Abstract
Aims
Frequent and extensive within-day glycemic variability (GV) in blood glucose levels may increase the risk of hypoglycemia and long-term mortality in hospitalized patients with diabetes. We aimed to assess the amplitude and frequency of within-day GV in inpatients with type 2 diabetes and to explore the factors influencing within-day GV.
Methods
We conducted a single-center, retrospective observational study by analyzing hospital records and 10-day real-time continuous glucose monitoring data. Within-day GV was assessed using the coefficient of variation (%CV). The primary outcome was the amplitude and frequency of within-day GV. The frequency of within-day GV was assessed by the consecutive days (CD) of maintaining within the target %CV range after first reaching it (CD after first reaching the target) and the maximum consecutive days of maintaining within the target %CV range (Max-CD). The target %CV range was less than 24.4%. We evaluated the factors influencing within-day GV using COX regression and Poisson regression models.
Results
A total of 1050 cases were analyzed, of whom 86.57% reduced the amplitude of within-day GV before the sixth day of hospitalization. Of the 1050 hospitalized patients, 66.57% stayed within the target %CV range for less than two days after first reaching the target and 69.71% experienced a Max-CD of fewer than four days. Reducing the average postprandial glucose excursion (hazard ratio [HR]: 0.81, 95% confidence interval [CI]: 0.77–0.85; incidence rate ratios [IRR]: 0.72, 95% CI: 0.69–0.74) and the use of α-glucosidase inhibitors (IRR: 1.1, 95% CI: 1.01–1.18) and glucagon-like peptide-1 agonist (IRR: 1.30, 95% CI: 1.02–1.65) contributed to reducing the amplitude and decreasing the frequency of within-day GV. However, the use of insulin (HR: 0.64, 95% CI: 0.55–0.75; IRR: 0.86, 95% CI: 0.79–0.93) and glinide (HR: 0.47, 95% CI: 0.31–0.73; IRR: 0.84, 95% CI: 0.73–0.97) may lead to an increased frequency of within-day GV.
Conclusions
An increasing frequency of within-day GV was observed during the hospitalization in patients with type 2 diabetes, despite the effective reduction in the amplitude of within-day GV. Using medications designed to lower postprandial blood glucose could contribute to minimize the risk of frequent within-day GV.
Similar content being viewed by others
Introduction
For a long time, glycated hemoglobin (HbA1c) has been viewed as a standard marker reflecting the level of glycemic control in patients with type 2 diabetes (T2D) [1, 2]. Achieving HbA1c values of 7% or less is associated with a decreased risk of micro- and macrovascular complications [3, 4]. However, by definition, HbA1c, reflects average blood glucose levels over the preceding 2–3 months, does not account for daily acute fluctuations. Those fluctuations in blood glucose are termed glycemic variability (GV). Thus, patients with T2D can still experience significant GV even when reaching target HbA1c levels [5, 6].
GV measures the extent of fluctuation in glucose levels over time. It primarily encompasses two components: amplitude, indicative of the magnitude of blood glucose excursions, and frequency (timing), denoting the time intervals during which these fluctuations occur [7]. Within-day GV refers to glucose fluctuations from peaks to nadirs within a single day [8]. Peaks, especially in T2D, usually correspond to postprandial hyperglycemia, while nadirs can indicate the risk of hypoglycemia [5]. Both the amplitude and the frequency of GV contribute to the risks for hypoglycemia and postprandial hyperglycemia associated with diabetes [9].
In a hospitalized setting, strict glycemic management ensures that uncontrolled T2D patients are less vulnerable to hyperglycemia’s detrimental effects [10]. However, swift reductions in overall blood glucose levels may increase within-day GV and thus potentiate the risk of hypoglycemia [11, 12]. Given this, it is imperative to monitor within-day GV, including amplitude and frequency, in hospitalized T2D patients. Accumulating evidence underscores the pivotal role of curtailing within-day GV amplitude in minimizing hypoglycemic risk [13,14,15,16]. However, present-day research is somewhat remiss in shedding light on the frequency of GV [12].[17]. Studies indicated that acute glycemic fluctuations within a day induce more oxidative stress compared to chronic hyperglycemia, believed to be the primary underlying mechanism behind glucose-induced vascular damage. This insight lends credence to exploring the hypothesis that recurrent acute glycemic fluctuations over time might amplify oxidative stress. Namely for healthcare practitioners, a continuous assessment of within-day GV stability could be more consequential than solely mitigating chronic hyperglycemia. Additionally, several studies have shown that increased GV is independently associated with extended hospitalizations, augmented short-term and long-term mortality in diabetic and critically ill or non-critically ill hospitalized patients [18,19,20,21,22]. Therefore, for hospitalized T2D patients, a dual evaluation approach focusing on both the amplitude of within-day GV and its temporal stability is crucial. The intent is to alleviate the accumulative strain of repetitive acute glycemic peaks and troughs. By dynamically adapting treatment modalities in sync with daily GV patterns, the overarching goal is to harmonize reduced hyperglycemia, limited hypoglycemia, and stable GV, culminating in ideal blood glucose control. However, current literature seems to be scant in its coverage of our knowledge. There is currently insufficient available evidence regarding the temporal within-day glycemic variability among hospitalized T2D patients with type 2 diabetes.
