Abstract
Noni is a fruit with potential medicinal use preventing elevated blood glucose levels in diabetes mellitus. Its effects have been attributed to an antioxidant property in several other diseases. However, the effects of noni-chronic supplementation on exercise performance in the presence of diabetes conditions are not known. Thirty-two male Wistar rats were used to verify the effects of chronic noni (Morinda citrifolia L) juice administration on glycemia, triglyceride levels, and its relation to physical performance. In addition, it was verified if chronic noni supplementation is safe for clinical use through kidney morphology analysis. In half of the rats, diabetes mellitus (DM) was induced with STZ. All rats were submitted to an incremental workload running test (IWT) until fatigued so that oxygen consumption and performance indexes (exercise time to fatigue and workload) could be analyzed before noni administration. Then, the control and DM groups received a placebo (saline solution) or noni juice (dilution 2:1) at a dose of 2 mL/kg once a day for 60 days. The result was four groups: control + placebo (CP), control + noni (CN), DM + placebo (DMP), and DM + noni (DMN). Our dose was based on in previous study by Nayak et al. (2011) that observed a significant reduction in glycemia with 2 ml/kg of the noni juice without any toxicity effect cited. Groups were then given a third IWT to verify the effect of the noni juice on exercise performance (exercise time to fatigue, workload, maximal oxygen consumption) and glycemia. Twenty-four hours after the third test, all animals were euthanized and blood and kidneys were removed for posterior analysis. The DM induction with STZ impaired the performance by 39%. Noni administration improved the time to fatigue and workload in DM rats beyond reducing hyperglycemia. These results could be associated with an improved energy efficiency promoted by noni ingestion, since the oxygen consumption was not different between the groups, although the exercise was longer in animals with noni ingestion. Our results provided evidence that chronic noni administration causes kidney damage since increased Bowman’s space area in the control rats, suggesting glomerular hyperfiltration at the same magnitude as the non-treated DM group.
In conclusion, chronic noni ingestion promoted glycemic control and improved the performance in DM rats but caused kidney toxicity.
Similar content being viewed by others
Introduction
Diabetes mellitus (DM) is a chronic disease characterized by an absolute or relative deficiency of insulin or its action that promotes hyperglycemia [1, 2]. Controlling blood glucose levels within physiological parameters is fundamental to avoiding complications associated with DM. In general, such control is possible by the administration of exogenous insulin or oral drugs, which reduces hyperglycemia events [3].
In this context, the ingestion of certain fruits and herbs seems to contribute to glycemic control through a hypoglycemic effect in DM people. For example, Morinda citrifolia L. (Rubiaceace), popularly known as noni, is a fruit native to Polynesia, Asia, Australia, and the Brazilian coast and has been extensively associated with preventing elevated blood glucose levels in diabetics. Its medicinal use has been attributed to a potential antioxidant effect (i.e., ascorbic acid and flavonoids) for the treatment of wounds, infections, menstrual and intestinal irregularities, hypertension, and cancer [4]. Specifically, it has been shown that ingestion of fermented noni juice (10x the noni sample amount w/v) reduced blood glucose levels and increased the use of lipids in genetically obese type II diabetes mice [5]. Furthermore, 70% ethanolic extract noni administration in vitro muscle cell (C2C12) cultures induced an increase in adenosine 5’-monophosphate-activated protein kinase (AMPK) pathway activation and GLUT4 translocation with increased glucose uptake [5]. Furthermore, ingestion of fermented noni juice seems to regulate metabolism through the gene expression levels of CPT-1 and PPARα increased which contribute to reducing the level of lipids synthesis and promote fatty acid β-oxidation [6].
Some results have indicated that fermented noni juice promotes lower glycemic levels [5, 7], however, several studies are mentioning the medicinal purposes of using the fruit diluted with water [8, 9]. The way noni is prepared has produced controversial results [8, 10]. In addition, the period of the noni juice supplementation could interfere with these responses and produce toxicity effects. The study by Leal-Silva et al. (2023) verified toxic effects for the liver and reproductive development in the mother and fetal abnormality after ingestion of noni aqueous extract with a dose higher than 400 mg/kg for 21 days. However, in a lower dose of the noni ingestion, any toxicity effect was not verified [11]. Thus, studies are necessary for clinical confirmation of the fruit and verification of any deleterious effects.
In the field of sports science, some studies have shown a similarity between the mechanisms produced by noni administration and exercise, which promotes glucose uptake as a consequence of AMPK-enhanced GLUT4 translocation, which is insulin-independent [12]. Briefly, the contraction muscle activates the increasing of intracellular Ca2 and consequently, translocation of GLUT4 induced by kinase protein dependent on the CA-calmodulin mechanism [12]. In addition, exercise promotes increased activity of the glycolytic pathway and consequently also activates ATP phosphorylation-dependent kinases, such as AMPK, inducing transporter translocation GLUT4 [12]. This mechanism has also already been proposed after noni supplementation, suggesting increasing mitochondrial biogenesis, since there was an increase in UCP3 and PGC1ɑ in muscle [8].
