Abstract
Background
Obesity is believed to be a risk factor for COVID-19 and unfavorable outcomes, although data on this remains to be better elucidated.
Objective
To evaluate the impact of obesity on the endpoints of patients hospitalized due to SARS-CoV-2.
Methods
This retrospective cohort study evaluated patients hospitalized at a tertiary hospital (Hospital das Clínicas da Faculdade de Medicina da USP) from March to December 2020. Only patients positive for COVID-19 (real-time PCR or serology) were included. Data were collected from medical records and included clinical and demographic information, weight and height, SAPS-3 score, comorbidities, and patient-centered outcomes (mortality, and need for mechanical ventilation, renal replacement therapy, or vasoactive drugs). Patients were divided into categories according to their BMI (underweight, eutrophic, overweight and obesity) for comparison porpoise.
Results
A total of 2547 patients were included. The mean age was 60.3 years, 56.2% were men, 65.2% were white and the mean BMI was 28.1 kg/m2. SAPS-3 score was a risk factor for all patient-centered outcomes (HR 1.032 for mortality, OR 1.03 for dialysis, OR 1.07 for vasoactive drug use, and OR 1.08 for intubation, p < 0.05). Male sex increased the risk of death (HR 1.175, p = 0.027) and dialysis (OR 1.64, p < 0.001), and underweight was protective for vasoactive drug use (OR 0.45, p = 0.027) and intubation (OR 0.31, p < 0.003).
Conclusion
Obesity itself was not an independent factor for worse patient-centered outcomes. Critical clinical state (indirectly evaluated by SAPS-3) appears to be the most important variable related to hard outcomes in patients infected with COVID-19.
Highlights
-
Obesity is present in up to 30% of patients hospitalized with COVID-19
-
Obesity itself is not related to key endpoints such as mortality and need for dialysis, orotracheal intubation, or vasoactive drug
-
SAPS-3 is the best predictor for worse outcomes in SARS-CoV-2 infection
Similar content being viewed by others
Introduction
During 2019, the world suffered a pandemic due to SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus 2) infection. The COVID-19 (Corona Virus Disease 2019) is believed to be responsible for almost 16 million deaths worldwide [1].
People with obesity are a risk population for this disease. In a cohort with almost 6000 hospitalized patients with COVID-19, half of them had obesity [2]. Obesity appeared to be also a risk factor for more severe disease and, consequently, increased mortality [3]. The mechanism behind this is probably related to premature immunosenescence, delayed hyperinflammation, and cytokine storm. In fact, adipocytes often secret more leptin, IL-6, TNF-alpha and INF-1, impairing residual immunological response [4].
In addition, concerning the pathophysiology, the virus uses the angiotensin-converting enzyme 2 receptor to infect the cell and replicate [5]. It is well-known that the adipose tissue may be vulnerable to more infection due to more expression of this receptor. People with obesity also have decreased chest-wall elastance, which leads to lower total respiratory compliance with a reduction of expiratory reserve volume and a higher susceptibility to infection. There is an impairment in total lung capacity and an increase in airway resistance as well as ventilation-perfusion mismatch [6].
Currently, COVID-19 treatment is based on supportive measures. In early stages of the pandemic, with the results of the RECOVERY trial, dexamethasone was a pillar treatment for hospitalized patients on invasive mechanical ventilation or oxygen supplementation alone, resulting in a lower 28-day mortality rate [7]. More recently, other drugs were studied for managing COVID-19 (such as remdesivir and tocilizumab) [8], but with an overall lower cost–benefit ratio than dexamethasone [9].
Currently, there are conflicting data on the role of obesity as an independent factor for mortality in hospitalized COVID-19 [10,11,12]. The disease severity itself (evaluated by desaturation, reduced level of consciousness, elevated creatinine) seemed to be the most relevant factor in predicting survival rate [13]. Therefore, the role of obesity alone as a factor for worse outcomes in patients with COVID-19 deserves better elucidation.