Using 10-day real-time continuous glucose monitoring (rtCGM) data, we scrutinized the amplitude variations of Within-day GV in 1050 hospitalized patients, and observed the frequency of Within-day GV based on several additional outcome measures. Furthermore, we conducted a regression model analysis to examine the influencing factors on both the amplitude and frequency of Within-day GV.
Methods
Data sources and study design
This retrospective, single-center observational study was conducted at the Kaifeng Hospital of Traditional Chinese Medicine in Henan Province, China. We performed a retrospective analysis of the hospital records and 10-day rtCGM (iPro(®)2 CGM system, Medtronic MiniMed, Northridge, CA) data for patients with T2D who were admitted due to poor glycemic control between January 1, 2018, and June 16, 2021. Only adult patients (aged ≥ 18 years) with type 2 diabetes who underwent rtCGM for more than 10 days were eligible for inclusion in the study. To investigate the factors influencing the temporal stability of within-day GV, we considered variables such as age, gender, duration, body mass index (BMI), low density lipoprotein (LDL), HbA1c, C-peptide, comorbidities, treatment modalities (oral antidiabetic drugs and insulin), and specific rtCGM data metrics (24-hour mean glucose, standard deviation of glucose, fasting plasma glucose (FPG), and postprandial glucose excursion (PPGE)). The baseline for the study was defined as 24 h after patient admission. All laboratory tests were drawn in a fasting state from the blood samples within 24 h after admission. All treatments were performed by the experienced physician after blood glucose profile assessments.
GV measurements and outcome assessments
Coefficient of variation (CV) was considered the most appropriate measure for evaluating GV [9]. CV was calculated as %. In this study, within-day %CV (%CVw) was used to evaluated within-day GV, with the within-day standard deviation (SDw) of glucose (mean SD of all measurements within a 24-h period) divided by the 24-hour mean glucose (Mean) [23]. The formula for calculating %CVw is shown in Eq. (1).
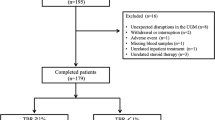
According to previous research [24], a threshold of 33% for %CV was established as an indicator of excessive GV in Chinese patients with diabetes. However, this value was primarily used to differentiate the upper limit of %CV between stable and unstable GV. Rodbard et al. [25] proposed the utilization of regions defined by the 25th, 75th, and 75th percentiles of %CV to categorize GV levels as excellent, good, fair, and poor. This approach allowed for a more precise evaluation of patients’ GV compared to a “one-size-fits-all”, such as a 33% threshold. In line with the findings of Mo et al. [24], %CV in Chinese patients with T2D was divided into four groups based on quartiles: excellent (≤ 19.3%), good (19.4–24.4%), fair (24.5–30.4%), and poor (> 30.4%). Our study included patients with baseline %CV rated as “fair” (Cohort 1) and “poor” (Cohort 2). Our final study population included 1050 patients (Fig. 1). The quartile levels of “excellent” and “good” for %CV, which were our study’s target %CV range, have been deemed more favorable for reducing the risk of hypoglycemia in T2D patients [26].
The primary outcome was the amplitude and frequency of within-day GV in hospitalized patients with type 2 diabetes. The design ideas of this research, according to Braithwaite’s proposed framework [12], were shown as follows: (1) the amplitude of within-day GV: aimed at investigating the likelihood and cumulative incidence of reaching the target %CV range for the first time, (2) frequency of within-day GV: aimed at assessing the continuous days of maintaining within the target %CV range, including the consecutive days (CD) of maintaining within the target %CV range after first reaching the target (CD after first reaching the target) and the maximum consecutive days of maintaining within the target %CV range (Max-CD) during the 10-day period. Furthermore, We considered the SD of %CV values and the proportion of Max-CD as additional measures of timing of within-day GV. The proportion of MAX-CD was defined as MAX-CD divided by the the remaining hospital days. The remaining hospital days were calculated as the total number of observational days (10 days) minus the number of days required to reach the target %CV range for the first time. The secondary objective was to determine the factors associated with amplitude and frequency of within-day GV. Additionally, we assessed the overall glycemic control via FPG and PPGE, while the PPGE provided insights into postprandial glycemic dynamics [27].
The continuous variables like diabetes duration (0 to ≤ 3, 3 to ≤ 10, and >10 years), LDL (< 2.6, and ≥ 2.6 mmol/L), and C-peptide (< 2.5, and ≥ 2.5 ng/mL) obtained from the baseline period were categorized into categorical variable. According to the consensus of Chinese experts on medical nutrition therapy for overweight/obesity [28], Participants were classified as normal weight (18.5 ≤ BMI ≤ 23.9 kg/m2), overweight (24 to ≤ BMI ≤ 27.9 kg/m2) and obesity (BMI ≥ 28 kg/m2). The age and HbA1c at baseline were calculated as continuous variables. The average FPG and PPGE during the 10-day period were also computed as continuous variables. To evaluate the influence of comorbidities on GV, 9 prevalent comorbidities in diabetes were included, such as liver diseases, hypertension, hyperlipidemia, coronary artery atherosclerosis, chronic kidney disease, cerebral infarction, ischemic cerebrovascular disease, diabetic retinopathy, diabetic polyneuropathy. Each comorbidity was treated as a categorical variable.