Furthermore, it is suggested that the blood glucose level may influence performance during exercise. However, such a result would conflict with studies that the effects of increased glucose availability on performance showed anticipation [13], delay [14], or no effect on fatigue [15].
Therefore, this study aimed to evaluate the effect of chronic noni juice administration on blood glucose levels and its relation to the physical performance of DM rats. In addition, to determine if chronic intake of noni juice is toxic, an evaluation was conducted using renal analysis.
Materials and methods
Animals
Thirty-two male Wistar rats weighing about 200 g (6 weeks of age) at the beginning of the study were used. They were acquired from the Central Bioterium ICB/UFMG and habituated to a local bioterium over seven days. They were housed in collective polypropylene cages (four rats per cage) under controlled light (0500–1900 h) and temperature (23.5 ± 1.0 °C) conditions with water and rat chow provided ad libitum.
DM induction
Half of the rats (n = 16) were injected intraperitoneally (i.p) with a single dose of streptozotocin (STZ), 60 mg/kg in a 2% solution of 0.1 M citrate buffer. In the Furman (2021) study is described that on the second day after the injection of a high dose of STZ, there is an increase in glycemia with values higher than the control which could reach superior 300 mg/dL values. However, there may still be remnants of pancreatic activity, representing the initial stage of DM1 [16]. In the present study, the experiments were started after one week after streptozotocin-induced diabetes. DM was confirmed by polydipsia, polyuria, and glucose levels greater than 300 mg/dL [17, 18]. The control group (n = 16) was administered saline i.p with the same volume. This procedure did not influence the glycemia of the control rats observed in the control group.
Preparation of noni juice in natural
Noni fruit was obtained from Colatina City, Espírito Santo State in Brazil. Mature noni was washed; the seeds were removed; and the pulp was put into a blender set on pulse mode. The pulp (in grams) was passed through an extra-fine sieve and diluted in 0.5 mL water at a ratio of 2:1.
The bromatological analysis and bioactive compounds of the noni juice were carried out by a commercial laboratory (Hidrocepe Serviços de Qualidade Ltda). The bioactive compounds, phenolics, vitamin C, and flavonoids were quantified by enzymatic gravimetric, gas chromatography, and fluorescence methods, according to industry standards.
Familiarization protocols
After arriving at the laboratory, all rats were introduced to a treadmill designed for small rodents (Modular Treadmill, Columbus Instruments, OH, USA). The familiarization protocol consisted of running on the treadmill for five consecutive days. The rats were encouraged to run by being given light electrical stimulation (0.5 mA, 0.5 mV) from a grid at the rear of the treadmill belt. Each daily session consisted of running at a constant speed (10 m⋅min-1) at an inclination of 5% for 5 min. Over the familiarization days, the speed increased gradually and ended at 15 m⋅min-1. This procedure was designed to teach the rats to run and avoid excessive stress during the tests [19].
In the same period, the rats were also familiarized with gavage, the technique chosen for the noni juice administration. This technique guarantees the ingestion of the correct amount established. This familiarization is also done to avoid stressing the animals during noni juice administration.
Experimental protocol
After the familiarization protocols, the rats were submitted to the incremental workload running test (IWT) until fatigued to measure three performance indexes: maximal oxygen consumption (VO2max), time to fatigue, and workload as performance indexes. Workload was calculated as body weight × exercise intensity × exercise time × treadmill inclination [20]. The workload tests began at a speed of 10 m⋅min-1 (5% inclination) with increments of 1 m⋅min-1 every 3 min until fatigue [21, 22]. Fatigue was defined as the point when the animals were no longer able to keep pace with the treadmill for 10 s [23]. From the result of this test, the rats were divided into four balanced groups to guarantee the homogeneity of the metabolic rate among the groups at the beginning of the study. Two of these groups received STZ i.p for DM induction, while the other two were control groups. The DM group was also treated with special-acting insulin (Humulin NPH®, São Paulo, SP): injections of two international units (UI) in the morning at 8:00 and other two UI in the evening at 6:00 (pilot data) to reduce mortality due to high levels of blood glucose. Control animals received the same volume of isotonic saline solution s.c for the same condition between groups to be guaranteed. Every three days, capillary glycemia was measured by a drop of blood formed from a small cut performed at the end of the tail. Glycemia was measured through the enzymatic analysis with the glucometer (Accu-Check Performa®; Roche Diabetes Care Brasil LTDA, São Paulo, Brazil). This glucometer uses an electrochemical method containing glucose oxidase.
