Abstract
Background
Women at risk of gestational diabetes mellitus (GDM) need preventative interventions.
Objective
To evaluate targeted interventions before and during pregnancy for women identified as being at risk of developing GDM.
Methods
Systematic review and meta-analysis conducted following PRISMA guidelines. MEDLINE, EMBASE and the Cochrane Library in addition to reference and citation lists were searched to identify eligible randomised controlled trials (RCTs) utilising risk stratification during the preconception period or in the first/early second trimester. Screening and data extraction were carried out by the authors independently. Quality assessment was conducted based on the Cochrane risk-of-bias tool. Random effects meta-analysis and narrative synthesis were performed.
Results
Eighty-four RCTs were included: two during preconception and 82 in pregnancy, with a pooled sample of 22,568 women. Interventions were behavioural (n = 54), dietary supplementation (n = 19) and pharmacological (n = 11). Predictive factors for risk assessment varied; only one study utilised a validated prediction model. Gestational diabetes was reduced in diet and physical activity interventions (risk difference − 0.03, 95% CI 0.06, − 0.01; I2 58.69%), inositol (risk difference − 0.19, 95% CI 0.33, − 0.06; I2 92.19%), and vitamin D supplements (risk difference − 0.16, 95% CI 0.25, − 0.06; I2 32.27%). Subgroup analysis showed that diet and physical activity interventions were beneficial in women with ≥ 2 GDM risk factors (risk difference − 0.16, 95% CI 0.25, − 0.07; I2 11.23%) while inositol supplementation was effective in women with overweight or obesity (risk difference − 0.17, 95% CI 0.22, − 0.11; I2 0.01%). Effectiveness of all other interventions were not statistically significant.
Conclusions
This review provides evidence that interventions targeted at women at risk of GDM may be an effective strategy for prevention. Further studies using validated prediction tools or multiple risk factors to target high-risk women for intervention before and during pregnancy are warranted.
Similar content being viewed by others
Introduction
Gestational diabetes mellitus (GDM) is a common pregnancy related complication affecting ~ 14% of pregnancies worldwide, although prevalence varies by country, population and diagnostic criteria [1]. Women who develop GDM have a higher risk of gestational hypertension, pre-eclampsia, caesarean and preterm delivery than women who do not develop the condition [2,3,4]. Infants of mothers with GDM are at increased risk of stillbirth, macrosomia, and neonatal hypoglycaemia [3, 4]. In the longer term, GDM is associated with a greater risk of metabolic disease for both the mother and her offspring [5,6,7], highlighting the importance of early screening and prevention.
Although the aetiology of GDM is not completely understood, there are obstetric, socio-demographic, clinical and metabolic risk factors implicated [8, 9]. The oral glucose tolerance test (OGTT), usually carried out between 24 and 28 weeks of gestation, is used to detect GDM as part of routine antenatal care [10]. To date there is no consensus on the strategies to identify women at high-risk of developing GDM earlier in pregnancy.
Several antenatal trials have aimed to prevent GDM, suggesting that behavioural interventions (e.g. diet and physical activity (PA)), supplementation (e.g. myo-inositol and vitamin D), and pharmacological intervention using metformin have possible benefits in reducing risk in the general antenatal population [11,12,13,14]. Moreover, studies have not been able to establish an effect of diet or exercise alone, probiotics, and/or other vitamins and minerals on GDM risk [11, 15]. It is not yet clear whether targeting interventions to women with specific risk factors for GDM is an effective approach to GDM prevention.
Additionally, research on the effectiveness of preconception interventions in preventing GDM is lacking [11], and it is a priortiy area for intervention research [16]. One preconception nutritional intervention was not successful in reducing maternal glycaemia or GDM in a large multi-site trial; however, this study did not target higher risk women [17]. Hence, a more selective approach in women who are at risk and planning to conceive might be more effective.
The aim of this review was to evaluate the effect of interventions (behavioural, supplementation and pharmacological) during the preconception period and/or in pregnancy on reducing GDM in women identified at higher risk for developing the condition.
Methods
This review was conducted in accordance with the relevant criteria of the PRISMA guidelines for reporting a systematic review and meta-analysis [18] and was registered in the PROSPERO database (CRD42020177976).
Eligibility criteria
Inclusion and exclusion criteria were developed using the PICOS (population, intervention, comparison, outcomes and study design) framework, summarised in Table 1. For inclusion, studies had to meet the following criteria: (1) randomised controlled trials (RCTs) that evaluated interventions, in the preconception period and/or during pregnancy compared with no intervention, standard care or placebo; (2) women identified as higher risk of developing GDM using any risk stratification in the preconception period or in early pregnancy; (3) data reporting GDM as a primary or secondary pregnancy outcome. All diagnostic criteria for GDM were deemed acceptable. Studies meeting the following criteria were excluded: (1) non-randomised and observational studies; (2) abstracts, reviews, letters, commentaries and editorials; (3) women aged less than 18 years or older than 50 years; (4) studies designed to treat GDM; (5) interventions starting too late in pregnancy (> 28 weeks’ gestation); (6) studies not published in English.
Database searches
Three electronic databases: MEDLINE, EMBASE and the Cochrane Central Register of Controlled Trials, were searched up to February 2023 with no date restriction. A comprehensive search strategy was developed (Additional file 1: Table A1, A2, A3) using search terms related to “pregnancy”, “adiposity” and “randomisation”. Reference lists from all included studies were examined for additional relevant articles to supplement the database searches as per PRISMA guideline recommendations [18]. Study authors were contacted when further information was required.
Study selection
Records obtained from all databases were imported into the EndNote X9 reference management software to eliminate duplicate publications. Subsequently, studies were imported into the screening management software Rayyan [19] for screening. All title and abstracts were screened by OFQ and a second independent reviewer (either KGN, AA, GSC, AP or NV). Full-text screening was also carried out independently in duplicate, with disagreements discussed and resolved by consensus opinion among 4 reviewers.
Data extraction and quality assessment
Data were extracted independently and in duplicate by two authors (OFQ, DA) using a standardised table created for this review. Data extraction included: title; authors; publication year; trial periods; study design; country; aim; sample size; population; inclusion/exclusion criteria; period of intervention (preconception and/or pregnancy); risk stratification; intervention characteristics and clinical outcomes. The Cochrane risk-of-bias tool for randomised trials (RoB 2) [20] was used to assess the validity and bias of each study included according to the Cochrane Handbook for Systematic Reviews of Interventions version 6.3 [21]. The domains used included randomisation bias (whether the allocation sequence was random and adequately concealed), deviations from the intended interventions (blinding of participants and trial personnel, adherence to intervention), bias due to missing outcome data (including biases introduced by procedures used to impute or otherwise account for missing outcome data), bias in measurements of the outcome (differential errors related to intervention assignment) and bias in selection of the reported results. The quality assessment was based on a series of signalling questions within each domain and was independently performed by two authors (OFQ, DA). Discrepancies were resolved by a third author (ACF). The overall risk was determined, and studies were classified as ‘low risk of bias’, ‘some concerns’ or ‘high risk of bias’.
Analysis
The interventions and outcomes were evaluated to determine the appropriateness of data pooling in order to perform a meta-analysis. The analysis was built around different intervention types including: behavioural (diet only, PA only, combined diet and PA), dietary supplementation (inositols, vitamin D, fibre, probiotics), and pharmacological (metformin). Any intervention that could not contribute to the analysis or could not be pooled was excluded from the meta-analysis (e.g., studies that are not sufficiently homogeneous to be combined under the prespecified interventions, Table 1); and a narrative synthesis was performed to provide a brief summary of these studies and their findings [22]. Where appropriate, summaries of exposure effect for each intervention were provided using a risk difference, performed using Stata software, version 16.0 (StataCorp, College Station, TX, USA). A random-effects meta-analysis model was used to estimate the effects of each intervention on GDM and an I2 value greater than 50% was considered an indication of significant heterogeneity across studies [23]. Furthermore, separate analyses were performed limited to studies where increased body mass index (BMI ≥ 25 kg/m2 or ≥ 24 kg/m2 depending on classification used) was the only risk factor as criteria for intervention and studies that utilised more than one risk factor which may or may not have included BMI. Publication bias was investigated using Egger's test and funnel plots, if there were more than 10 RCTs per meta-analysis.