Antidiabetic medications administered during hospitalization were classified as follows: dipeptidyl peptidase-4 inhibitors (DPP-4i), sulfonylurea, metformin, α-glucosidase inhibitors, SGLT2 inhibitors (SGLT2i), thiazolidinediones (TZDs), glinide, glucagon-like peptide-1 (GLP-1) agonist, and insulin. In our research, each antidiabetic medication was considered a categorical variable.
Statistical analysis
Descriptive analysis was used to evaluate admission characteristics. Continuous variables were reported as mean ± SD if normally distributed, alternatively as median with interquartile range (IQR). Categorical variables were reported as frequencies and percentages.
We employed the Kaplan-Meier method to delineate the cumulative incidence rate of both patient cohorts, achieving the target %CV range for the first time. Differences between the two cohorts were assessed using the log-rank test, with a significance level set at p < 0.05. We used descriptive statistics to calculate the CD after first reaching the target, as well as the MAX-CD and the proportions of MAX-CD for each patient. The SD of %CV values during hospitalization for each patient was calculated and presented graphically.
Cox proportional hazards regression model was used to determine the risk factors affecting the number of days required to reach the target %CV range for the first time. As for factors that might affect timing of within-day GV, logistic and Poisson regression models were used for evaluation. The primary analysis involves using a Poisson regression model to determine the influencing factors on the MAX-CD. In the sensitivity analysis, we employed a logistic regression model to analyze the factors influencing the proportions of MAX-CD and the SD of %CV values as additional outcomes. The results were visualized in forest plots, showing the hazard ratio (HR, for Cox regression), incidence rate ratios (IRR, for Poisson regression) and odds ratios (OR, for logistic regression) alongside the 95% confidence interval (CI) for each outcome. All analyses were carried out with R 4.3.1.
Results
The study cohort consisted of 1050 individuals: 358 patients in Cohort 1 and 692 in Cohort 2. Participant demographics and baseline characteristics were similar across the two study groups. Most participants were male (62.3% for Cohort 1 and 57.9% for Cohort 2), overweight or obese (74.0% for Cohort 1 and 68.2% for Cohort 2), with a disease duration of fewer than 3 years or 3–10 years (75.7% for Cohort 1 and 73% for Cohort 2). Subjects in Cohort 1 (56.3 ± 14.0 years) had a slightly younger average age than those in Cohort 2 (59.5 ± 13.1 years), while higher median HbA1c values were reported in Cohort 1 (8.5% [69.4 mmol/mol]). (Table 1).
The most prevalent comorbidities were diabetic polyneuropathy (48.3%), hypertension (42.7%), and chronic kidney disease (32.6%). The most frequently used antidiabetic medications were insulin (58.1%), α-glucosidase inhibitors (31.4%), and TZDs (21.1%) (Table 1).
Overall glycemic control
From day 1 to day 10, median (IQR) FPG decreased from 8.7 mmol/L (8.1 mmol/L, 10.4 mmol/L) to 7.4 mmol/L (6.4 mmol/L, 8.5 mmol/L) (p < 0.01), and median (IQR) PPGE decreased from 4.7 mmol/L (3.6 mmol/L, 6.6 mmol/L) to 3.15 mmol/L (1.9 mmol/L, 4.5mmol/L) (p < 0.01) in all paticipants. (Fig. 2)
Amplitude of within-day GV: the cumulative incidence of first reaching the target %CV range
Over the 10-day observation period, the estimated probability of reaching the target %CV range for the first time was highest on Day 2 (38.9%; Table 2). Nearly all (94.8%) of the patients reached the target %CV range for the first time within 10 days (Table 2). From day 1 to day 6, 86.5% of the patients reached the target %CV range for the first time. The cumulative probability of first reaching the target %CV range remained stable from day 6 to day 10 (Table 2). The cumulative incidence of first reaching the target %CV range was higher in cohort 1 compared to cohort 2 (P <0.01; Fig. 3).
Frequency of within-day GV: the continuous days of maintaining within the target %CV range
Of the 1050 hospitalized patients, 66.6% stayed within the target %CV range for less than two days after first reaching the target and 69.7% experienced a Max-CD of fewer than four days. (Table 3 and Supplementary Material Table S1)
The average remaining hospitalization days for 1050 patients after first reaching the target %CV range was 7 days, whereas 63.9% of patients had MAX-CD less than or or equal to half of the remaining hospitalization days (The proportion of MAX-CD less than or equal to 50% was observed in 63.9% of the patients.). (Table 3 and Supplementary Material Table S1)
67.3% of patients in Cohort 1 had a SD of %CV ranging between 0.05 and 0.1 (Fig. 4a), while 82.1% of patients in Cohort 2 exhibited a SD of %CV within the range of 0.05 to 0.15 (Fig. 4b).
Factors influencing the within-day GV
According to the COX proportional hazards model, factors influencing the number of days required to reach the target %CV range encompass sex, age, HbA1c, use of glinide, insulin, sulfonylurea and TZDs, and the mean PPGE. Male patients (HR: 0.86, 95% CI: 0.75–0.98, P < 0.05) were less likely to achieve the target %CV range for the first time than female patients. The likelihood of reaching the target %CV range for the first time diminished with advancing age (HR: 0.99, 95% CI: 0.99-1.00, P < 0.05). Individuals with higher levels of HbA1c had a higher probability of reaching the target %CV range for the first time (HR: 1.26, 95% CI: 1.21–1.32, P < 0.05). Conversely, the usage of Glinide (HR: 0.47, 95% CI: 0.31–0.73, P < 0.05), Insulin (HR: 0.64, 95% CI: 0.55–0.75, P < 0.05), Sulfonylurea (HR: 0.62, 95% CI: 0.45–0.87, P < 0.05) and TZDs (HR: 0.76, 95% CI: 0.60–0.96, P < 0.05) markedly reduced this probability. Moreover, an increasing mean PPGE value reduced the likelihood of reaching the target %CV range for the first time (HR: 0.81, 95% CI: 0.77–0.85, P < 0.05)(Fig. 5).