Twenty-four hours after DM confirmation, all rats were submitted to the second IWT to verify its effects on performance and VO2 max. Following each group was divided into rats that received noni juice or a placebo (water) administered by gavage. Noni juice or placebo was administrated at a dose of 2 mL/kg once a day at 9:00 a.m., for 60 days. The groups were denominated as control + placebo (CP); control + noni (CN); DM + placebo (DMP); DM + noni (DMN). The dose was based on in previous study by Nayak et al. (2011) that observed a significant reduction in glycemia with 2 ml/kg of the noni juice.
After the noni administration period, all groups were submitted to a third IWT to verify the effect of noni juice on exercise performance and glycemia. All four groups performed the IWT between 2:00 and 5:00 p.m. to prevent circadian interference on performance or metabolism. After 24 h, all animals were euthanized (Fig. 1).
Euthanasia
Six hours before the euthanasia, chow was removed from the cages, and the animals were left to fast. At approximately 8:00 a.m., the animals were euthanized by decapitation. The blood from the trunk was collected for fasting analysis and the tissues were removed. Kidney samples from euthanized rats were obtained and processed for histopathological evaluation.
Histopathological analysis
Kidney samples were fixed in 10% buffered formalin for 24 h and embedded in paraffin for tissue sectioning (5 μm thickness). The sections were stained with hematoxylin and eosin (H&E) and evaluated under a microscope (BX53, Olympus Latin America Inc.) adapted to a microcamera (Q-Color3, Olympus Latin America Inc., SP, Brazil).
For the histopathological analysis measurements, the H&E sections images were digitized using a color video camera attached to a microscope. After digitalization, Bowman’s capsule and the glomerular tuft were traced and their areas were calculated using image analysis software (ImageJ). The area of Bowman’s space was determined by the difference between the area of the glomerular tuft and Bowman’s capsule [24]. Fifty glomeruli were measured in each histological sample from each animal. The H&E sections were examined and scored by an observer in a blinded manner for the described parameters.
Statistical analysis
The data were reported as mean ± SEM. The normality and homoscedasticity of data distribution were verified using the Ryan–Joiner and Levene test. The differences among the groups were evaluated by one-way analysis of variance (ANOVA). To evaluate groups and time points, a two-way analysis of variance (ANOVA) followed by Student–Newman–Keuls tests was conducted. The effect size (ES), measured on the Cohen’s d scale was considered for the analysis of data having a coefficient of variation above 30%. ES values were considered trivial (< 0.2), small (0.2–0.5), medium (0.5–0.8), or large (≥ 0.8). Correlations were assessed using Pearson’s coefficient.
All statistical analyses were performed using the SigmaPlot software (version 11.0; SYSTAT software, Bangalore, Karnataka, India), adopting a significance level of α = 5% (p < 0.05).
Results
Results of bromatological analysis
The results of the bromatological and bioactive compound analyses of noni juice are demonstrated in Table 1. Data indicated the presence of antioxidant and anti-inflammatory compounds such as flavonoids (0.017 g/100 mL), phenolic compounds (0.95 g/100 mL), and vitamin C (50.989 mg/100 g).
IWT until fatigue before DM induction
Body weight, performance, and metabolic indexes were measured during the first IWT. Values indicated that body weight was not different among groups (p = 0.70). In addition, time to fatigue, maximal velocity, workload, and maximal oxygen consumption did not differ among the groups (p > 0.05 for all indexes; Table 2). Data indicated that groups were divided in a balanced way, with neither variable had shown differences among them (Table 2).
Effect of DM induction on IWT
The IWT induced a progressive increase in VO2 in both DMP and DMN groups from the beginning of the exercise (p < 0.001). As illustrated in Fig. 2, DM induced a marked decrease in VO2max in both groups DMP (64.3 ± 4.0 °C, 1° IWT vs. 59.9 ± 4.1 °C, 2° IWT; p < 0.001, Fig. 2A) and DMN (67.5 ± 2.8 °C, 1º IWT vs. 58.7 ± 2.9 °C, 2° IWT; p < 0.001, Fig. 2B). In addition, DM induction reduced by 39% the time to fatigue from the first IWT to the second in the DMP and DMN groups (Figs. 2A and 2Bp < 0.001).
Effect of diabetes induction on exercise time and VO2max in DMP (Panel A) and DMN (Panel B) rats. White and black symbols represent the values measured in 1° IWT (before DM induction) and 2° IWT (after DM induction), respectively. Data were expressed as mean ± SEM. Significant differences were considered if p < 0.05. * represents a difference from the beginning of the exercise. (n = 8 in each group). # represents a difference between groups
Effect of chronic noni juice ingestion on performance indexes
As a result of the third IWT, the performance of the control groups CP and CN was lower performance than that of the second. This result may have been related to the weight gain and growth of the rats since a body mass of approximately 400 g was observed. Thus, to compensate for the body mass effect, the workload of the animals was calculated (Table 3).