Results
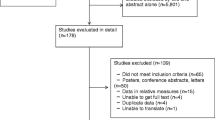
We identified 29,205 results through database searches and an additional 10 through citation searches and reference lists: 4,337 were removed as duplicates and of the remaining 24,878 articles, 24,470 were excluded during title and abstract screening. There were 408 full-texts screened against the eligibility criteria, and 84 met the inclusion criteria (Fig. 1). Major reasons for exclusion were: no risk stratification, ongoing RCTs and GDM not a primary/secondary outcome.
Risk stratification for GDM
The included studies used different risk stratification strategies incorporating a variety of risk factors to identify women at high-risk of developing GDM. The number of variables ranged from one to sixteen (Additional file 1: Table A5). Fifty-four studies recruited women with increased BMI [24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77], with three of these using additional risk factors; polycystic ovary syndrome (PCOS) [41], previous history of GDM [41, 42, 56], family history of diabetes [41, 42], high-risk ethnicity [41] or history of unexplained intrauterine fetal death or macrosomic infant [41].
Two studies considered a family history of diabetes [78, 79], one utilised previous history of GDM [80] and one targeted women who had previously delivered an infant with macrosomia [81]. Three studies used elevated fasting blood glucose (FBG) [82,83,84] or/and haemoglobin A1c (HbA1c) [84]. Six studies used a history of PCOS [85,86,87,88,89,90] with Valdés et al. [90] additionally specifying pregestational insulin resistance (PIR) with at least one PIR clinical sign or the diagnosis of PCOS.
Fourteen studies required women to have at least one risk factor for the development of GDM including advanced maternal age [91,92,93,94,95,96,97,98,99], PCOS [93, 95, 98,99,100], BMI above a particular threshold (obesity/overweight) [91,92,93,94,95,96,97, 99,100,101,102,103], a family history of diabetes [91,92,93,94,95,96, 99,100,101, 104], signs of glucose intolerance [91, 92, 99], previous GDM [91,92,93,94,95,96,97,98,99,100,101,102, 104] or previous macrosomic infant [91,92,93,94, 96,97,98,99,100,101, 104]. Seven [94, 97,98,99,100,101, 103] of these specified other factors including high-risk ethnicity, chronic hypertension, twin pregnancies, abnormal lipid metabolism, glycosuria, previous pregnancy complications (e.g. gestational hypertension, pre-eclampsia, premature rupture of membranes, small for gestational age (SGA), large for gestational age (LGA), intrauterine growth restriction, low Apgar score, preterm deliveries, fetal anomaly, recurrent abortion, intrauterine fetal death and family history of either GDM or adverse obstetrical outcomes).
One study enrolled women with two or more GDM risk factors; including pre-pregnancy obesity, PCOS, high-risk ethnicity, previous GDM or macrosomic infant and family history of diabetes [105], while Mohsenzadeh-Ledari et al. [106] enrolled participants who had at least three components of the metabolic syndrome. One study used a validated prediction tool (simple scoring system) for identification of women at high-risk of developing GDM; this included history of GDM, maternal age, BMI, Asian descent, history of poor obstetric outcome and family history of diabetes [107].
Characteristics of included studies
Characteristics of the studies are shown in Additional file 1: Table A4. The majority of the studies (n = 82) were RCTs targeting high-risk women in the antenatal period. Two studies in the preconception period were identified. Among the eighty-four studies included, fifteen were conducted in United States [29,30,31, 35, 42, 48, 49, 55, 56, 61, 62, 66, 71, 74, 84], eight in China [24, 25, 67, 73, 93, 95, 96, 99], eight in Italy [38, 39, 44, 59, 63, 78, 82, 83], eight in Australia [36, 47, 57, 58, 72, 75, 80, 107], seven in Iran [65, 68, 69, 88, 94, 98, 106], five in the United Kingdom [26, 40, 51, 100, 103], four in Finland [60, 91, 92, 102], four in the Republic of Ireland [27, 37, 79, 81], four in Denmark [33, 43, 46, 77], three in Norway [54, 85, 86], two in each of India [97, 101], Netherlands [41, 104], New Zealand [28, 64], Brazil [34, 50] and one in each of Canada [45], France [70], Chile [90], Belgium [53], Spain [32], Bangladesh [89] and the United Arab Emirates [105]. Three studies were multi-country; Norway, Sweden and Iceland [87]; United Kingdom, Republic of Ireland, Austria, Poland, Italy, Spain and Belgium [52, 76]; Netherlands, and Denmark [76].
The sample size of the studies ranged from 40 [86] to 2,122 [58] participants and the pooled sample size was 22568. Thirty studies included women of all BMI categories [78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107], while 54 included pregnant women living with overweight and/or obesity [24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77].
The intervention design varied between studies (Additional file 1: Table A5). The majority (n = 34) evaluated the effect of combined diet and PA [30,31,32,33,34,35, 37, 48, 51, 53, 55, 56, 58, 59, 61, 63, 65,66,67, 69,70,71, 74, 84, 91,92,93, 95, 96, 99, 102, 105,106,107]. Seven studies focused on modifying diet only [24, 29, 46, 49, 57, 81, 103], while ten aimed at modifying PA alone [25, 27, 45, 54, 62, 64, 72, 80, 97, 104]. A multidisciplinary approach (consisting of continuity of care, assessment of weight gain, a brief dietary intervention and psychological approach using solution-focused therapy) was used in one study [75]. Nine studies based their intervention on the supplement inositol (myo-inositol, d-chiro-inositol or combination of both) [38, 39, 44, 68, 78, 79, 82, 83, 100] while four used probiotic supplementation (Lactobacillus and Bifidobacterium species) [36, 60, 77, 98]. In three studies, the impact of vitamin D was assessed [52, 94, 101] and two evaluated the intake of soluble fibre [42, 73] of which one study additionally provided women with frozen blueberries [42]. Metformin was used as a pharmacological intervention in eleven RCTs [40, 41, 47, 50, 85,86,87,88,89,90, 108]. Three studies included more than two arms; Renault et al. [43] compared PA only and combined diet and PA with standard care, whilst Simmons et al. [76] compared the effect of diet alone, PA alone, combined diet and PA with standard care. Okesene-Gafa et al. [28] compared diet alone and probiotic to standard care and placebo arms.
Risk of bias (quality) assessment
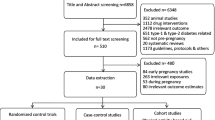
The overall quality of the included studies varied and is summarised in Fig. 2. Twenty-three studies were assessed as ‘low risk of bias’ [25, 28, 31, 43, 47, 49, 52, 55, 58, 67,68,69, 75,76,77, 79, 80, 82, 85, 87, 99, 102, 103], 30 as ‘high risk of bias’ [30, 33,34,35, 37,38,39, 46, 50, 54, 56, 57, 61, 65, 66, 78, 84, 86, 88,89,90, 93,94,95, 97, 98, 104,105,106,107] and the remaining 31 studies as ‘some concerns’ [24, 26, 27, 29, 32, 36, 40,41,42, 44, 45, 48, 51, 53, 59, 60, 62,63,64, 70,71,72,73,74, 81, 83, 91, 92, 96, 100, 101]. The main source of bias across all studies was the non-adherence to the assigned intervention regimen.
Behavioural intervention (diet only, PA only, combined diet and PA)
In preconception women, the two included studies [55, 56] assessed the impact of behavioural intervention (combined diet and PA) on GDM risk and were pooled in a meta-analysis (Additional file 1: Table A6). Diet and PA-based interventions prior to pregnancy did not reduce GDM development among those who were considered at higher risk prior to pregnancy (risk difference − 0.01, 95% CI 0.24 to 0.23; I2 63.72%) (Fig. 3).