From the Poisson regression analysis, the principal factors affecting the maximum consecutive days of staying within the target %CV range (Max-CD) were HbA1c, use of glinide, insulin,α-glucosidase inhibitors and GLP-1 agonists, along with the mean FPG and PPGE. Patients with higher HbA1c values were more likely to have an increased MAX-CD (IRR: 1.23, 95% CI: 1.21–1.25, P < 0.05). Similarly, the use of α-glucosidase inhibitors (IRR: 1.10, 95% CI: 1.01–1.18, P < 0.05) and GLP-1 agonists (IRR: 1.30, 95% CI: 1.02–1.65, P < 0.05) contributed to increasing the MAX-CD. Conversely, the administration of glinide (IRR: 0.84, 95% CI: 0.73–0.97, P < 0.05) and insulin (IRR: 0.86, 95% CI: 0.79–0.93, P < 0.05), along with an increase in average FPG (IRR: 0.97, 95% CI: 0.94–0.99, P < 0.05) and PPGE (IRR: 0.72, 95% CI: 0.69–0.74, P < 0.05) values, may contribute to a decrease in the MAX-CD (Fig. 6). Sensitivity analysis showed similar results (Supplementary Figs. S1 and S2).
Discussion
We observed that glycemic control of all hospitalized patients with type 2 diabetes, assessed by FPG and PPGE, was effectively managed. An impressive 86.5% of patients successfully managed to lower their within-day GV amplitude by the sixth day of hospitalization, however, a concomitant increase in the frequency of within-day GV is observed. Upon admission, patients with higher within-day GV typically experienced relatively shorter durations of stable within-day GV.
Medical institutions prioritize reducing FPG and PPGE to address hyperglycemia, given their significant contribution to overall hyperglycemia [29, 30]. Increasingly, evidence suggests that hyperglycemia activates oxidative stress (OS) by hyperglycemia through various pathways, significantly contributing to micro- and macrovascular complications in T2D [31,32,33,34]. However, past research has indicated that glucose fluctuations, including within-day GV, might exert more adverse effects than persistent hyperglycemia on OS [17, 35, 36], a crucial factor in exacerbating diabetic cardiovascular complications. Several studies also suggested that short-term GV could heighten the risk of adverse clinical outcomes in individuals with T2D [37,38,39,40]. Another study highlighted that short-term GV holds more weight than the mean daily glucose concentration in predicting the risk of unequivocal hypoglycemia, a pressing issue in enhancing the life quality of T2D patients [41]. Such insights underscore the need for patients with T2D, that is, persons with diabetes should strike a balance between managing chronic hyperglycemia and curbing glucose fluctuations. This would minimize both the long-term risk of developing diabetic complications and the acute hypoglycemia risks [42]. However, our results indicate that achieving this balance remains difficult.
The key to breaking this balance was the volatile shift of within-day GV from peaks to troughs. We found a robust correlation between within-day GV stability and factors like use of antidiabetes drugs, PPGE, and HbA1c values. Our data indicates that heightened postprandial glucose spikes were linked to increased within-day GV. Echoing the findings of Monnier et al. [43] and Candido et al. [44], half of the overall within-day GV resulted from postprandial glucose spikes. It is plausible to conclude that PPGE is pivotal in the intricate dynamics between within-day GV and factors like HbA1c. Our data suggests that patients with higher HbA1c values are conducive to stabilizing within-day GV. This is corroborated by findings from an observational study indicating that the contribution of PPGE to total glucose decreases as HbA1c increases [45]. Several studies have arrived at similar conclusions [30, 46,47,48].
Furthermore, regarding the interplay between within-day GV and therapeutic drugs in T2DM medications, our findings align with previous studies underscoring the efficacy of GLP-1 agonists in curtailing postprandial glucose spikes and overall within-day GV [49, 50]. However, we also discerned a positive link between high within-day GV fluctuations and the use of sulfonylurea, insulin, and glinide due to their heightened hypoglycemia risks [51]. This association is consistent with extensive literature suggesting that hypoglycemia risks are pronounced with insulin therapy, both basal insulin and intensive strategies [52,53,54,55], sulfonylurea [56,57,58], and glinide [59,60,61].
Our observations were partially congruent with findings from two studies [62, 63] on the positive relationship between an increase or a reduction in within-day GV and clinical factors, including age, HbA1c, or antidiabetic drugs by 72-h continuous glucose monitoring. However, the two studies did not focus on the stability of within-day GV across the same period. Conversely, we assessed both the overall glycemic control and the within-day GV stability in 499 hospitalized patients with T2D by using 10-day rtCGM data to acquire a more precise evaluation. Additionally, we used %CVw([mean SD of all of the measurements in a 24-h period] / [[mean glucose] × 100) to evaluate within-day GV given the existence of three different calculation methods of %CV(%CVT, %CVw, %CVb) for the evaluation of GV. All studies with an evaluation of GV through %CV should specify which %CV was used, according to the study of Julla et al. [23]. However, the calculation method of %CV was not reported in the two studies, although they both evaluated within-day GV through %CV. Furthermore, in our cohorts, the %CV threshold for patients with high within-day GV to be considered as low within-day GV post-treatment was 24%. This was lower than the %CV value (< 27%) reported by Uemura et al. [64] and Monnier et al. [26] to help maintain a minimal risk of hypoglycemia in patients with T2D treated with either insulin and/or noninsulin glucose-lowering agents.