The DMN group recorded the highest values for the third IWT after noni ingestion compared to the second (Table 3). This improved performance could be also observed through the exercise time (Fig. 3B) and exercise time variation between after and before noni ingestion (DMP − 2.1 ± 5.0 min vs. DMN 2.6 ± 3.6 min; p = 0.04, Cohen d = 0.53).
Effect of chronic noni (Morinda citrifolia L) administration on exercise time until fatigue and VO2max in diabetic rats. Black and gray symbols represent the values measured in the IWT test before and after the 60 days of noni administration, respectively. DMP: Rats with diabetes that were supplemented with placebo. DMN: Rats with diabetes that were supplemented with noni juice. Data were expressed as mean ± SEM. Significant differences were considered if p < 0.05. * represents a difference from the beginning of the exercise. (n = 8 in each group)
Since performance parameters improved after chronic noni administration, the energetic efficiency was analyzed by oxygen consumption as a function of the exercise time percentage. As illustrated in Fig. 4, oxygen consumption between groups DMP and DMN showed no difference, indicating that noni increased energy efficiency over the same time of exercise.
Effect of chronic noni (Morinda citrifolia L) administration on exercise time until fatigue and VO2 max in diabetic rats. Black and gray symbols represent the values measured in IWT test before and after the 60 days of the noni administration, respectively. DMP: Rats with diabetes that were supplemented with placebo. DMN: Rats with diabetes that were supplemented with noni juice. Data were expressed as mean ± SEM. Significant differences were considered if p < 0.05. * represents a difference from the beginning of the exercise. (n = 8 in each group)
Effect of chronic noni juice ingestion on glucose and triglycerides blood concentration
Chronic noni administration reduced the mean blood glucose calculated by the values obtained during the 60 days (large effect size Cohen’s d = 0.86, Fig. 5A). This result may imply improved glycated hemoglobin values (DMC 13.2% vs. DMN 10.9%).
The diabetic rats showed increased triglyceridemia compared to the control group (CP 147.6 ± 5.5 mg/dL vs. DMP 228.9 ± 21.5 mg/dL, p < 0.001, Fig. 5B). Triglyceride concentrations were determined by enzymatic colorimetric assays (Lab Test® Diagnostic S.A., Lagoa Santa, Brazil). After diabetic rats were supplemented with noni triglycerides, the values returned to the control condition and were lowered compared to those of the DMP group (DMP 228.9 ± 21.5 mg/dL vs. DMN 171.2 ± 14.2 mg/dL, p = 0.04).
From triglycerides and glucose concentration was calculated TYG index. The TYG index was calculated according to the following formula [Ln (fasting triglycerides (mg/dl) X fasting glucose (mg/dl)]/2; Ln is the neperian logarithm [25, 26]. The TYG index of DMP was higher than CP (CP 8.95 ± 0.06 vs. DMP 10.56 ± 0.16, p < 0.001). Rats that received noni supplementation attenuated the insulin resistance evaluated by TYG index, with a cutoff point of 7.88 was used for insulin resistance risk screening (DMP 10.56 ± 0.16 vs. DMN 9.97 ± 0.21, p = 0.04).
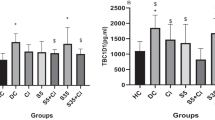
Effect of chronic noni juice ingestion on kidney morphology
In Fig. 6, noni ingestion produced an important increase in Bowman’s space area in the control rats, suggesting glomerular hyperfiltration (CP 0.60 ± 0.02 × 106 µm2 vs. CN 1.48 ± 0.08 × 106 µm2 ; p < 0.001). However, in diabetic rats, the noni supplementation did not produce additional impairment compared to CN (CN 1.48 ± 0.08 × 106 µm2 vs. DMP 1.70 ± 0.02 × 106 µm2 vs. DMN 1.98 ± 0.08 × 106 µm2).
Photomicrography of kidney tissue of the control and diabetic rats after chronic placebo or noni (Morinda citrifolia L) administration. (CP: control + placebo; CN: control + noni; DMP: DM + placebo; DMN: DM + noni). Sections stained with H&E; Arrowhead indicates Bowman’s Space in groups with structural alterations. 10x increase in Microscope Nikon
Discussion
The present study showed that chronic noni administration in rats induced an improved mechanical efficiency associated with greater exercise time until fatigue. In addition, noni supplementation produced an antihyperglycemic effect and attenuated insulin resistance in DM rats. However, noni shows kidney toxicity through the increased Bowman’s Space, which can indicate their use is not safe in clinical or nutritional applications.
Comparable to our results, other studies have already demonstrated an ergogenic effect after noni administration [8, 27, 28]. Shalan et al. [8] observed that four weeks of noni supplementation tripled the swimming effort in rats. This increase in exercise performance was attributed to the peripheral and also to central effects induced by noni.