Fifty-three antenatal studies [24, 25, 27,28,29,30,31,32,33,34,35, 37, 43, 45, 46, 48, 49, 51, 53, 54, 57,58,59, 61,62,63,64,65,66,67, 69,70,71,72, 74,75,76, 80, 81, 84, 91,92,93, 95,96,97, 99, 102,103,104,105,106,107] reported the effect of a behavioural intervention during pregnancy on the development of GDM with 52 of those included in the meta-analysis (Additional file 1: Table A6). One study was considered insufficiently homogenous to be pooled for meta-analysis and was excluded from the analysis since only brief dietary intervention (5 min) was provided as part of the multidisciplinary approach [75]. In the 34 studies that examined the effect of combined diet and PA interventions on GDM risk [30,31,32,33,34,35, 37, 43, 48, 51, 53, 58, 59, 61, 63, 65,66,67, 69,70,71, 74, 76, 84, 91,92,93, 95, 96, 99, 102, 105,106,107], women who received the intervention were 3% less likely to develop GDM (risk difference − 0.03, 95% CI 0.06 to − 0.01; I2 58.69%) with significant heterogeneity across studies (Fig. 4). Nine studies of diet only [24, 28, 29, 46, 49, 57, 76, 81, 103] (Fig. 5) and twelve of PA only [25, 27, 43, 45, 54, 62, 64, 72, 76, 80, 97, 104] interventions (Fig. 6), showed no significant difference in GDM risk (risk difference − 0.01, 95% CI 0.05 to 0.02; I2 48.72 and − 0.04, 95% CI 0.09 to 0.01; I2 38.76% respectively).
In the subgroup analyses, three antenatal studies that used combined diet and PA interventions for women who had ≥ 2 risk factors reduced GDM risk (risk difference − 0.16, 95% CI 0.25 to − 0.07; I2 11.23%; Fig. 7). There was no effect in sub-group analyses for BMI as the only risk factor (combined diet and PA: risk difference − 0.02, 95% CI − 0.05 to 0.00; I2 51.50%; diet only: risk difference − 0.02, 95% CI − 0.07 to 0.03; I2 22.43%; PA only: risk difference − 0.03, 95% CI − 0.12 to 0.05; I2 49.46%; Fig. 8).
Supplementation interventions (inositols, vitamin D, probiotics, fibre)
There were 19 studies [28, 36, 38, 39, 42, 44, 52, 60, 68, 73, 77,78,79, 82, 83, 94, 98, 100, 101] that assessed the effect of dietary supplementation on risk of GDM during pregnancy and all were pooled in the meta-analysis (Additional file 1: Table A6). Nine used inositol [38, 39, 44, 68, 78, 79, 82, 83, 100] and three vitamin D [52, 94, 101]; both of which reduced risk of GDM (risk difference − 0.19, 95% CI 0.33 to − 0.06; I2 92.19 and − 0.16, 95% CI 0.25 to − 0.06; I2 32.27% respectively); however, there was significant heterogeneity among the inositol interventions (Fig. 9, 10). Two studies that tested the use of fibre [42, 73] showed a reduction in GDM (risk difference − 0.13, 95% CI 0.25 to − 0.02; I2 0.01%; Fig. 11). There was no evidence of an effect of probiotic use on the prevention of GDM (risk difference 0.03, 95% CI 0.01 to 0.07; I2 0.01%; Fig. 12). In the subgroup analysis, four studies used inositol interventions where BMI was the only risk factor and demonstrated a reduction in GDM (risk difference − 0.17, 95% CI 0.22 to − 0.11; I2 0.01%; Fig. 8). It was not possible to perform a sub-group analysis due to lack of supplementation studies among women with multiple risk factors.
Pharmacological intervention
There were eleven studies [40, 41, 47, 50, 85,86,87,88,89,90, 108] that evaluated the effect of metformin on GDM and all were included in the meta-analysis (Additional file 1: Table A6). There was no significant effect of metformin in the prevention of GDM either overall (risk difference − 0.00, 95% CI − 0.04 to 0.03; I2 8.82%; Fig. 13) or in the subgroup analyses of women with multiple risk factors (risk difference − 0.03, 95% CI − 0.37 to 0.30; I2 77.04%; Fig. 7) or those with overweight and obesity (risk difference 0.00, 95% CI − 0.04 to 0.04; I2 0.03%; Fig. 8).
Narrative synthesis
It was not possible to conduct a meta-analysis for one study that used a multidisciplinary approach intervention. Therefore, data has been synthesised narratively. The study reported GDM as a primary outcome in pregnant women with increased BMI (> 25 kg/m2) and the intervention resulted in a significant reduction in the incidence of GDM (n = 4, 6% vs. n = 17, 29%, OR 0.17, 95% CI 0.03–0.95, p = 0.04) [75].
Publication bias
There was no evidence of small study effects for any intervention except antenatal combined diet and PA interventions (p < 0.05), which may signal publication bias (Additional file 1: Figure A1, Table A7).
Discussion
This review aimed to evaluate the effectiveness of preconception and pregnancy interventions in reducing GDM among women at increased risk. The findings from the meta-analysis showed that GDM was reduced using combined diet and PA, inositol and vitamin D supplementation in women identified in early pregnancy as higher risk. The effect was greatest with the dietary supplements. In a sub-group analysis, diet and PA interventions were most effective in women with multiple GDM risk factors while in pregnant women living with overweight and obesity, inositol was effective. The results were limited by high levels of heterogeneity between the included studies, while some studies were not sufficiently powered to detect a difference in GDM. Additionally, there was a lack of preconception studies.
Whilst this analysis showed that the use of antenatal combined diet and PA intervention modestly reduced the risk of GDM when higher risk women were recruited, this same approach did not appear effective when higher BMI was considered the sole risk factor. These findings are consistent with a meta-regression examining moderators of intervention effectiveness for preventing GDM; it showed that behavioural interventions in populations with higher risk of GDM demonstrated greater effectiveness, but also highlighted that BMI before or in early pregnancy was not related to the effect size of the intervention [109]. This suggests that BMI stratification alone is not an effective strategy for either risk prediction or response to intervention [109]. Conversely, the current review showed that using a targeted recruitment strategy that includes multiple risk factors for GDM helped maximise the effectiveness of a combined diet and PA intervention. Given the limited number of studies available (n = 3) and the high risk of bias associated with these studies, we still lack certainty and more research is needed to examine targted interventions particularly in women with multiple risk factors.
The current review found that antenatal interventions using inositol in higher-risk women, including those with increased BMI as a sole risk factor, were effective in reducing GDM. This correlates with findings of a recent meta-analysis of inositol interventions in pregnant women living with overweight and obesity [110]. The insulin-mimetic effects of myo-inositol or its isomers are thought to be related to the production of inositol glycan secondary messengers, resulting in improved glycogen synthesis and glucose peripheral tissue uptake [111, 112]. Moreover, a deficit in intracellular d-chiro-inositol (DCI) has been observed in women with PCOS and overweight or obesity, resulting in impaired coupling between insulin action and the release of d-chiro-inositol-containing inositolphosphoglycan (DCI-IPG) that acts as an insulin mediator and sensitizer [113]. Although further studies of pregnant women with overweight and obesity are required to confirm inositol effectiveness, our analysis suggests that inositol supplementation may reduce the incidence of GDM among pregnant women at increased risk, regardless of the factors used in the risk assessment.
The present meta-analysis also suggests a preventative effect of antenatal vitamin D supplementation on GDM risk, and Cochrane reviews have also previously provided evidence for this possible benefit [11]. Several mechanistic pathways elucidating an influence of vitamin D on glucose homeostasis and GDM development have been described which impact upon metabolic markers including blood glucose concentrations, insulin resistance and inflammatory biomarkers [114,115,116]. Given that only three studies were identified in the current review that used vitamin D interventions, there is a need for further well-designed trials using larger cohorts among pregnant women identified as high-risk.