From a clinical standpoint, the findings obtained suggest that most inpatients with T2D struggle to maintain stable, low within-day GV levels, even when they exhibit reasonably good in-hospital glycemic control. Healthcare professionals should recognize potential factors affecting GV that disrupt the balance between glycemic control and glycemic stability, potentially increasing the risk of hypoglycemia. The significance of GV is not widely acknowledged in China currently due to a dearth of relevant recommendations in authoritative guidelines [65]. A prospective observational study drawing from EMR data noted that 71.4% of patients in China were administered insulin upon admission [66]. Nevertheless, our data reveals a strong correlation between insulin therapy and unstable high within-day GV fluctuations; thus, healthcare professionals in China should exercise caution when intensifying glycemic control too aggressively in hospitalized T2D patients. The goal should be to minimize the occurrences of pronounced within-day GV and subsequent hypoglycemia. Furthermore, based on our observations, therapeutic agents that curtail post-meal glucose spikes can enhance within-day GV based on our finding that increased postprandial glucose decreased glycemic stability.
We report several significant limitations in our study. Unlike research grounded in expansive, prospectively gathered datasets, our single-center retrospective data might have variances in accuracy. We were also hamstrung by the absence of specific data on crucial clinical variables, such as the duration of diabetes, due to incomplete records or significant data gaps in the inpatient logs. We should have devoted more scrutiny to the effect of lifestyle factors, like diet and physical activity, and their influence on within-day GV, as such details were absent from our datasets. Moreover, the diverse methods employed to quantify GV can lead to varied conclusions. However, %CV is highly recommended for within-day GV assessments [11].
To conclude, the results highlight the instability of glycemic fluctuations in patients hospitalized with T2D patients and shed light on the existing disparity between glycemic stability and glycemic control in clinical settings. While our findings underscore the imperative for healthcare professionals to prioritize within-day GV in future practices, they also necessitate further investigations conducted within a structured data collection framework to mitigate measurement biases. Our observations also advocate for a measured approach to understanding the interplay between anti-glycemic medications, post-meal glucose surges, and within-day GV instability. This emphasizes the importance of devising improved glycemic management tactics. Further studies are essential to explore therapeutic measures that can effectively reduce post-meal glucose spikes and positively influence within-day GV.
Data availability
The datasets analysed during the current study are not publicly available due to the hospital’s request and China’s legal regulations on clinical medical data but are available from the corresponding author on reasonable request.
Abbreviations
- HbA1c:
-
Glycated hemoglobin
- T2D:
-
Type 2 diabetes
- GV:
-
Glycemic variability
- rtCGM:
-
Real-time continuous glucose monitoring
- BMI:
-
Body mass index
- LDL:
-
Low density lipoprotein
- FPG:
-
Fasting plasma glucose
- PPGE:
-
Postprandial glucose excursion
- %CV:
-
Coefficient of variation
- SD:
-
Standard deviation
- CD:
-
Continuous days
- CD after first reaching the target:
-
The consecutive days of maintaining within the target %CV range after first reaching the target
- MAX-CD:
-
The maximum consecutive days of maintaining within the target %CV range
- DPP-4i:
-
Dipeptidyl peptidase-4 inhibitors
- SGLT2i:
-
SGLT2 inhibitors
- TZDs:
-
Thiazolidinediones
- GLP-1 agonist:
-
Glucagon-like peptide-1 agonist
- IQR:
-
Interquartile range
- HR:
-
Hazard ratio
- IRR:
-
Incidence rate ratios
- OR:
-
Odds ratios
- CI:
-
Confidence interval
References
Ding L, Xu Y, Liu S, Bi Y, Xu Y. Hemoglobin A1c and diagnosis of diabetes. J Diabetes. 2018;10(5):365–72.
Gomez-Peralta F, Choudhary P, Cosson E, Irace C, Rami-Merhar B, Seibold A. Understanding the clinical implications of differences between glucose management indicator and glycated haemoglobin. Diabetes Obes Metab. 2022;24(4):599–608.
The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the diabetes control and complications trial. Diabetes 1995;44(8):968–83.
Lachin JM, Genuth S, Nathan DM, Zinman B, Rutledge BN. Effect of glycemic exposure on the risk of microvascular complications in the diabetes control and complications trial–revisited. Diabetes. 2008;57(4):995–1001.
Dandona P. Minimizing Glycemic fluctuations in patients with type 2 diabetes: approaches and importance. Diabetes Technol Ther. 2017;19(9):498–506.
Kovatchev B, Cobelli C. Glucose variability: timing, risk analysis, and relationship to hypoglycemia in diabetes. Diabetes Care. 2016;39(4):502–10.
Cobelli C, Facchinetti A. Yet another glucose variability index: time for a paradigm change? Diabetes Technol Ther. 2018;20(1):1–3.