Centrally, our results demonstrated a reduced relative effort perception in DMN compared to the DMP group (Fig. 4). This fact could be evidenced by similar oxygen consumption values between groups when analyzed at the same relative performance, which was expressed as a percentage of running time. It is worth mentioning that both the time of exercise and workload (Table 3) were higher in the DMN rats. These data suggest a change in the central modulation that coordinates the motor drive and consequently induces delayed fatigue [29, 30].
Changes in the turnover of neuronal systems could play an important role in the peripheral adaptations associated with the development of fatigue. The Shalan et al. [8] study verified alterations to the central neurotransmitter systems, such as serotonin (5-HT) and dopamine (DA) receptors and transporters, associated with fatigue development in rats with noni supplementation. Furthermore, it has already been shown that peripheral signaling integrated with the central brain areas could modify the effort perception and consequently delay the end of the running time [31]. The neuronal 5-HT and DA system profile during exercise could change the running time until fatigue as a result of modifications to the lethargy, rating of perceived exertion, and motivation, which interfered with central brain signaling to the active musculature [32, 33]. It is important to point out that the experiment in this study was not designed to verify central fatigue. However, the observed peripheral effects could be attributed to both the drive from the central areas and feedback signaling changes.
Peripherally, the results of this study, such as increased energy efficiency in the DMN group (Fig. 4) could have been related to a direct action on central homeostasis neuromodulators, such as 5-HT and DA, contributing to energetic control to exercise but also a feedback from different muscle and metabolic conditions after noni supplementation. In addition, the DMN group showed a reduced hyperglycemic effect compared to the DMP group (Fig. 5). This result had already been reported by Osman et al. [28] and Shalan et al. [8] during swim exercise protocols and was thought to be the cause of an observed ergogenic effect. It is interesting to note that this study is the first to relate the effects of noni supplementation in rats with diabetes during controlled-intensity exercise performed according to a running protocol until fatigue.
Wang et al. [7] reported improvements in carbohydrate and lipid metabolism via the AMPK pathway in rats supplemented with noni. With specific regard to glucose metabolism, it had already been demonstrated an improved insulin receptor sensitivity beyond an increase in glycogen stores. It has been suggested that noni improves glycogen stores either by increasing glycogen storage, delaying glycogen consumption during exercise, or both [28]. These data contribute to blood glucose disappearing, probably through the improvement the carbohydrate muscle and liver uptake.
In the present study, the improved glucose metabolism in DMN rats could have been supported by a reduction in triglyceride plasma concentrations, which could indicate higher lipid oxidation (Fig. 5). This possibility is suggested by the reduction in the relative effort perception in the DMN group associated with a change in substrate use (Fig. 5). Data from Zhang et al. (2020) support that lipid metabolism was improved with noni supplementation accompanied by the increased expression of CPT-1 and PPARα, changing the fatty acid β oxidation [6].
In addition, some studies have associated the ergogenic and metabolic effects of noni administration with the influence of antioxidant compounds such as phenols and flavonoids (e.g., epicatechin and catechin) [7, 34, 35]. During exercise, there is an increase in the production of oxidative molecules, which may function as intracellular messengers in several physiological processes [36]. On the other hand, evidence shows that highly oxidative molecule concentrations represent possible toxicity and damage to the cell that could interfere with the excitation–contraction muscle process and consequently performance [37, 38]. The phenolic compounds and flavonoids present in noni have antioxidant and anti-inflammatory characteristics that may contribute to reducing the oxidative status promoting the effects observed on glucose and triglycerides metabolism, beyond the performance in DM rats with noni supplementation. Furthermore, it is suggested that the antioxidant and anti-inflammatory effects induced by noni supplementation may have contributed to the increased glucose uptake and consequent reduction in insulin resistance through pathways activation to GLUT4 translocation such as CA-calmodulin and AMPK [5]. In the present study, rats with noni supplementation exhibited reduced insulin resistance, calculated by the TYR index. However, this reduction did not reach similar values to the CP.
Although noni supplementation has increased performance and energy efficiency beyond having lowered glycemia and triglyceridemia, it appears to have a potentially toxicity effect on the kidneys as demonstrated by morphological analysis (Fig. 6). The increase in the Bowman’s space area in rats with noni supplementation (CN and DMN) suggested glomerular hyperfiltration. Our data also demonstrated an increase in Bowman’s space with the same magnitude of diabetic impairment in the control group that ingested noni. In the case of poorly controlled diabetes, there may be glycosuria and proteinuria as results of the deleterious inflammatory effect resulting from the reactive oxygen species formation, especially superoxide anion, which react with the glomerular matrix increasing the membrane permeability and consequent hyperfiltration.
Souza et al. [39] did not observe functional or histological disturbances in the kidneys or liver after nine days of noni juice consumption. Stands out that the juice dilution was lower compared to those mentioned in our study (1:10 for the Sousa et al. vs. 2:1 present study). In addition, the period of noni juice consumption in our study was extended (60 days), which may have contributed to the effect on the kidneys.