No effect of probiotics on the development of GDM in higher-risk women was found, consistent with the results of a Cochrane review [11]. However, in contrast, a recent meta-analysis (n = 10) showed a significant reduction of GDM with probiotic supplementation in the general antenatal population [117]. Furthermore, metformin was not found to be effective in preventing GDM in higher risk pregnant women. This is consistent with a previous meta-analysis in which the use of metformin started at conception or before 20 weeks of pregnancy did not reduce GDM when BMI was the only risk factor, or another selective risk assessment strategy such as PCOS and/or PIR was used [39]. A Cochrane systematic review of metformin use to prevent the development of type 2 diabetes mellitus (T2DM) (n = 20 RCTs) in individuals at increased risk supported efficacy in prevention (with or without behavioural interventions) when taken over a minimum of at least a 1-year [118]. This may imply that given the relatively short period of gestation, women at increased risk might benefit from metformin intervention if commenced during the preconception period. Moreover, our analysis highlights the limited evidence for metformin interventions (n = 2) in women with multiple risk factors, and the significant heterogeneity between them. Again, further research is needed as a potential effect of prolonged preconception metformin use in preventing GDM in such a population cannot be discounted.
Several different approaches for the identification of women at higher risk were utilised in the included studies for this review. Notably, only one study used a validated tool to identify risk while five studies intervened in women with ≥ 2 GDM risk factors. Our finding that interventions with women who had multiple risk factors reduced GDM prevalence supports the use of multiple risk factor clustering or validated tools to screen for risk of GDM. One such tool for identifying which women living with obesity are at higher risk of GDM development early in pregnancy has been developed [119] and is currently being validated. Better identification of risk amongst women with obesity, most of whom will not develop GDM, should facilitate interventions to be targeted at those most likely to benefit [119].
This review highlights the paucity of interventions targeting higher risk women in the preconception period, mirroring a lack of interventions globally in individuals preparing for pregnancy [120]. Moreover, we found no evidence of benefit of preconception behavioural interventions on the development of GDM in the two identified studies. The current interest in the importance of improving preconception health has stimulated recent attempts [121]. NiPPeR, a double-blind multicentre RCT in healthy women planning pregnancy, examined the effect of a nutritional formulation containing myo-inositol, probiotics, and multiple micronutrients taken preconception and throughout pregnancy, on gestational glycaemia and preterm delivery [17]. Whilst preterm delivery was reduced, there was no effect on the prevalence of GDM [17]. Additionally, NiPPeR did not demonstrate any benefit of the intervention in women with overweight or obesity or those with documented dysglycaemia; however, the trial was not powered to detect differences between subgroups [17]. Appropriately designed RCTs which encompass behavioural, supplementation and pharmacological interventions in high-risk women contemplating pregnancy are needed to evaluate the role of these interventions at the population level.
Strengths and limitations
To date, this study represents the largest review on this subject, with the inclusion of recently published studies targeting higher risk antenatal populations. A robust comprehensive search strategy was utilised using well-defined eligibility criteria. The screening, risk of bias assessment and data extraction were performed independently in duplicate. However, meta-analyses were limited by the quality and methodological variability of studies available. There was considerable variation in criteria for risk stratification and the gestational age at which the intervention was introduced across the trials. The inclusion of studies using different GDM diagnostic criteria may also have contributed to heterogeneity between studies. Due to the limited number of studies in women with multiple risk factors, a subgroup analysis comprising other types of intervention (e.g., diet only, PA only, dietary supplements, preconception interventions) could not be performed. Potential publication bias for combined diet and PA intervention effects was found. Therefore, the interpretation of the findings is limited by the possible bias from selective reporting. Moreover, exclusion of non-English studies may contribute to publication bias.
Recommendations for further research and practice
Further large-scale studies are needed, with higher methodological quality in women with multiple risk factors for GDM to determine if interventions, whether behavioural, dietary supplement or pharmacological, are more effective in reducing GDM and improving other related pregnancy outcomes than unselected population-based approaches or a single risk factor strategy. Future studies in the preconception period should consider risk stratification to identify women who may derive greater benefit. We report here that a variety of strategies were used to identify women at risk of GDM. Validation of groups of risk factors or predictive tools to identify high-risk populations should therefore be considered to harmonise risk assessment and develop effective preventative interventions to improve maternal and infant health in those women who would benefit the most.
Conclusion
This study suggests that identification of women at high-risk of developing GDM in early pregnancy and targeted intervention using combined diet and physical activity, inositol or vitamin D reduces GDM, indicating that a targeted approach provides a promising strategy. The results should be interpreted with caution due to differences in risk stratification strategies, diagnostic criteria for GDM, gestational age for intervention, and in intervention design. Further RCTs using validated prediction tools or multiple risk factors to target high-risk women for interventions before and during pregnancy are required.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files.
Abbreviations
- BMI:
-
Body mass index
- DCI:
-
D-chiro-inositol
- DCI-IPG:
-
D-chiro-inositol-containing inositolphosphoglycan
- FBG:
-
Fasting blood glucose
- GDM:
-
Gestational diabetes mellitus
- HbA1c:
-
Haemoglobin a1c
- LGA:
-
Large-for-gestational age
- OGTT:
-
Oral glucose tolerance test
- PA:
-
Physical activity
- PCOS:
-
Polycystic ovary syndrome
- PIR:
-
Pregestational insulin resistance
- PRISMA:
-
Preferred reporting items for systematic reviews and meta-analyses
- RCTs:
-
Randomised controlled trials
- RoB 2:
-
The Cochrane risk-of-bias tool for randomised trials
- SGA:
-
Small for gestational age
- T2DM:
-
Type 2 diabetes mellitus
References
Wang H, Li N, Chivese T, Werfalli M, Sun H, Yuen L, et al. IDF diabetes atlas: estimation of global and regional gestational diabetes mellitus prevalence for 2021 by international association of diabetes in pregnancy study group’s criteria. Diabetes Res Clin Pract. 2022;183:109050.
Huet J, Beucher G, Rod A, Morello R, Dreyfus M. Joint impact of gestational diabetes and obesity on perinatal outcomes. J Gynecol Obstet Hum Reprod. 2018;47:469–76.
Billionnet C, Mitanchez D, Weill A, Nizard J, Alla F, Hartemann A, et al. Gestational diabetes and adverse perinatal outcomes from 716,152 births in France in 2012. Diabetologia. 2017;60:636–44.
Ye W, Luo C, Huang J, Li C, Liu Z, Liu F. Gestational diabetes mellitus and adverse pregnancy outcomes: systematic review and meta-analysis. BMJ. 2022; 377.
Kramer CK, Campbell S, Retnakaran R. Gestational diabetes and the risk of cardiovascular disease in women: a systematic review and meta-analysis. Diabetol. 2019. https://doi.org/10.1007/s00125-019-4840-2.
Song C, Lyu Y, Li C, Liu P, Li J, Ma RC, et al. Long-term risk of diabetes in women at varying durations after gestational diabetes: a systematic review and meta-analysis with more than 2 million women. Obes Rev. 2018;19:421–9. https://doi.org/10.1111/obr.12645.
Pathirana MM, Lassi ZS, Ali A, Arstall MA, Roberts CT, Andraweera PH. Association between metabolic syndrome and gestational diabetes mellitus in women and their children: a systematic review and meta-analysis. Endocrine. 2020;71:310–20. https://doi.org/10.1007/s12020-020-02492-1.
Lee KW, Ching SM, Ramachandran V, Yee A, Hoo FK, Chia YC, et al. Prevalence and risk factors of gestational diabetes mellitus in Asia: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2018. https://doi.org/10.1186/s12884-018-2131-4.