The aim of this article is to evaluate how obesity influenced the evolution and outcomes of hospitalized patients with COVID-19 in a single tertiary hospital center.
Methods.
This was a retrospective cohort study in a tertiary hospital center (Hospital das Clínicas da Faculdade de Medicina da USP), a reference for treatment care during the COVID pandemic, receiving complex cases with moderate-to-severe SARS-CoV-2 infection.
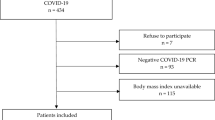
Data were collected from medical records during March 2020 to December 2020. The inclusion criteria were the presence of COVID-19 (symptoms alongside a confirmatory test, such as serology or real-time polymerase chain reaction), more than 18 years of old, moderate-to-severe disease that needed hospitalization, and presence of weight and height at admission. Patients with less than 18 years, incomplete data (without weight and height) or incorrect data (typeset errors) were excluded (Fig. 1).
For comparison porpoise, patients were stratified by body mass index (BMI, in kg/m2) in underweight (BMI < 18.5), eutrophic (18.6–24.9), overweight (25–29.9), class I obesity (30–34.9), class II obesity (35–39.9) and class III obesity (> 40). The primary endpoint was all-cause mortality. Secondary outcomes evaluated were the use of vasoactive drugs and the need for dialysis or mechanical ventilation. The SAPS-3 Score [14] was also calculated for staging severity, being overall a good predictor of mortality. Classically the variables computed for this score are age, PaO2, arterial pH, heart rate, creatinine, Glasgow coma scale, total bilirubin, leukocytes, systolic blood pressure, vasoactive drug, among others.
Descriptive and comparative analyses were presented. Data were summarized as mean ± standard deviation for continuous variables and as counts and percentages for categorical variables. For group comparisons, the chi-square test was used for categorical variables. Comparative analysis of the quantitative variables was presented using the analysis of variance (ANOVA) with Bonferroni correction and the likelihood ratio test. For the primary outcome, mortality was analyzed as a time-to-event measurement, with hazard ratio (HR) calculated by Cox regression. First, a univariate logistic regression was made and the variables that presented p < 0.10 were included in the multivariable Cox regression, as a stepwise selection of covariates. Odds ratios (OR) with 95% confidence intervals (CI) were calculated in a multivariable logistic regression test for the determination of risk factors for the secondary endpoints. The stepwise approach used in Cox regression was also used for logistic regression.
The variables used in the multivariable Cox regression for the primary endpoint were BMI, gender, and SAPS-3. The variables used in the multivariable logistic regression for the secondary endpoints for dialysis required were gender, and SAPS-3, and for vasoactive drugs use and need for mechanical ventilation were underweight, gender and SAPS-3.
The p-value < 0.05 was considered statistically significant. Statistical analysis of the data was performed using Statistical Package for Social Science (SPSS) version 17.0. The study was approved by the local Ethics Committee.
Results
A total of 2547 patients were included. The mean age was 60.3 ± 15.7 years, 1431 (56.2%) were men and 603 (65.2%) were white. The most common comorbidity was hypertension in 1530 (60.1%) patients, followed by type 2 diabetes in 38.7% and smoking in 23.1%. The mean BMI was 28.1 ± 7.5 kg/m2.
Table 1 shows baseline variables in the groups stratified by BMI. Class III obesituently observed at younger ages and in women. This population also had more hypertension, asthma, and diabetes, but a lower SAPS-3 score.
Concerning the primary outcome, multivariate Cox analysis determined only the male sex and SAPS-3 score being significantly associated with the risk of death (Table 2). Men had almost 20% more chance of dying than women and for each point in SAPS-3, the mortality likelihood increased by 3%.
The evaluation of patient-centered secondary outcomes (dialysis, vasoactive drug, and mechanical ventilation) showed that a higher SAPS-3 score is a risk factor for all of them (Table 3). Male sex was associated with the intrahospital need for dialysis. Interestingly, underweight was found to be protective against vasoactive drugs and mechanical ventilation.