Monnier L, Colette C, Owens DR. The application of simple metrics in the assessment of glycaemic variability. Diabetes Metab. 2018;44(4):313–9.
Danne T, Nimri R, Battelino T, Bergenstal RM, Close KL, DeVries JH, Garg S, Heinemann L, Hirsch I, Amiel SA, Beck R, Bosi E, Buckingham B, Cobelli C, Dassau E, Doyle FJ 3rd, Heller S, Hovorka R, Jia W, Jones T, Kordonouri O, Kovatchev B, Kowalski A, Laffel L, Maahs D, Murphy HR, Nørgaard K, Parkin CG, Renard E, Saboo B, Scharf M, Tamborlane WV, Weinzimer SA, Phillip M. International Consensus on Use of continuous glucose monitoring. Diabetes Care. 2017;40(12):1631–40.
Egi M, Bellomo R, Stachowski E, French CJ, Hart GK, Hegarty C, Bailey M. Blood glucose concentration and outcome of critical illness: the impact of diabetes. Crit Care Med. 2008;36(8):2249–55.
Akirov A, Amitai O, Masri-Iraqi H, Diker-Cohen T, Shochat T, Eizenberg Y, Shimon I. Predictors of hypoglycemia in hospitalized patients with diabetes mellitus. Intern Emerg Med. 2018;13(3):343–50.
Braithwaite SS. Glycemic variability in hospitalized patients: choosing metrics while awaiting the evidence. Curr Diab Rep. 2013;13(1):138–54.
Bergenstal RM, Mullen DM, Strock E, Johnson ML, Xi MX. Randomized comparison of self-monitored blood glucose (BGM) versus continuous glucose monitoring (CGM) data to optimize glucose control in type 2 diabetes. J Diabetes Complications. 2022;36(3):108106.
Monnier L, Wojtusciszyn A, Colette C, Owens D. The contribution of glucose variability to asymptomatic hypoglycemia in persons with type 2 diabetes. Diabetes Technol Ther. 2011;13(8):813–8.
Kauffmann RM, Hayes RM, Buske BD, Norris PR, Campion TR Jr., Dortch M, Jenkins JM, Collier BR, May AK. Increasing blood glucose variability heralds hypoglycemia in the critically ill. J Surg Res. 2011;170(2):257–64.
Bajaj HS, Venn K, Ye C, Patrick A, Kalra S, Khandwala H, Aslam N, Twum-Barima D, Aronson R. Lowest glucose variability and hypoglycemia are observed with the combination of a GLP-1 receptor agonist and basal insulin (VARIATION Study). Diabetes Care. 2017;40(2):194–200.
Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, Colette C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295(14):1681–7.
Akirov A, Diker-Cohen T, Masri-Iraqi H, Shimon I. High glucose variability increases mortality risk in hospitalized patients. J Clin Endocrinol Metab. 2017;102(7):2230–41.
Mendez CE, Mok KT, Ata A, Tanenberg RJ, Calles-Escandon J, Umpierrez GE. Increased glycemic variability is independently associated with length of stay and mortality in noncritically ill hospitalized patients. Diabetes Care. 2013;36(12):4091–7.
Timmons JG, Cunningham SG, Sainsbury CA, Jones GC. Inpatient Glycemic variability and long-term mortality in hospitalized patients with type 2 diabetes. J Diabetes Complications. 2017;31(2):479–82.
Krinsley JS. Glycemic variability: a strong independent predictor of mortality in critically ill patients. Crit Care Med. 2008;36(11):3008–13.
Olsen MT, Dungu AM, Klarskov CK, Jensen AK, Lindegaard B, Kristensen PL. Glycemic variability assessed by continuous glucose monitoring in hospitalized patients with community-acquired pneumonia. BMC Pulm Med. 2022;22(1):83.
Julla JB, Jacquemier P, Fagherazzi G, Vidal-Trecan T, Juddoo V, Jaziri A, Mersel H, Venteclef N, Roussel R, Massin P, Couturier A, Gautier JF, Riveline JP. Is the consensual threshold for defining high glucose variability implementable in clinical practice? Diabetes Care. 2021;44(7):1722–5.
Mo Y, Ma X, Lu J, Shen Y, Wang Y, Zhang L, Lu W, Zhu W, Bao Y, Zhou J. Defining the target value of the coefficient of variation by continuous glucose monitoring in Chinese people with diabetes. J Diabetes Investig. 2021;12(6):1025–34.
Rodbard D. Clinical interpretation of indices of quality of glycemic control and glycemic variability. Postgrad Med. 2011;123(4):107–18.
Monnier L, Colette C, Owens D. Below which threshold of glycemic variability is there a minimal risk of hypoglycemia in people with type 2 diabetes? Diabetes Technol Ther. 2022;24(6):453–4.
Donahue SR, Turner KC, Patel S. Pharmacokinetics and pharmacodynamics of glyburide/metformin tablets (glucovance) versus equivalent doses of glyburide and metformin in patients with type 2 diabetes. Clin Pharmacokinet. 2002;41(15):1301–9.
Chinese Expert Consensus on Medical Nutrition Therapy for Overweight/Obesity. Chin J Diabetes Mellitus. 2016;10(10):451–5.
Shaefer C, Reid T, Vlajnic A, Zhou R, DiGenio A. FASTING VERSUS POSTPRANDIAL HYPERGLYCEMIA AS A TREATMENT TARGET TO LOWER ELEVATED HEMOGLOBIN A1C. Endocr Pract. 2015;21(12):1323–32.