Huvinen E, Eriksson JG, Stach-Lempinen B, Tiitinen A, Koivusalo SB. Heterogeneity of gestational diabetes (GDM) and challenges in developing a GDM risk score. Acta Diabetol Acta Diabetol. 2018;55:1251–9.
Chiefari E, Arcidiacono B, Foti D, Brunetti A. Gestational diabetes mellitus: an updated overview. J Endocrinol Invest. 2017;40:899–909.
Griffith RJ, Alsweiler J, Moore AE, Brown S, Middleton P, Shepherd E, et al. Interventions to prevent women from developing gestational diabetes mellitus: an overview of cochrane reviews. Cochrane Database Syst Rev. 2020. https://doi.org/10.1002/14651858.CD012394.pub3/full.
Motuhifonua SK, Lin L, Alsweiler J, Crawford TJ, Crowther CA. Antenatal dietary supplementation with myo-inositol for preventing gestational diabetes. Cochrane database Syst Rev. 2023;2:CD011507.
Zhao R, Zhou L, Wang S, Xiong G, Hao L. Association between maternal vitamin D levels and risk of adverse pregnancy outcomes: a systematic review and dose-response meta-analysis. Food Funct. 2022;13:14–37.
Chan KY, Wong MMH, Pang SSH, Lo KKH. Dietary supplementation for gestational diabetes prevention and management: a meta-analysis of randomized controlled trials. Arch Gynecol Obstet. 2021;303:1381–91.
Davidson SJ, Barrett HL, Price SA, Callaway LK, Dekker NM. Probiotics for preventing gestational diabetes. Cochrane database Syst Rev. 2021;4:CD009951.
Barker M, Dombrowski SU, Colbourn T, Fall CHD, Kriznik NM, Lawrence WT, et al. Intervention strategies to improve nutrition and health behaviours before conception. Lancet. 2018;391:1853–64.
Godfrey KM, Barton SJ, El-Heis S, Kenealy T, Nield H, Baker PN, et al. Myo -inositol, probiotics, and micronutrient supplementation from preconception for glycemia in pregnancy: nipper international multicenter double-blind randomized controlled trial. Diabetes Care. 2021;44:1091–9. https://doi.org/10.2337/dc20-2515.
Moher D, Liberati A, Tetzlaff J, Altman DG, Altman D, Antes G, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009. https://doi.org/10.1371/journal.pmed.1000097.
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016. https://doi.org/10.1186/s13643-016-0384-4.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019. https://doi.org/10.1136/bmj.l4898.
Higgins JP, Savovic J, Page MJ, Sterne JA. RDG. Revised Cochrane risk-of-bias tool for randomized trials (RoB 2). 2019. p. 1–72.
Detsky AS, Naylor CD, O’Rourke K, McGeer AJ, L’Abbé KA. Incorporating variations in the quality of individual randomized trials into meta-analysis. J Clin Epidemiol Pergamon. 1992;45:255–65.
Ryan R, Hill S. How to GRADE the quality of the evidence. Cochrane Consum. 2016.
Zhang Y, Wang L, Yang W, Niu D, Li C, Wang L, et al. Effectiveness of low glycemic index diet consultations through a diet glycemic assessment app tool on maternal and neonatal insulin resistance: a randomized controlled trial. JMIR mHealth uHealth. 2019. https://doi.org/10.2196/12081.
Wang C, Wei Y, Zhang X, Zhang Y, Xu Q, Sun Y, et al. A randomized clinical trial of exercise during pregnancy to prevent gestational diabetes mellitus and improve pregnancy outcome in overweight and obese pregnant women. Am J Obstet Gynecol. 2017;216:340–51.
Chiswick C, Reynolds RM, Denison F, Drake AJ, Forbes S, Newby DE, et al. Effect of metformin on maternal and fetal outcomes in obese pregnant women (EMPOWaR): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2015;3:778–86.
Daly N, Farren M, McKeating A, O’Kelly R, Stapleton M, Turner MJ. A medically supervised pregnancy exercise intervention in obese women: a randomized controlled trial. Obstet Gynecol. 2017;130:1001–10.
Okesene-Gafa KAM, Li M, McKinlay CJD, Taylor RS, Rush EC, Wall CR, et al. Effect of antenatal dietary interventions in maternal obesity on pregnancy weight-gain and birthweight: healthy mums and babies (HUMBA) randomized trial. Am J Obstet Gynecol. 2019;221:1521–15213.
Phillips JK, Skelly JM, Roberts LM, Bernstein IM, Higgins ST. Combined financial incentives and behavioral weight management to enhance adherence with gestational weight gain guidelines: a randomized controlled trial. Am J Obstet Gynecol MFM. 2019;1:42–9.
Peccei A, Blake-Lamb T, Rahilly D, Hatoum I, Bryant A. Intensive prenatal nutrition counseling in a community health setting: a randomized controlled trial. Obstet Gynecol. 2017;130:423–32.
Ferrara A, Hedderson MM, Brown SD, Ehrlich SF, Tsai AL, Feng J, et al. A telehealth lifestyle intervention to reduce excess gestational weight gain in pregnant women with overweight or obesity (GLOW): a randomised, parallel-group, controlled trial. Lancet Diabetes Endocrinol. 2020;8:490–500.
Gonzalez-Plaza E, Bellart J, Arranz Á, Luján-Barroso L, Mirasol EC, Seguranyes G. Effectiveness of a step counter smartband and midwife counseling intervention on gestational weight gain and physical activity in pregnant women with obesity (Pas and Pes study): randomized controlled trial. JMIR mHealth uHealth. 2022;10:e28886.
Vinter CA, Jensen DM, Ovesen P, Beck-Nielsen H, Jørgensen JS. The LiP (lifestyle in pregnancy) study: a randomized controlled trial of lifestyle intervention in 360 obese pregnant women. Diabetes Care. 2011;34:2502–7.
Sartorelli DS, Crivellenti LC, Baroni NF, de Andrade Miranda DEG, da Silva SI, Carvalho MR, et al. Effectiveness of a minimally processed food-based nutritional counselling intervention on weight gain in overweight pregnant women: a randomized controlled trial. Eur J Nutr. 2022;62:443–54. https://doi.org/10.1007/s00394-022-02995-9.
Van Horn L, Peaceman A, Kwasny M, Vincent E, Fought A, Josefson J, et al. Dietary approaches to stop hypertension diet and activity to limit gestational weight: maternal offspring metabolics family intervention trial, a technology enhanced randomized trial. Am J Prev Med. 2018;55:603–14.
Callaway LK, McIntyre HD, Barrett HL, Foxcroft K, Tremellen A, Lingwood BE, et al. Probiotics for the prevention of gestational diabetes mellitus in overweight and obese women: findings from the SPRING double-blind randomized controlled trial. Diabetes Care. 2019;42:364–71.
Kennelly MAA, Kate Lindsay KL, O’Sullivan E, Gibney ER, McCarthy M, et al. Pregnancy exercise and nutrition with smartphone application support: a randomized controlled trial. Obstet Gynecol. 2018;131:818–26.
Santamaria A, Di Benedetto A, Petrella E, Pintaudi B, Corrado F, D’Anna R, et al. Myo-inositol may prevent gestational diabetes onset in overweight women: a randomized, controlled trial. J Matern Fetal Neonatal Med. 2016;29:3234–7.
Vitale SG, Corrado F, Caruso S, Di Benedetto A, Giunta L, Cianci A, et al. Myo-inositol supplementation to prevent gestational diabetes in overweight non-obese women: bioelectrical impedance analysis, metabolic aspects, obstetric and neonatal outcomes - a randomized and open-label, placebo-controlled clinical trial. Int J Food Sci Nutr. 2021;72:670–9.
Syngelaki A, Nicolaides KH, Balani J, Hyer S, Akolekar R, Kotecha R, et al. Metformin versus placebo in obese pregnant women without diabetes mellitus. N Engl J Med. 2016;374:434–43.