Discussion
Classically critical patients are at greater risk of unfavorable endpoints. Several trials have shown directly (by SatO2, acute renal failure, coma) or indirectly (by scores) higher mortality rates in more severe patients. The HOPE-COVID-19-Registry [13] evaluated retrospectively more than 3000 patients with COVID-19. The Cox multivariate analysis for mortality determined age ≥ 70 years, ICU admission, SpO2 < 92%, Glasgow Coma Scale < 15, connective tissue disease, and elevated creatinine as independent predictors for mortality. BMI, on the other hand, did not affect the mortality rate.
Some authors have proposed an obesity paradox during COVID-19. People with obesity would have a greater chance for ICU admission [10, 15] and mechanical ventilation [16], but not for mortality. Others, on the other hand, have shown that obesity is a neutral factor for these outcomes [11, 12].
An interesting study [17] evaluated the relationship between the mortality of COVID-19 and obesity classes according to BMI, visceral adipose tissue and muscle area. Patients > 70 years, with low muscle area (< 92 cm2) and BMI < 30 had a lower survival rate (HR 3.89–9.66, p < 0.0006). Patients with obesity and muscle area > 92 cm2 had a higher survival rate and obesity as an isolated parameter was not associated with mortality. This shows that the evaluation of BMI alone is very complex. Muscle area was significantly reduced in critical patients compared to noncritical patients, indirectly characterizing a more severe disease.
In contrast to the previous data, a recent Cochrane Review [18] identified those with class III obesity to be at increased odds for mortality (OR 1.67, 95% CI 1.39–2.00) compared to normal BMI or patients without obesity. Another study [19], set in Brazil (state of Espírito Santo), showed a twofold risk for death in people with obesity. It is unclear if these studies included adjustments in mortality rate by the baseline severity of the disease, so controversy remains. It is fair to imagine that patients with BMI > 40 have more comorbidities and a higher risk for severe disease, the latter being the true responsible for increased mortality. In fact, eutrophic people with multiple organ dysfunction have a higher chance of unfavorable outcomes than a person with obesity alone. In our study, people with BMI > 40 had significantly lower SAPS-3 score, which partially explained why more severe obesity itself was not correlated with mortality. Some hypothesis justifies the lower SAPS-3 in this group: by chance; a lower threshold for hospitalization due to severe obesity; earlier mortality or difficulty in accessing primary or secondary medical care, affecting the referral rate to a tertiary hospital center.
The Cochrane Review [18] also observed, for mechanical ventilation, increasing odds with higher classes of obesity in comparison to normal BMI or patients without obesity (class I: OR 1.38, 95% CI 1.20–1.59; class II: OR 1.67, 95% CI 1.42–1.96; class III: OR 2.17, 95% CI 1.59–2.97). In our study, underweight was protective against intubation. Interestingly, the already cited HOPE-COVID-19-Registry [13] also demonstrated that BMI < 25 was an independent factor for a lower rate of respiratory insufficiency.
Another meta-analysis [20] found that obesity prevalence rates were 32% in hospitalized patients, 43% in patients needing invasive mechanical ventilation, and 33% in those who died. Obesity was associated with a higher risk for hospitalization, ICU admission, and intubation requirement, but no increase in risk of death. Of note, the prevalence of pooled obesity (class I, II and III) in our study was similar, about 30%. As a matter of comparison, the recent VIGITEL inquiry revealed a 24.3% rate of BMI > 30 kg/m2 in the city of São Paulo [21]. This indirectly shows that people with obesity have an increased risk for hospitalization [19], since our rate in hospitalized patients was 6% higher than the observed in an overall healthy population.