Woerle HJ, Neumann C, Zschau S, Tenner S, Irsigler A, Schirra J, Gerich JE, Göke B. Impact of fasting and postprandial glycemia on overall glycemic control in type 2 diabetes importance of postprandial glycemia to achieve target HbA1c levels. Diabetes Res Clin Pract. 2007;77(2):280–5.
Papachristoforou E, Lambadiari V, Maratou E, Makrilakis K. Association of Glycemic Indices (Hyperglycemia, glucose variability, and hypoglycemia) with oxidative stress and Diabetic complications. J Diabetes Res. 2020;2020:7489795.
Volpe CMO, Villar-Delfino PH, Dos Anjos PMF, Nogueira-Machado JA. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018;9(2):119.
Raz I, Wilson PW, Strojek K, Kowalska I, Bozikov V, Gitt AK, Jermendy G, Campaigne BN, Kerr L, Milicevic Z, Jacober SJ. Effects of prandial versus fasting glycemia on cardiovascular outcomes in type 2 diabetes: the HEART2D trial. Diabetes Care. 2009;32(3):381–6.
PJ. Gycemic control, mealtime glucose excursions, and diabetic complications in type 2 diabetes mellitus. Mayo Clin Proc. 2001;76(6):609–18.
Ceriello A, Esposito K, Piconi L, Ihnat MA, Thorpe JE, Testa R, Boemi M, Giugliano D. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes. 2008;57(5):1349–54.
Ohara M, Fukui T, Ouchi M, Watanabe K, Suzuki T, Yamamoto S, Yamamoto T, Hayashi T, Oba K, Hirano T. Relationship between daily and day-to-day glycemic variability and increased oxidative stress in type 2 diabetes. Diabetes Res Clin Pract. 2016;122:62–70.
Andersen A, Jørgensen PG, Bagger JI, Baldassarre MPA, Christensen MB, Pedersen-Bjergaard U, Lindhardt TB, Gislason G, Knop FK, Vilsbøll T. Acute changes in plasma glucose increases left ventricular systolic function in insulin-treated patients with type 2 diabetes and controls. Diabetes Obes Metab. 2022;24(6):1123–31.
Foreman YD, van Doorn W, Schaper NC, van Greevenbroek MMJ, van der Kallen CJH, Henry RMA, Koster A, Eussen S, Wesselius A, Reesink KD, Schram MT, Dagnelie PC, Kroon AA, Brouwers M, Stehouwer CDA. Greater daily glucose variability and lower time in range assessed with continuous glucose monitoring are associated with greater aortic stiffness: the Maastricht Study. Diabetologia. 2021;64(8):1880–92.
Mo Y, Wang C, Lu J, Shen Y, Chen L, Zhang L, Lu W, Zhu W, Xia T, Zhou J. Impact of short-term glycemic variability on risk of all-cause mortality in type 2 diabetes patients with well-controlled glucose profile by continuous glucose monitoring: a prospective cohort study. Diabetes Res Clin Pract. 2022;189:109940.
Hirakawa Y, Arima H, Zoungas S, Ninomiya T, Cooper M, Hamet P, Mancia G, Poulter N, Harrap S, Woodward M, Chalmers J. Impact of visit-to-visit glycemic variability on the risks of macrovascular and microvascular events and all-cause mortality in type 2 diabetes: the ADVANCE trial. Diabetes Care. 2014;37(8):2359–65.
Monnier L, Wojtusciszyn A, Molinari N, Colette C, Renard E, Owens D. Respective contributions of Glycemic Variability and Mean Daily glucose as predictors of hypoglycemia in type 1 diabetes: are they equivalent? Diabetes Care. 2020;43(4):821–7.
Monnier L, Colette C, Owens D. Glucose variability and diabetes complications: risk factor or biomarker? Can we disentangle the Gordian knot? Diabetes Metab. 2021;47(3):101225.
Guerci B, Monnier L, Serusclat P, Petit C, Valensi P, Huet D, Raccah D, Colette C, Quéré S, Dejager S. Continuous glucose profiles with vildagliptin versus sitagliptin in add-on to metformin: results from the randomized Optima study. Diabetes Metab. 2012;38(4):359–66.
Candido R. Which patients should be evaluated for blood glucose variability? Diabetes Obes Metab. 2013;15(Suppl 2):9–12.
Schernthaner G, Guerci B, Gallwitz B, Rose L, Nicolay C, Kraus P, Kazda C. Impact of postprandial and fasting glucose concentrations on HbA1c in patients with type 2 diabetes. Diabetes Metab. 2010;36(5):389–94.
Fysekidis M, Cosson E, Banu I, Duteil R, Cyrille C, Valensi P. Increased glycemic variability and decrease of the postprandial glucose contribution to HbA1c in obese subjects across the glycemic continuum from normal glycemia to first time diagnosed diabetes. Metabolism. 2014;63(12):1553–61.
Monnier L, Colette C, Dejager S, Owens D. Residual dysglycemia when at target HbA(1c) of 7% (53mmol/mol) in persons with type 2 diabetes. Diabetes Res Clin Pract. 2014;104(3):370–5.
