Bergamaschi L, Angeli F, Fabrizio M, Donati F, et al. Impact of admission hyperglycemia on short and long-term prognosis in Acute myocardial infarction: Minoca Versus Mioca. Cardiovasc Diabetol. 2021;20(1):192. https://doi.org/10.1186/s12933-021-01384-6. IF: 9.3 Q1 B1 IF: 9.3 Q1 B1 IF: 9.3 Q1 B1 IF: 9.3 Q1 B1 IF: 9.3 Q1 B1 IF: 9.3 Q1 B1 IF: 9.3 Q1 B1. Epub 2021/09/26.
Mjos OD. Effect of free fatty acids on myocardial function and oxygen consumption in Intact Dogs. J Clin Invest. 1971;50(7):1386–9. https://doi.org/10.1172/JCI106621. IF: 15.9 Q1 B1 IF: 15.9 Q1 B1 IF: 15.9 Q1 B1 IF: 15.9 Q1 B1 IF: 15.9 Q1 B1 IF: 15.9 Q1 B1 IF: 15.9 Q1 B1. Epub 1971/07/01.
Acknowledgements
The authors wish to thank all of the investigators who participated in this study.
Funding
This work was supported by grants from the Medical Science and Technology Project of Zhejiang Province (2022KY1378).
Author information
Authors and Affiliations
Contributions
L. H. designed the study, collected data and performed statistical analyses, and wrote and edited the manuscript; J. W. and X. J. reviewed and revised the manuscript; G. L. and M. F. collected the data; J. S. reviewed the manuscript; T. T. designed and conceptualized the study, and reviewed the manuscript; B. S. had the idea for the study, designed and conceptualized the study, and reviewed the manuscript. All authors reviewed and approved the final draft of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval
Medical Ethics Committee of Taizhou Hospital of Zhejiang Province. (Identification code: K20220211, date: 15 February 2022).
Competing interests
The authors declare no competing financial interests.
Consent for publication
All authors have read and approved the final version of the manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
13098_2024_1275_MOESM1_ESM.tiff
Supplementary Material 1 Supplementary figure S. Boxplots of blood glucose concentration between patients with and without glucocorticoid pre-treatment before admission
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hu, L., Wang, J., Jin, X. et al. Stress-induced hyperglycemia is associated with the mortality of thrombotic thrombocytopenic purpura patients. Diabetol Metab Syndr 16, 44 (2024). https://doi.org/10.1186/s13098-024-01275-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13098-024-01275-2