Abstract
Background
During Ramadan fasting, postprandial hyperglycemia is commonly observed after iftar (break of fast at sunset) meal. d-allulose is a rare sugar and is reported to have several health benefits, including the suppression of increase in postprandial glucose levels. This study investigates whether d-allulose (a C-3 epimer of d-fructose) improves the postprandial glucose in patients with type 2 diabetes mellitus (T2DM) during Ramadan.
Methods
This was a pilot, prospective single-arm study design that was conducted for 10 consecutive days; 5 days of control and 5 days of consumption. The primary outcome was postprandial peak glucose levels. During the consumption period, 8.5 g of d-allulose was consumed by the participants before iftar meal. Postprandial glucose was measured using a continuous glucose monitoring system.
Results
A total of 12 participants completed the study. Significant lower (p < 0.01) postprandial glucose values and the glucose incremental area under the curve (iAUC) were observed from 0 to 180 min during the consumption period compared to the control period. The consumption period demonstrated significantly higher percentages of time in which glucose values were found in the target range (p = 0.0032), and when the glucose levels above the target range were reduced (p = 0.0015).
Conclusions
The supplementation with d-allulose has the potential to improve postprandial hyperglycemia in patients with T2DM after iftar during Ramadan. Further studies are needed to confirm these findings.
Trial registration ClinicalTrials.gov NCT05071950. Retrospectively registered, 8 October 2021.
Similar content being viewed by others
Background
Reports from the International Diabetes Federation revealed that about 451 million people worldwide had diabetes in 2017, and the number is projected to increase to 693 million by 2045 [1]. Approximately 90 million Muslims worldwide have diabetes [2]. In Malaysia, the prevalence of diabetes is estimated as 18.3% affecting 3.9 million adults aged 18 years and above. The prevalence of diabetes increased rapidly from 13.4% in 2015 to 18.3% in 2019 [3]. Obesity due to higher intake of sweetened foods, carbohydrates, and sedentary lifestyles was revealed as the main factor of type 2 diabetes mellitus (T2DM) in Malaysia [4, 5].
Fasting during the month of Ramadan (the ninth month of the Islamic Calendar) is one of the five pillars of Islam. During Ramadan, all healthy Muslims are required to fast for a whole month from dawn to sunset. Fasting durations vary by geographical area [6], and in Malaysia, the duration is approximately 14 hours. Despite Ramadan fasting having several benefits [7, 8], fasting in individuals with uncontrolled diabetes increased the risk of hypoglycemia, hyperglycemia [9], diabetic ketoacidosis, and thrombosis due to dehydration [10, 11]. Although the holy Quran has explicitly exempted sick people from this obligation [12], many Muslim diabetic patients choose to fast despite the associated risks [10, 13, 14].
One of the underlining events leading to the onset of diabetes, especially in healthy individuals, is postprandial hyperglycemia. Postprandial hyperglycemia refers to a sudden increase in blood glucose levels above 140 mg/dL (7.8 mmol/L) within the first two hours after a meal [15]. It is a well-established risk factor for the onset and progression of vascular complications among diabetes patients. Postprandial hyperglycemia is usually observed during Ramadan, especially after iftar (break of fast at sunset) [16]. Despite the lower eating frequency during Ramadan, energy intake remains unpredictable and differs between individuals in line with dietary practices which are influenced by economic status, and dietary behaviors [16]. Ramadan is recognized as a month of feasting in several Muslim societies, including Malaysia [17]. A sudden rise in blood glucose levels occurs after iftar given that the meals are typically carbohydrate-rich, high-calorie, and usually sweet food. Additionally, most individuals consume more calories during iftar than suhoor (pre-dawn meal) and engage in less physical activities at night [16]. These events make it difficult for diabetes patients to effectively control and manage their blood glucose levels.
A potential solution to these issues may be d-allulose (a C-3 epimer of d-fructose) that is found in small quantities in nature. d-allulose is a monosaccharide, a rare sugar type with 70% the sweetness of sucrose and has zero calorie. Extensive basic and clinical studies [18,19,20] have reported beneficial outcomes from d-allulose for human health, including improved hypoglycemia, reduced postprandial hyperglycemia [19, 21] hypolipidemia, and antioxidant activities [21]. Based on the reports by the U.S. Food and Drug Administration (FDA), d-allulose is well-known as safe (GRAS) for use as a food ingredient and with other sweeteners [22]. Consumption of d-allulose led to significant glucose suppressive effects in healthy individuals after meals [18, 23, 24]. Similar findings were detected in pre-diabetes patients following the administration of d-allulose [19]. However, there is limited information and data paucity regarding the effects of d-allulose in diabetic patients. This study is the first attempt to elucidate the effects of d-allulose in response to normal routine meals consumed by patients with type 2 diabetes mellitus during Ramadan iftar. In this study, we hypothesized that supplementation with 8.5 g d-allulose prior to carbohydrate intake during iftar will reduce the postprandial blood glucose.