Brink HS, Alkemade M, van der Lely AJ, van der Linden J. Metformin in women at high risk of gestational diabetes mellitus. Diabetes Metab. 2018;44:300–2.
Basu A, Feng D, Planinic P, Ebersole JL, Lyons TJ, Alexander JM. Dietary blueberry and soluble fiber supplementation reduces risk of gestational diabetes in women with obesity in a randomized controlled trial. J Nutr. 2021. https://doi.org/10.1093/jn/nxaa435.
Renault KM, Nørgaard K, Nilas L, Carlsen EM, Cortes D, Pryds O, et al. The Treatment of Obese Pregnant Women (TOP) study: a randomized controlled trial of the effect of physical activity intervention assessed by pedometer with or without dietary intervention in obese pregnant women. Am J Obstet Gynecol. 2014;210:134.e1-134.e9.
D’Anna R, Di Benedetto A, Scilipoti A, Santamaria A, Interdonato ML, Petrella E, et al. Myo-inositol supplementation for prevention of gestational diabetes in obese pregnant women: a randomized controlled trial. Obstet Gynecol. 2015;126:310–5.
Bisson M, Alméras N, Dufresne SS, Robitaille J, Rhéaume C, Bujold E, et al. A 12-week exercise program for pregnant women with obesity to improve physical activity levels: an open randomised preliminary study. PLoS One. 2015. https://doi.org/10.1371/journal.pone.0137742.
Wolff S, Legarth J, Vangsgaard K, Toubro S, Astrup A. A randomized trial of the effects of dietary counseling on gestational weight gain and glucose metabolism in obese pregnant women. Int J Obes. 2008;32:495–501.
Dodd JM, Louise J, Deussen AR, Grivell RM, Dekker G, McPhee AJ, et al. Effect of metformin in addition to dietary and lifestyle advice for pregnant women who are overweight or obese: the GRoW randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019;7:15–24.
Vesco KK, Karanja N, King JC, Gillman MW, Leo MC, Perrin N, et al. Efficacy of a group-based dietary intervention for limiting gestational weight gain among obese women: a randomized trial. Obesity. 2014;22:1989–96.
Thornton YS, Smarkola C, Kopacz SM, Ishoof SB. Perinatal outcomes in nutritionally monitored obese pregnant women: a randomized clinical trial. J Natl Med Assoc. 2009;101:569–77.
Sales WB, Do Nascimento IB, Dienstmann G, De Souza MLR, Da Silva GD, Silva JC. Effectiveness of metformin in the prevention of gestational diabetes mellitus in obese pregnant women. Rev Bras Ginecol Obstet. 2018;40:180–7.
Poston L, Bell R, Croker H, Flynn AC, Godfrey KM, Goff L, et al. Effect of a behavioural intervention in obese pregnant women (the UPBEAT study): a multicentre, randomised controlled trial. Lancet Diabetes Endocrinol. 2015;3:767–77.
Corcoy R, Mendoza LC, Simmons D, Desoye G, Adelantado JM, Chico A, et al. The DALI vitamin D randomized controlled trial for gestational diabetes mellitus prevention: no major benefit shown besides vitamin D sufficiency. Clin Nutr. 2020;39:976–84.
Bogaerts AFL, Devlieger R, Nuyts E, Witters I, Gyselaers W, Van Den Bergh BRH. Effects of lifestyle intervention in obese pregnant women on gestational weight gain and mental health: a randomized controlled trial. Int J Obes. 2013;37:814–21.
Garnæs KK, Mørkved S, Salvesen Ø, Moholdt T. Exercise Training and weight gain in obese pregnant women: a randomized controlled trial (ETIP Trial). PLOS Med. 2016;13:e1002079. https://doi.org/10.1371/journal.pmed.1002079.
LeBlanc ES, Smith NX, Vesco KK, Paul IM, Stevens VJ. Weight loss prior to pregnancy and subsequent gestational weight gain: prepare, a randomized clinical trial. Am J Obstet Gynecol. 2021;224:99.e1-99.e14.
Phelan S, Jelalian E, Coustan D, Caughey AB, Castorino K, Hagobian T, et al. Randomized controlled trial of prepregnancy lifestyle intervention to reduce recurrence of gestational diabetes mellitus. Am J Obstet Gynecol. 2023. https://doi.org/10.1016/j.ajog.2023.01.037.
McCarthy EA, Walker SP, Ugoni A, Lappas M, Leong O, Shub A. Self-weighing and simple dietary advice for overweight and obese pregnant women to reduce obstetric complications without impact on quality of life: a randomised controlled trial. BJOG. 2016;123:965–73.
Dodd JM, Cramp C, Sui Z, Yelland LN, Deussen AR, Grivell RM, et al. The effects of antenatal dietary and lifestyle advice for women who are overweight or obese on maternal diet and physical activity: the LIMIT randomised trial. BMC Med. 2014;12:1–19. https://doi.org/10.1186/s12916-014-0161-y.
Petrella E, Malavolti M, Bertarini V, Pignatti L, Neri I, Battistini NC, et al. Gestational weight gain in overweight and obese women enrolled in a healthy lifestyle and eating habits program. J Matern Fetal Neonatal Med. 2013;27:1348–52.
Pellonperä O, Mokkala K, Houttu N, Vahlberg T, Koivuniemi E, Tertti K, et al. Efficacy of fish oil and/or probiotic intervention on the incidence of gestational diabetes mellitus in an at-risk group of overweight and obese women: a randomized, placebo-controlled, double-blind clinical trial. Diabetes Care. 2019;42:1009–17.
Liu J, Wilcox S, Wingard E, Turner-McGrievy G, Hutto B, Burgis J. A behavioral lifestyle intervention to limit gestational weight gain in pregnant women with overweight and obesity. Obesity. 2021;29:672–80.
Kong KL, Campbell CG, Foster RC, Peterson AD, Lanningham-Foster L. A pilot walking program promotes moderate-intensity physical activity during pregnancy. Med Sci Sports Exerc. 2014;46:462–71.
Bruno R, Petrella E, Bertarini V, Pedrielli G, Neri I, Facchinetti F. Adherence to a lifestyle programme in overweight/obese pregnant women and effect on gestational diabetes mellitus: a randomized controlled trial. Matern Child Nutr. 2017;13:e12333. https://doi.org/10.1111/mcn.12333.
Seneviratne SN, Jiang Y, Derraik JGB, McCowan LME, Parry GK, Biggs JB, et al. Effects of antenatal exercise in overweight and obese pregnant women on maternal and perinatal outcomes: a randomised controlled trial. BJOG. 2016;123:588–97.
Motahari-Tabari NS, Nasiri-Amiri F, Faramarzi M, Shirvani MA, Bakhtiari A, Omidvar S. The effectiveness of information-motivation-behavioral skills model on self-care practices in early pregnancy to prevent gestational diabetes mellitus in Iranian overweight and obese women: a randomized controlled trial. Int Q Community Health Educ. 2021;43:257.
Gallagher D, Rosenn B, Toro-Ramos T, Paley C, Gidwani S, Horowitz M, et al. Greater neonatal fat-free mass and similar fat mass following a randomized trial to control excess gestational weight gain. Obesity. 2018;26:578.
Ding B, Gou B, Guan H, Wang J, Bi Y, Hong Z. WeChat-assisted dietary and exercise intervention for prevention of gestational diabetes mellitus in overweight/obese pregnant women: a two-arm randomized clinical trial. Arch Gynecol Obstet. 2021;304(609):18. https://doi.org/10.1007/s00404-021-05984-1.
Esmaeilzadeh S, Ghadimi R, Mashayekh-Amiri S, Delavar MA, Basirat Z. The effect of myo-inositol supplementation on the prevention of gestational diabetes in overweight pregnant women: a randomized, double-blind, controlled trial. Minerva Obstet Gynecol. 2023. https://doi.org/10.23736/S2724-606X.22.05036-9.