These impair the function of cycloxygenases and hydroelectrolytics, which then inhibit prostaglandin synthesis leading to chronic renal failure [40]. In addition, the potassium content present in noni can negatively impact kidney function in patients with renal failure (Mueller, 2000).
In addition, a other possible mechanism for kidney dysfunction is the hepatic changes induced by hepatotoxic components like as anthraquinones found in noni, which reduces anti-inflammatory molecules and initiate the lipid peroxidation [40]. In theory, noni could affect the kidneys from the liver since the organs are closely and are related in terms of metabolism and elimination of toxic substances.
However, it is crucial to understand that the effects of the bioactive compounds can vary depending on several factors, including dose, bioavailability and form of consumption.
Conclusion
In the present study, chronic noni administration contributed to attenuating the hyperglycemia and avoiding the DM effects on performance during exercise until fatigue. However, the use of noni as a clinical and nutritional strategy in the treatment of DM must be understood with caution, since renal morphological change was observed.
Limitation of the study
The results of the present study demonstrate glycemic and metabolic improvement with noni ingestion under conditions of experimental DM. This result cannot be directly transposed to humans. Thus further studies, including in humans, are needed for the use of noni as a nutritional strategy in glycemic control. Further, DM experimental induction does not promote similar whole organic conditions as compared to the natural disease induction, representing a limitation of the studies.
Data Availability
The datasets analyzed in this study are available from the corresponding author (juliana.guimaraes@uemg.br) upon reasonable request.
References
American Diabetes Association. : Standards of Medical Care in Diabetes—2023 Diabetes Care, 46(january) (2023).
van Belle TL, Coppieters KT, von Herrath MG. Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiol Rev. 2011;91(1):79–118. https://doi.org/10.1152/physrev.00003.2010
American Diabetes Association. The official pocket guide to diabetic food choices. Arlington, VA: American Diabetes Association; 2020.
Wang MY, West BJ, Jensen CJ, Nowicki D, Su C, Palu AK, Anderson G. Morinda citrifolia (Noni): a literature review and recent advances in Noni research. Acta Pharmacol Sin. 2002;23(12):1127–41.
Lee SY, Park SL, Hwang JT, Yi SH, Nam YD, Lim SI. Antidiabetic Effect of Morinda citrifolia (Noni) Fermented by Cheonggukjang in KK-A(y) Diabetic Mice. Evidence-based complementary and alternative medicine: eCAM, 2012, 163280 (2012) https://doi.org/10.1155/2012/163280
Zhang KM, Li J, Tang X, Ma X. Shuhua; Lv,Yipin; Yang, Shaojun Noni (Morinda citrifolia L.) wine prevents the oxidative stress and obesity in mice induced by high-fat diet. J Food Biochem (e13460) (2020).
Wang R, Zhang L, Zhang Q, Zhang J, Liu S, Li C, Wang L. Glycolipid Metabolism and Metagenomic Analysis of the therapeutic effect of a Phenolics-Rich Extract from Noni Fruit on type 2 Diabetic mice. J Agric Food Chem. 2022;70(9):2876–88. https://doi.org/10.1021/acs.jafc.1c07441
Shalan NAAMM, Mohamed NM. Suhaila Morinda citrifolia leaf enhanced performance by improving angiogenesis, mitochondrial biogenesis, antioxidant, anti-inflammatory & stress responses. Food Chem. 2016;212:443–52.
Yang XCLSCWLJTXW. Therapeutic effects of noni fruit water extract and polysaccharide on oxidative stress and inflammation in mice under high-fat diet. Food Funct, 1 (2020).