The finding that men had higher mortality rates and dialysis appears to be something established and consolidated in the literature [19, 22, 23]. There are several mechanisms for worse COVID-19-related outcomes in the male sex: woman have a higher rate of vaccination and seek medical care more often and at early stages of the disease [24], inherent immunological differences (X chromosome has the largest number of genes related to the integrity of immune system) [23], and the higher testosterone levels (which facilitates SARS-CoV-2 entry via angiotensin-converting enzyme 2 expressed on cell surfaces) [25]. However, it should be highlighted that at the time of our study, vaccines were not yet universally available.
This study had some limitations. The data was retrospective and being a reference hospital for COVID-19 treatment, more severe cases were evaluated. Another limitation was the restriction to hospitalized patients. However, the high sample included should be noticed as a strength.
Conclusion
Clinical critical condition (as evaluated by SAPS-3 score) is the best predictor for undesirable outcomes in COVID-19, namely mortality, need for dialysis, mechanical ventilation, or vasoactive drug. BMI itself was not an independent predictor of mortality; however, underweight patients might have lower intubation rates and vasoactive drug use than the control population.
More studies are needed to correctly assess the burden of obesity in COVID-19. Ideally, controlled trials with cases at the same severity levels but with different BMI should be conducted, trying to evaluate the isolated impact of obesity on clinically relevant outcomes.
Availability of data and materials
Our institution has an institution-wide data management plan for COVID-19 datasets which includes making anonymized data publicly available to contribute to nationwide and international registries of COVID-19 patients according to a pre-defined schedule.
References
Jha P, Brown PE, Ansumana R. Counting the global COVID-19 dead. Lancet Lond Engl. 2022;399(10339):1937–8. https://doi.org/10.1016/S0140-6736(22)00845-5.
Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA. 2020;323(20):2052–9. https://doi.org/10.1001/jama.2020.6775.
Brandão SCS, Godoi ETAM Oliveira Cordeiro LH de, et al. COVID-19 and obesity: the meeting of two pandemics. Arch Endocrinol Metab. 2021;65(1):3–13. https://doi.org/10.20945/2359-3997000000318
Alarcon PC, Damen MSMA, Madan R, et al. Adipocyte inflammation and pathogenesis of viral pneumonias: an overlooked contribution. Mucosal Immunol. 2021;14(6):1224–34. https://doi.org/10.1038/s41385-021-00404-8.
Bourgonje AR, Abdulle AE, Timens W, et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J Pathol. 2020;251(3):228–48. https://doi.org/10.1002/path.5471.
Bazurro S, Ball L, Pelosi P. Perioperative management of obese patient. Curr Opin Crit Care. 2018;24(6):560–7. https://doi.org/10.1097/MCC.0000000000000555.
Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with covid-19. N Engl J Med. 2021;384(8):693–704.
Berlin DA, Gulick RM, Martinez FJ. Severe covid-19. N Engl J Med. 2020;383(25):2451–60. https://doi.org/10.1056/NEJMcp2009575.
Li G, Hilgenfeld R, Whitley R, De Clercq E. Therapeutic strategies for COVID-19: progress and lessons learned. Nat Rev Drug Discov. 2023;22(6):449–75. https://doi.org/10.1038/s41573-023-00672-y.
Biscarini S, Colaneri M, Ludovisi S, et al. The obesity paradox: analysis from the SMAtteo COvid-19 REgistry (SMACORE) cohort. Nutr Metab Cardiovasc Dis. 2020;30(11):1920–5. https://doi.org/10.1016/j.numecd.2020.07.047.
Sezer H, Canbaz HB, Yurdakul F, Özserezli B, Yazıcı D. Is obesity paradox valid for critically-ill COVID-19 patients with respiratory failure? Turk Thorac J. 2022;23(4):268–76. https://doi.org/10.5152/TurkThoracJ.2022.21139.
Sprockel Díaz JJ, Coral Zuñiga VE, Angarita Gonzalez E, et al. Obesity and the obesity paradox in patients with severe COVID-19. Med Intensiva. 2023;47(10):565–74. https://doi.org/10.1016/j.medine.2023.03.009.