Eslami E, Mohammad Alizadeh Charandabi S, Khalili AF, Jafarabadi MA, Mirghafourvand M. The effect of a lifestyle-based training package on weight gain and frequency of gestational diabetes in obese and overweight pregnant females. Iran Red Crescent Med J. 2018. https://doi.org/10.5812/ircmj.62576.
Parat S, Nègre V, Baptiste A, Valensi P, Bertrand AM, Chollet C, et al. Prenatal education of overweight or obese pregnant women to prevent childhood overweight (the ETOIG study): an open-label, randomized controlled trial. Int J Obes. 2019;43:362–73.
Herring SJ, Cruice JF, Bennett GG, Rose MZ, Davey A, Foster GD. Preventing excessive gestational weight gain among African American women: a randomized clinical trial. Obesity. 2015;24:30–6.
Callaway LK, Colditz PB, Byrne NM, Lingwood BE, Rowlands IJ, Foxcroft K, et al. Prevention of gestational diabetesfeasibility issues for an exercise intervention in obese pregnant women. Diabetes Care. 2010;33:1457–9.
Zhang DY, Cheng DC, Cao YN, Su Y, Chen L, Liu WY, et al. The effect of dietary fiber supplement on prevention of gestational diabetes mellitus in women with pre-pregnancy overweight/obesity: a randomized controlled trial. Front Pharmacol. 2022;13:3409.
Phelan S, Wing RR, Brannen A, McHugh A, Hagobian TA, Schaffner A, et al. Randomized controlled clinical trial of behavioral lifestyle intervention with partial meal replacement to reduce excessive gestational weight gain. Am J Clin Nutr. 2018;107:183.
Quinlivan JA, Lam LT, Fisher J. A randomised trial of a four-step multidisciplinary approach to the antenatal care of obese pregnant women. Aust N Z J Obstet Gynaecol. 2011;51:141–6.
Simmons D, Devlieger R, Van Assche A, Jans G, Galjaard S, Corcoy R, et al. Effect of physical activity and/or healthy eating on GDM risk: the DALI lifestyle study. J Clin Endocrinol Metab. 2017;102:903–13.
Halkjær SI, De Knegt VE, Lo B, Nilas L, Cortes D, Pedersen AE, et al. Multistrain probiotic increases the gut microbiota diversity in obese pregnant women: results from a randomized, double-blind placebo-controlled study. Curr Dev Nutr. 2020. https://doi.org/10.1093/cdn/nzaa095.
D’Anna R, Scilipoti A, Giordano D, Caruso C, Cannata ML, Interdonato ML, et al. Myo-inositol supplementation and onset of gestational diabetes mellitus in pregnant women with a family history of type 2 diabetes: a prospective, randomized, placebo-controlled study. Diabetes Care Diabetes Care. 2013;36:854–7.
Farren M, Daly N, McKeating A, Kinsley B, Turner MJ, Daly S. The prevention of gestational diabetes mellitus with antenatal oral inositol supplementation: a randomized controlled trial. Diabetes Care. 2017;40:759–63.
Guelfi KJ, Ong MJ, Crisp NA, Fournier PA, Wallman KE, Grove JR, et al. Regular exercise to prevent the recurrence of gestational diabetes mellitus: a randomized controlled trial. Obstet Gynecol. 2016;128:819–27.
Walsh JM, McGowan CA, Mahony R, Foley ME, McAuliffe FM. Low glycaemic index diet in pregnancy to prevent macrosomia (ROLO study): randomised control trial. BMJ. 2012;345:e5605.
Matarrelli B, Vitacolonna E, D’Angelo M, Pavone G, Mattei PA, Liberati M, et al. Effect of dietary myo-inositol supplementation in pregnancy on the incidence of maternal gestational diabetes mellitus and fetal outcomes: a randomized controlled trial. J Matern Neonatal Med. 2013;26:967–72.
Celentano C, Matarrelli B, Pavone G, Vitacolonna E, Mattei PA, Berghella V, et al. The influence of different inositol stereoisomers supplementation in pregnancy on maternal gestational diabetes mellitus and fetal outcomes in high-risk patients: a randomized controlled trial. J Matern Neonatal Med. 2018;33:743–51.
Roeder HA, Moore TR, Wolfson MT, Gamst AC, Ramos GA. Treating hyperglycemia in early pregnancy: a randomized controlled trial. Am J Obstet Gynecol MFM. 2019;1:33–41.
Vanky E, Stridsklev S, Heimstad R, Romundstad P, Skogøy K, Kleggetveit O, et al. Metformin versus placebo from first trimester to delivery in polycystic ovary syndrome: a randomized, controlled multicenter study. J Clin Endocrinol Metab. 2022;95:E448-55.
Vanky E, Salvesen KÅ, Heimstad R, Fougner KJ, Romundstad P, Carlsen SM. Metformin reduces pregnancy complications without affecting androgen levels in pregnant polycystic ovary syndrome women: results of a randomized study. Hum Reprod. 2004;19:1734–40.
Løvvik TS, Carlsen SM, Salvesen Ø, Steffensen B, Bixo M, Gómez-Real F, et al. Use of metformin to treat pregnant women with polycystic ovary syndrome (PregMet2): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019;7:256–66.
Jamal A, Milani F, Al-Yasin A. Evaluation of the effect of metformin and aspirin on utero placental circulation of pregnant women with PCOS. Iran J Reprod Med. 2012;10:265.
Begum MR, Khanam NN, Quadir E, Ferdous J, Begum MS, Khan F, et al. Prevention of gestational diabetes mellitus by continuing metformin therapy throughout pregnancy in women with polycystic ovary syndrome. J Obstet Gynaecol Res. 2009;35:282–6.
Valdés E, Sepúlveda-Martínez A, Candia P, Abusada N, Orellana R, Manukian B, et al. Metformin as a prophylactic treatment of gestational diabetes in pregnant patients with pregestational insulin resistance: a randomized study. J Obstet Gynaecol Res. 2018;44:81–6.
Korpi-Hyövälti EAL, Laaksonen DE, Schwab US, Vanhapiha TH, Vihla KR, Heinonen ST, et al. Feasibility of a lifestyle intervention in early pregnancy to prevent deterioration of glucose tolerance. BMC Public Health. 2011;11:179.
Luoto R, Kinnunen TI, Aittasalo M, Kolu P, Raitanen J, Ojala K, et al. Primary prevention of gestational diabetes mellitus and large-for-gestational-age newborns by lifestyle counseling: a cluster-randomized controlled trial. PLoS Med. 2011. https://doi.org/10.1371/journal.pmed.1001036.
Wang S, Ma J-M, Yang H-X. Lifestyle intervention for gestational diabetes mellitus prevention: a cluster-randomized controlled study. Chronic Dis Transl Med. 2015;1:169–74.
Shahgheibi S, Farhadifar F, Pouya B. The effect of vitamin D supplementation on gestational diabetes in high-risk women: results from a randomized placebo-controlled trial. J Res Med Sci. 2016;21:2.
Lin X, Yang T, Zhang X, Wei W. Lifestyle intervention to prevent gestational diabetes mellitus and adverse maternal outcomes among pregnant women at high risk for gestational diabetes mellitus. J Int Med Res. 2020;48:1–10.
Chan RSM, Tam WH, Ho ICH, Kwan MWC, Li LS, Sea MMM, et al. Randomized trial examining effectiveness of lifestyle intervention in reducing gestational diabetes in high risk Chinese pregnant women in Hong Kong. Sci Rep. 2018. https://doi.org/10.1038/s41598-018-32285-6.
Rakhshani A, Nagarathna R, Mhaskar R, Mhaskar A, Thomas A, Gunasheela S. The effects of yoga in prevention of pregnancy complications in high-risk pregnancies: a randomized controlled trial. Prev Med Prev Med. 2012;55:333–40.