Nayak BS, Marshall JR, Isitor G, Adogwa A. Hypoglycemic and Hepatoprotective Activity of Fermented Fruit Juice of Morinda citrifolia (Noni) in Diabetic Rats. Evidence-based complementary and alternative medicine: eCAM, 2011, 875293 (2011) https://doi.org/10.1155/2011/875293
Leal-Silva T, Souza MR, Cruz LL, Moraes-Souza RQ, Paula VG, Soares TS, Dela Justina V, Giachini FR, Damasceno DC, Americo MF, Volpato GT. Toxicological effects of the Morinda citrifolia L. fruit extract on maternal reproduction and fetal development in rats. Drug Chem Toxicol. 2023;46(3):609–15. https://doi.org/10.1080/01480545.2022.2070197
Rose AJ, Richter EA. Skeletal muscle glucose uptake during exercise: how is it regulated? Physiology. 2005;20:260–70. https://doi.org/10.1152/physiol.00012.2005
Marliss EB, Vranic M. Intense exercise has unique effects on both insulin release and its roles in glucoregulation: implications for diabetes. Diabetes. 2002;51(Suppl 1):271–83. https://doi.org/10.2337/diabetes.51.2007.s271
Coyle EF, Hagberg JM, Hurley BF, Martin WH, Ehsani AA, Holloszy JO. Carbohydrate feeding during prolonged strenuous exercise can delay fatigue. J Appl Physiol Respir Environ Exerc Physiol. 1983;55(1 Pt 1):230–5. https://doi.org/10.1152/jappl.1983.55.1.230
Coyle EF, Hamilton MT, Alonso JG, Montain SJ, Ivy JL. Carbohydrate metabolism during intense exercise when hyperglycemic. J Appl Physiol (1985). 1991;70(2):834–40. https://doi.org/10.1152/jappl.1991.70.2.834
Furman BL. Streptozotocin-induced diabetic models in mice and rats. Curr Protocols. 2021;1. https://doi.org/10.1002/cpz1.78
Reisi P, Babri S, Alaei H, Sharifi MR, Mohaddes G, Noorbakhsh SM, Lashgari R. Treadmill running improves long-term potentiation (LTP) defects in streptozotocin-induced diabetes at dentate gyrus in rats. Pathophysiology. 2010;17(1):33–8. https://doi.org/10.1016/j.pathophys.2009.06.001
Ghanbari EN, Khazaei V. Mozafar Improvement in serum biochemical alterations and oxidative stress of liver and pancreas following use of royal jelly in streptozotocin-induced diabetic rats. Cell J. 2016;18(3):362–70.
Guimaraes JB, Wanner SP, Machado SC, Lima MR, Cordeiro LM, Pires W, La Guardia RB, Silami-Garcia E, Rodrigues LO, Lima NR. Fatigue is mediated by cholinoceptors within the ventromedial hypothalamus independent of changes in core temperature. Scand J Med Sci Sports. 2013;23(1):46–56. https://doi.org/10.1111/j.1600-0838.2011.01350.x
Brooks GA, Donovan CM, White TP. Estimation of anaerobic energy production and efficiency in rats during exercise. J Appl Physiol Respir Environ Exerc Physiol. 1984;56(2):520–5. https://doi.org/10.1152/jappl.1984.56.2.520
Santiago HPL, Laura HR, Lima, Paulo Marcelo A, Rodovalho GV, Szawka, Raphael E, Coimbra CândidoC. The improvement of exercise performance by physical training is related to increased hypothalamic neuronal activation. Clin Exp Pharmacol Physiol, 43 (2015).
de Barcellos LAM, Goncalves WA, Esteves de Oliveira MP, Guimaraes JB, Queiroz-Junior CM, de Resende CB, Russo RC, Coimbra CC, Silva AN, Teixeira MM. Rezende and V. Pinho: Effect of Physical Training on Exercise-Induced inflammation and performance in mice. Front Cell Dev Biol. 2021;9:625680. https://doi.org/10.3389/fcell.2021.625680
Fonseca CG, Pires W, Lima MR, Guimaraes JB, Lima NR, Wanner SP. Hypothalamic temperature of rats subjected to treadmill running in a cold environment. PLoS ONE. 2014;9(11):e111501. https://doi.org/10.1371/journal.pone.0111501
Kashif AW, Verma N, Verma S, Boruah D, Sahu R, Kalra S, Malik A. Utility of glomerular morphometry in diagnosing pediatric renal disease. Med J Armed Forces India. 2021;77(2):194–9. https://doi.org/10.1016/j.mjafi.2020.08.007
Guerrero-Romero FS-M, Gonza´ lez-Ortiz L, Martínez-Abundis M, Ramos-Zavala E, Jacques-Camarena MaríaG. Omar; Rodríguez-Mora´ n, Martha The Product of Triglycerides and Glucose, a Simple Measure of Insulin Sensitivity. Comparison with the Euglycemic-Hyperinsulinemic Clamp. J Clin Endocrinol Metab, 95 (7), 3347–3351 (2010).
Lopes WO, Locateli GH, Simões JC. TyG na predic¸ão da resistência à insulina. J Pediatr. 2020;96:132–3.