Suh S, Joung JY, Jin SM, Kim MY, Bae JC, Park HD, Lee MS, Lee MK, Kim JH. Strong correlation between glycaemic variability and total glucose exposure in type 2 diabetes is limited to subjects with satisfactory glycaemic control. Diabetes Metab. 2014;40(4):272–7.
Investigators F-ST. Glucose variability in a 26-Week randomized comparison of Mealtime Treatment with Rapid-Acting insulin Versus GLP-1 agonist in participants with type 2 diabetes at High Cardiovascular Risk. Diabetes Care. 2016;39(6):973–81.
Lee H, Park SE, Kim EY. Glycemic variability impacted by SGLT2 inhibitors and GLP 1 agonists in patients with diabetes Mellitus: a systematic review and Meta-analysis. J Clin Med 2021;10(18).
Kilpatrick ES, Rigby AS, Goode K, Atkin SL. Relating mean blood glucose and glucose variability to the risk of multiple episodes of hypoglycaemia in type 1 diabetes. Diabetologia. 2007;50(12):2553–61.
Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129–39.
Gerstein HC, Miller ME, Byington RP, Goff DC Jr., Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH Jr., Probstfield JL, Simons-Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–59.
Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–72.
Tuttle KR. More is not always better: intensive glycemic control in type 2 diabetes. Am J Kidney Dis. 2009;53(1):12–5.
UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837–53.
Choudhary P, Lonnen K, Emery CJ, MacDonald IA, MacLeod KM, Amiel SA, Heller SR. Comparing hormonal and symptomatic responses to experimental hypoglycaemia in insulin- and sulphonylurea-treated type 2 diabetes. Diabet Med. 2009;26(7):665–72.
van Dalem J, Brouwers MC, Stehouwer CD, Krings A, Leufkens HG, Driessen JH, de Vries F, Burden AM. Risk of hypoglycaemia in users of sulphonylureas compared with metformin in relation to renal function and sulphonylurea metabolite group: population based cohort study. BMJ. 2016;354:i3625.
Davis HA, Spanakis EK, Cryer PE, Davis SN. Hypoglycemia During Therapy of Diabetes. In: Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, Levy M, McGee EA, McLachlan R, New M, Purnell J, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, eds. Endotext. South Dartmouth (MA): MDText.com, Inc. Copyright © 2000–2023, MDText.com, Inc.; 2000.
Nakhleh A, Shehadeh N. Hypoglycemia in diabetes: an update on pathophysiology, treatment, and prevention. World J Diabetes. 2021;12(12):2036–49.
Wei Y, Lin FJ, Lin SY, Wang CC. Risk of hypoglycemia and concomitant use of Repaglinide and Clopidogrel: a Population-based nested case-control study. Clin Pharmacol Ther. 2019;106(6):1346–52.
Jin SM, Kim TH, Bae JC, Hur KY, Lee MS, Lee MK, Kim JH. Clinical factors associated with absolute and relative measures of glycemic variability determined by continuous glucose monitoring: an analysis of 480 subjects. Diabetes Res Clin Pract. 2014;104(2):266–72.
Ohara M, Hiromura M, Nagaike H, Kohata Y, Fujikawa T, Goto S, Sato N, Kushima H, Terasaki M, Yamamoto T, Mori Y, Hayashi T, Fukui T, Yamagishi SI, Hirano T. Relationship between glucose variability evaluated by continuous glucose monitoring and clinical factors, including glucagon-stimulated insulin secretion in patients with type 2 diabetes. Diabetes Res Clin Pract. 2019;158:107904.
Uemura F, Okada Y, Mita T, Torimoto K, Wakasugi S, Katakami N, Yoshii H, Matsushita K, Nishida K, Inokuchi N, Tanaka Y, Gosho M, Shimomura I, Watada H. Risk factor analysis for type 2 diabetes patients about Hypoglycemia using continuous glucose monitoring: results from a prospective observational study. Diabetes Technol Ther. 2022;24(6):435–45.
Clinical guidelines for prevention and treatment of type 2 diabetes mellitus in the elderly in China (2022 edition). Zhonghua Nei Ke Za Zhi 2022;61(1):12–50.
Dong Y, Gao W, Zhang L, Wei J, Hammar N, Cabrera CS, Wu X, Qiao Q. Patient characteristics related to metabolic disorders and chronic complications in type 2 diabetes mellitus patients hospitalized at the Qingdao Endocrine and Diabetes Hospital from 2006 to 2012 in China. Diab Vasc Dis Res. 2017;14(1):24–32.
Acknowledgements
Not applicable.
Funding
This study was supported by the Science and Technology Innovation Project of China Academy of Chinese Medical Sciences (CI2021A00509 and CI2021A05501).
Author information
Authors and Affiliations
Contributions
Y.X and M.W wrote the main manuscript and prepared figures. HP.L assisted with data processing. PH.L and GM.P provided the data. H.Z and TC.W provided financial support and assistance in manuscript revision. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Our study adhered to the ethical principles pertinent to real-world research and non-interventional studies. All data related to the research participants were approved by the Ethics Committee of Kaifeng Traditional Chinese Medicine Hospital (Appendix 1).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Xing, Y., Wu, M., Liu, H. et al. Assessing the temporal within-day glycemic variability during hospitalization in patients with type 2 diabetes patients using continuous glucose monitoring: a retrospective observational study. Diabetol Metab Syndr 16, 56 (2024). https://doi.org/10.1186/s13098-024-01269-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13098-024-01269-0