Shahriari A, Karimi E, Shahriari M, Aslani N, Khooshideh M, Arab A. The effect of probiotic supplementation on the risk of gestational diabetes mellitus among high-risk pregnant women: a parallel double-blind, randomized, placebo-controlled clinical trial. Biomed Pharmacother. 2021;141: 111915.
Deng Y, Hou Y, Wu L, Liu Y, Ma L, Yao A. Effects of diet and exercise interventions to prevent gestational diabetes mellitus in pregnant women with high-risk factors in China: a randomized controlled study. Clin Nurs Res. 2021;31:836–47.
Amaefule CE, Drymoussi Z, Gonzalez Carreras FJ, Pardo Llorente MDC, Lanz D, Dodds J, et al. Myo-inositol nutritional supplement for prevention of gestational diabetes (EMmY): a randomised, placebo-controlled, double-blind pilot trial with nested qualitative study. BMJ Open. 2022;12: e050110.
Ajmani SN, Simantini S. Role of vitamin D supplementation in preventing development of gestational diabetes mellitus. An Int J Obstet Gynaecol. 2020. https://doi.org/10.1136/bmjopen-2021-050110.
Koivusalo SB, Rönö K, Klemetti MM, Roine RP, Lindström J, Erkkola M, et al. Gestational diabetes mellitus can be prevented by lifestyle intervention: the Finnish gestational diabetes prevention study (RADIEL): a randomized controlled trial. Diabetes Care. 2016;39:24–30.
Wattar HAIB, Dodds J, Placzek A, Beresford L, Spyreli E, Moore A, et al. Mediterranean-style diet in pregnant women with metabolic risk factors (ESTEEM): A pragmatic multicentre randomised trial. PLOS Med. 2019;16:e1002857.
Oostdam N, Van Poppel MNM, Wouters MGAJ, Eekhoff EMW, Bekedam DJ, Kuchenbecker WKH, et al. No effect of the FitFor2 exercise programme on blood glucose, insulin sensitivity, and birthweight in pregnant women who were overweight and at risk for gestational diabetes: results of a randomised controlled trial. BJOG. 2012;119:1098–107.
Sadiya A, Jakapure V, Shaar G, Adnan R, Tesfa Y. Lifestyle intervention in early pregnancy can prevent gestational diabetes in high-risk pregnant women in the UAE: a randomized controlled trial. BMC Pregnancy Childbirth. 2022;22:1–8. https://doi.org/10.1186/s12884-022-04972-w.
Mohsenzadeh-ledari F, Taghizadeh Z, Keramat A, Moosazadeh M, Yazdani S, Najafi A, et al. The effect of caring intervention (physical activity, diet and counseling) on gestational diabetes for pregnant women with metabolic syndrome. J Matern Neonatal Med. 2020. https://doi.org/10.1080/14767058.2020.1849088.
Harrison CL, Lombard CB, Strauss BJ, Teede HJ. Optimizing healthy gestational weight gain in women at high risk of gestational diabetes: a randomized controlled trial. Obesity. 2013;21:904–9.
Chiswick C, Reynolds RM, Denison F, Drake AJ, Forbes S, Newby DE, et al. Effect of metformin on maternal and fetal outcomes in obese pregnant women (EMPOWaR): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2015;3:778.
Guo X-Y, Shu J, Fu X-H, Chen X-P, Zhang L, Ji M-X, et al. Improving the effectiveness of lifestyle interventions for gestational diabetes prevention: a meta-analysis and meta-regression. BJOG An Int J Obstet Gynaecol. 2019;126:311–20.
Mashayekh-Amiri S, Mohammad-Alizadeh-Charandabi S, Abdolalipour S, Mirghafourvand M. Myo-inositol supplementation for prevention of gestational diabetes mellitus in overweight and obese pregnant women: a systematic review and meta-analysis. Diabetol Metab Syndr. 2022. https://doi.org/10.1186/s13098-022-00862-5.
Pintaudi B, Di Vieste G, Bonomo M. The effectiveness of myo-inositol and d-chiro inositol treatment in type 2 diabetes. Int J Endocrinol. 2016. https://doi.org/10.1155/2016/9132052.
Celentano C, Matarrelli B, Mattei PA, Pavone G, Vitacolonna E, Liberati M. Myo-inositol supplementation to prevent gestational diabetes mellitus. Curr Diab Rep. 2016. https://doi.org/10.1007/s11892-016-0726-6.
Baillargeon J-P, Iuorno MJ, Apridonidze T, Nestler JE. Uncoupling between insulin and release of a D-chiro-inositol-containing inositolphosphoglycan mediator of insulin action in obese women With polycystic ovary syndrome. Metab Syndr Relat Disord United States. 2009;8:127–36.
Asemi Z, Samimi M, Tabassi Z, Shakeri H, Esmaillzadeh A. Vitamin D supplementation affects serum high-sensitivity C-reactive protein, insulin resistance, and biomarkers of oxidative stress in pregnant women. J Nutr United States. 2013;143:1432–8.
Asemi Z, Hashemi T, Karamali M, Samimi M, Esmaillzadeh A. Effects of vitamin D supplementation on glucose metabolism, lipid concentrations, inflammation, and oxidative stress in gestational diabetes: a double-blind randomized controlled clinical trial. Am J Clin Nutr. 2013;98:1425–32.
Soheilykhah S, Mojibian M, Moghadam MJ, Shojaoddiny-Ardekani A. The effect of different doses of vitamin D supplementation on insulin resistance during pregnancy. Gynecol Endocrinol Off J Int Soc. 2013;29:396–9.
Pakmehr A, Ejtahed H-S, Shirzad N, Hemmatabadi M, Farhat S, Larijani B. Preventive effect of probiotics supplementation on occurrence of gestational diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. Front Med. 2022;9:1031915.
Madsen KS, Chi Y, Metzendorf M-I, Richter B, Hemmingsen B. Metformin for prevention or delay of type 2 diabetes mellitus and its associated complications in persons at increased risk for the development of type 2 diabetes mellitus. Cochrane database Syst Rev. 2019;12:CD008558.
White SL, Lawlor DA, Briley AL, Godfrey KM, Nelson SM, Oteng-Ntim E, et al. Early antenatal prediction of gestational diabetes in obese women: development of prediction tools for targeted intervention. PLoS One. 2016;11:e0167846.
Hanson M, Barker M, Dodd JM, Kumanyika S, Norris S, Steegers E, et al. Interventions to prevent maternal obesity before conception, during pregnancy, and post partum. Lancet Diabetes Endocrinol. 2017;5:65–76.
Poston L, Caleyachetty R, Cnattingius S, Corvalán C, Uauy R, Herring S, et al. Preconceptional and maternal obesity: epidemiology and health consequences. Lancet Diabetes Endocrinol. 2016;4:1025–36.
Acknowledgements
OFQ is supported by King Abdulaziz University, Jeddah, Saudi Arabia.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Concept and design: OFQ, ACF and SLW. Literature search: OFQ, KGN, AA, GSC, AP and NV. Quality assessment and data extraction: OFQ & DA. Data analysis and interpreting data: OFQ. Critical discussion and manuscript drafting: OFQ. Revising manuscript: OFQ, ACF, SLW, LP and NH. Manuscript submission: OFQ. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table A1.
Literature search strategy (MEDLINE). Table A2. Literature search strategy (EMBASE). Table A3. Literature search strategy (Cochrane Library). Table A4. Characteristics of the included studies. Table A5. Summary of criteria used for GDM risk stratification and intervention characteristic. Table A6. Pregnancy outcomes. Table A7. Eggers test of publication bias for antenatal interventions. Figure A1. Funnel plots of publication bias for antenatal interventions.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Quotah, O.F., Andreeva, D., Nowak, K.G. et al. Interventions in preconception and pregnant women at risk of gestational diabetes; a systematic review and meta-analysis of randomised controlled trials. Diabetol Metab Syndr 16, 8 (2024). https://doi.org/10.1186/s13098-023-01217-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13098-023-01217-4