Abumayyaleh M, Núñez Gil IJ, El-Battrawy I, et al. Does there exist an obesity paradox in COVID-19? Insights of the international HOPE-COVID-19-registry. Obes Res Clin Pract. 2021;15(3):275–80. https://doi.org/10.1016/j.orcp.2021.02.008.
Moreno RP, Metnitz PGH, Almeida E, et al. SAPS 3–from evaluation of the patient to evaluation of the intensive care unit. Part 2: development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med. 2005;31(10):1345–55. https://doi.org/10.1007/s00134-005-2763-5.
Paravidino VB, Leite TH, Mediano MFF, et al. Association between obesity and COVID-19 mortality and length of stay in intensive care unit patients in Brazil: a retrospective cohort study. Sci Rep. 2022;12(1):13737. https://doi.org/10.1038/s41598-022-17197-w.
Goyal P, Ringel JB, Rajan M, et al. Obesity and COVID-19 in New York city: a retrospective cohort study. Ann Intern Med. 2020;173(10):855–8. https://doi.org/10.7326/M20-2730.
Beltrão FE de L, Beltrão DC de A, Carvalhal G, et al. Low muscle mass and high visceral fat mass predict mortality in patients hospitalized with moderate-to-severe COVID-19: a prospective study. Endocr Connect. 2022;11(10):e220290.
Tadayon Najafabadi B, Rayner DG, Shokraee K, et al. Obesity as an independent risk factor for COVID-19 severity and mortality. Cochrane Database Syst Rev. 2023;5(5):CD015201. https://doi.org/10.1002/14651858.CD015201.
Reis ECD, Rodrigues P, de Jesus TR, de Freitas Monteiro EL, Virtuoso Junior JS, Bianchi L. Risk of hospitalization and mortality due to COVID-19 in people with obesity: an analysis of data from a Brazilian state. PLoS ONE. 2022;17(3): e0263723. https://doi.org/10.1371/journal.pone.0263723.
Helvaci N, Eyupoglu ND, Karabulut E, Yildiz BO. Prevalence of obesity and its impact on outcome in patients with COVID-19: a systematic review and meta-analysis. Front Endocrinol. 2021;12: 598249. https://doi.org/10.3389/fendo.2021.598249.
Vigitel Brasil 2023 - Vigilância de Fatores de Risco e Proteção para Doenças Crônicas por Inquérito Telefônico—Ministério da Saúde. https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/svsa/vigitel/vigitel-brasil-2023-vigilancia-de-fatores-de-risco-e-protecao-para-doencas-cronicas-por-inquerito-telefonico/view. Accessed 17 Dec 2023.
Zhang J, Pang Q, Zhou T, et al. Risk factors for acute kidney injury in COVID-19 patients: an updated systematic review and meta-analysis. Ren Fail. 2023;45(1):2170809.
Fabião J, Sassi B, Pedrollo EF, et al. Why do men have worse COVID-19-related outcomes? A systematic review and meta-analysis with sex adjusted for age. Braz J Med Biol Res. 2022;55: e11711. https://doi.org/10.1590/1414-431X2021e11711.
Kaim A, Shetrit SB, Saban M. Women are more infected and seek care faster but are less severely ill: gender gaps in COVID-19 morbidity and mortality during two years of a pandemic in Israel. Healthc Basel Switz. 2022;10(12):2355. https://doi.org/10.3390/healthcare10122355.
Yeap BB, Marriott RJ, Manning L, et al. Higher premorbid serum testosterone predicts COVID-19-related mortality risk in men. Eur J Endocrinol. 2022;187(1):159–70. https://doi.org/10.1530/EJE-22-0104.
Acknowledgements
We would like to acknowledge the outstanding work performed by healthcare workers and staff in our hospital during the COVID-19 crisis. We would also like to thank the Hospital das Clínicas COVID-19 crisis committee and the informatics department (NETI) for their support for this project. We would like the names of the individual members of the Group to be searchable through their individual PubMed records.