Abdul Majid N, Abdul Hamid A, Salleh SZ, Saari N, Abas F, Pak Dek MS, Ramli NS, Jaafar AH. Metabolomics approach to investigate the ergogenic effect of Morinda citrifolia L. leaf extract on obese Sprague Dawley rats. Phytochem Anal. 2020;31(2):191–203. https://doi.org/10.1002/pca.2880
Osman WNW, Mohamed S. Standardized Morinda citrifolia L. and Morinda elliptica L. leaf extracts alleviated fatigue by improving glycogen storage and lipid/carbohydrate metabolism. Phytother Res. 2018;32(10):2078–85. https://doi.org/10.1002/ptr.6151
Kayser B. Exercise starts and ends in the brain. Eur J Appl Physiol. 2003;90(3–4):411–9. https://doi.org/10.1007/s00421-003-0902-7
Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81(4):1725–89. https://doi.org/10.1152/physrev.2001.81.4.1725
Meeusen R, Roelands B. Fatigue: is it all neurochemistry? Eur J Sport Sci. 2018;18(1):37–46. https://doi.org/10.1080/17461391.2017.1296890
Balthazar CH, Leite LH, Rodrigues AG, Coimbra CC. Performance-enhancing and thermoregulatory effects of intracerebroventricular dopamine in running rats. Pharmacol Biochem Behav. 2009;93(4):465–9. https://doi.org/10.1016/j.pbb.2009.06.009
Soares DD, Coimbra CC, Marubayashi U. Tryptophan-induced central fatigue in exercising rats is related to serotonin content in preoptic area. Neurosci Lett. 2007;415(3):274–8. https://doi.org/10.1016/j.neulet.2007.01.035
Salleh SZ, Hamid AA, Jaafar AH, Abdul Majid ND, Saari N, Halim HH, Ismail A, Abdul Razis AF, Ramli NS. Pak dek: ergogenic property of Morinda citrifolia L. leaf extract affects energy metabolism in obese Sprague Dawley rats. J Food Biochem. 2022;46(1). https://doi.org/10.1111/jfbc.14027. e14027.
Lohani M, Majrashi M, Govindarajulu M, Patel M, Ramesh S, Bhattacharya D, Joshi S, Fadan M, Nadar R, Darien B, Maurice DV, Kemppainen B, Dhanasekaran M. Immunomodulatory actions of a polynesian herb Noni (Morinda citrifolia) and its clinical applications. Complement Ther Med. 2019;47:102206. https://doi.org/10.1016/j.ctim.2019.102206
Niess AM, Simon P. Response and adaptation of skeletal muscle to exercise–the role of reactive oxygen species. Front Biosci. 2007;12:4826–38. https://doi.org/10.2741/2431
Vollaard NB, Shearman JP, Cooper CE. Exercise-induced oxidative stress:myths, realities and physiological relevance. Sports Med. 2005;35(12):1045–62. https://doi.org/10.2165/00007256-200535120-00004
Powers SK, Talbert EE, Adhihetty PJ. Reactive oxygen and nitrogen species as intracellular signals in skeletal muscle. J Physiol. 2011;589(9):2129–38. https://doi.org/10.1113/jphysiol.2010.201327
Sousa BCdM, Rodrigues CB, Machado WF, Silva JR, Marcos Vinicius da; Costa, Thiago Alvares da; Lazo-Chica, Javier Emilio; Degasperi,Thatiane do Prado;, Sales-Campos H, Bucek EU. ; Oliveira, Carlo José Freire Effects of short-term consumption of Morinda citrifolia (Noni) fruit juice on mice intestine, liver and kidney immune modulation. Food and Agricultural Immunology, 28(3) (2017).
Stadlbauer V, Fickert P, Lackner C, Schmerlaib J, Krisper P, Trauner M, Stauber RE. Hepatotoxicity of NONI juice: report of two cases. World J Gastroenterol. 2005;11(30):4758–60. https://doi.org/10.3748/wjg.v11.i30.4758
Acknowledgements
The authors thank the Exercise Physiology Laboratory at the Federal University of Minas Gerais represented by Ph.D. Danusa Dias Soares and Ph.D. Samuel Penna Wanner for their assistance in the whole study.
Funding
This work was financed and supported by the FAPEMIG – Fundação de Amparo à Pesquisa do Estado de Minas Gerais (CDS - APQ-03450-16 and CBB - BIP-00346-17). Furthermore, this study received contributions and funding (FAPEMIG - CDS - APQ-03546-15 from Brazil) by Ph.D. Danusa Dias Soares. In addition, the authors are thankful for support from PIBIC/UEMG/FAPEMIG and PROPPG/UEMG (Universidade do Estado de Minas Gerais).
Author information
Authors and Affiliations
Contributions
DOF; FGC; JSFB; KJS; JBG collected data with the animals; BPM; MTA; DDS performed additional experiments with diabetic rats that support some methodological choices; MCFC; LAMB; DDS; JBG experiments idealization and academic and technical support; DOF; MVC; LAMB; JBG statistical and graphical analysis; DOF; LAMB; JBG wrote the main manuscript text; all authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethical approval
All experimental procedures were approved by the Ethics Committee of the Federal University of Minas Gerais for the Care and Use of Laboratory Animals and were conducted following the regulations described in the Committee’s Guiding Principles Manual (protocol Nº 109/2016).
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
de Oliveira Fernandes, D., César, F.G., Melo, B.P. et al. Chronic supplementation of noni in diabetic type 1-STZ rats: effects on glycemic levels, kidney toxicity and exercise performance. Diabetol Metab Syndr 15, 191 (2023). https://doi.org/10.1186/s13098-023-01171-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13098-023-01171-1