EPICCoV study group: Adriana Hirota, Alberto Kendy Kanasiro, Alessandra Crescenzi, Amanda Coelho Fernandes, Anna Miethke-Morais, Arthur Petrillo Bellintani, Artur Ribeiro Canasiro, Bárbara Vieira Carneiro, Beatriz Keiko Zanbon, Bernardo Pinheiro Senna Nogueira Batista, Bianca Ruiz Nicolao, Bruno Adler Maccagnan Pinheiro Besen, Bruno Biselli, Bruno Rocha De Macedo, Caio Machado Gomes De Toledo, Carlos Roberto Ribeiro De Carvalho, Caroline Gomes Mol, Cassio Stipanich, Caue Gasparotto Bueno, Cibele Garzillo, Clarice Tanaka, Daniel Neves Forte, Daniel Joelsons, Daniele Robira, Eduardo Leite Vieira Costa, Elson Mendes Da Silva Júnior, Fabiane Aliotti Regalio, Gabriela Cardoso Segura, Giulia Sefrin Louro, Gustavo Brasil Marcelino, Yeh-Li Ho, Isabela Argollo Ferreira, Jeison Oliveira Gois, Joao Manoel Da Silva-Jr, Jose Otto Reusing Junior, Julia Fray Ribeiro, Juliana Carvalho Ferreira, Karine Vusberg Galleti, Katia Regina Silva, Larissa Padrao Isensee, Larissa Santos Oliveira, Leandro Utino Taniguchi, Leila Suemi Letaif, Lígia Trombetta Lima, Lucas Yongsoo Park, Lucas Chaves Netto, Luciana Cassimiro Nobrega, Luciana Bertocco Paiva Haddad, Ludhmila Abrahao Hajjar, Luiz Marcelo Sa Malbouisson, Manuela Cristina Adsuara Pandolfi, Marcelo Park, Maria José Carvalho Carmona, Maria Castilho Prandini H. Andrade, Mariana Moreira Santos, Matheus Pereira Bateloche, Mayra Akimi Suiama, Mayron Faria de Oliveira, Mayson Laercio Sousa, Michelle Louvaes Garcia, Natassja Huemer, Pedro Vitale Mendes, Paulo Ricardo Gessolo Lins, Pedro Gaspar Dos Santos, Pedro Ferreira Paiva Moreira, Renata Mello Guazzelli, Renato Batista Dos Reis, Renato Daltro-Oliveira, Roberta Muriel Longo Roepke, Rodolpho Augusto Moura Pedro, Rodrigo Kondo, Samia Zahi Rached, Sergio Roberto Silveira Da Fonseca, Thais Sousa Borges, Thalissa Ferreira, Vilson Cobello Junior, Vivian Vieira Tenório Sales, and Willaby Serafim Cassa Ferreira. We would like the names of the individual members of the group to be searchable through their individual PubMed records. All investigators above are from Hospital das Clínicas HCFMUSP, Faculdade de Medicina, Universidade de Sao Paulo, SP, BR.
Funding
No funding was obtained for this study.
Author information
Authors and Affiliations
Consortia
Contributions
MCM and MEM conceived the study and participate in its design, coordination, data interpretation, statistical analysis and provided critical revision of the manuscript; FAC and MAMS helped to draft the manuscript and participate in its design, coordination, data interpretation, and statistical analysis. CC and AEF helped to draft the manuscript and participated in its design and revision. All authors read and approved the final manuscript. The EPICCoV study group participated in data collection and data interpretation.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Research Ethics Committee of Hospital das Clínicas da Universidade de São Paulo and registered in a public registry (CAAE: 35995920.2.0000.0068). Informed consent was waived due to the observational nature of the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Carra, F.A., de Melo, M.E., Stumpf, M.A.M. et al. The impact of obesity in hospitalized patients with COVID-19: a retrospective cohort study. Diabetol Metab Syndr 16, 20 (2024). https://doi.org/10.1186/s13098-023-01246-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13098-023-01246-z