Methods
Study design
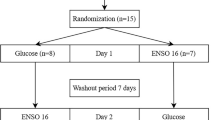
This research entailed a pilot, prospective intervention study involving a single-arm group. The study was carried out during Ramadan from April 13 to May 12, 2021. Specifically, the study duration was 10 consecutive days in the Month of Ramadan, comprising a control period and consumption period of 5 days each (Fig. 1).
The study protocol was registered on ClinicalTrials.gov (identifier: NCT05071950) and the National Medical Research Register (NMRR-19–3457-51288), whereas approval to conduct the study was obtained from the Medical Research Ethics Committee (MREC), Ministry of Health Malaysia (KKM/NIHSEC/P19-2734). Written informed consent was provided by the participants prior to enrollment and the study procedure were performed according to the principles of the Declaration of Helsinki 1964 and later versions.
Participants
This study recruited Muslim T2DM patients aged 20–70 years old. The inclusion criteria were: patients with HbA1c levels of < 8% before Ramadan, treated with oral hypoglycemic agents (OHAs) and/or diet control, intending to fast for Ramadan, and categorized in the low-risk group [25]. Patients were excluded if they had serious complications of diabetes, serum creatinine level of 1.5 mg/dl or higher, pregnant, breastfeeding, or who were advised not to fast by a doctor. This study was conducted at the diabetes outpatient clinic of a tertiary government hospital located in East Peninsular Malaysia. The G*Power 3.1.9.4 software was used to estimate the sample size of the study [26]. Using a statistical power of 95%, significance level of 0.05, and effect size of 1, based on previous study [18] it was estimated that at least 40 participants (20% of expected attrition rate was added) would be needed to show a statistically significant difference in glycemic control between 2 weeks of Ramadan fasting.
Data collection
The screening and enrollment processes were conducted within 2 months before Ramadan. This study required participants to visit the research site twice. Vital and physical parameters such as heart rate, blood pressure, height, weight, and waist circumference were measured on the first visit. Participants received seven packs of 8.5 g d-allulose and a food diary along with oral and written instructions. A sensor of flash continuous glucose monitoring (FCGM) device was attached to each participant. On the final visit, the sensors were removed and data were downloaded using a reader. Thereafter, the food diaries were collected and the side effects were evaluated. Hypoglycemia and hyperglycemia symptoms were recorded based on participants’ self-reports.
Clear zipped packs were used to collect 8.5 g of d-allulose with over 99% purity (Matsutani Chemical Industry Co., Ltd.). Each pack was numbered (1 to 7), indicating the corresponding days and dates. During the consumption period, participants dissolved one pack of d-allulose in plain water and finish the drink before consuming their main iftar meal. The quantity of plain water was based on participants’ preferences. During the study, no additional sugar/sweetener drinks were allowed before the iftar meals. Participants recorded the type and amount of iftar meals and drinks they consumed, and intakes of any additional sugar after the iftar in the food diary. During both periods, participants were instructed to adhere to the standard diabetes management provided by their health professionals, as there were adjustments in timing and doses of OHAs, as well as caloric intakes for Ramadan [25, 27]. No new or modified drugs were added or changed during the period. Therefore, no changes in diabetes treatment were recorded either before or when consuming d-allulose.
Glycemic measures
The FreeStyle Libre Pro Flash Glucose Monitory system (Abbott Japan Diabetes Care Inc., Tokyo, Japan) was used to measure the glucose values. This continuous glucose monitoring device measures glucose in the interstitial fluid. This device is reliable, non-invasive, and consists of a reader and a small sensor. The range target glucose values were set between 70 and 140 mg/dl (3.9–7.8 mmol/L). The FCGM sensor recorded interstitial glucose levels in 15-min intervals for a total of 96 readings every 24 h. However, only 10 days (5 days each of the control and consumption period) of data at 0 min (before iftar/ d-allulose consumption) and 15, 30, 45, 60, 75, 90, 105, 120, 135, 150, 165 and 180 min after iftar/ d-allulose consumption were extracted for data analysis. Data from the first two days and the last two days were excluded as they were considered unstable [28]. Participants took approximately 15–30 min to complete their meal during iftar. Therefore, data at the beginning of iftar were included in the analysis.
Outcome measures
The primary outcome was the peak postprandial glucose level documented by the FCGM. Secondary outcomes were: (1) percentage of time in which postprandial glucose levels were in the target range (%TIR = 70–140 mg/dl), (2) percentage of time in which glucose levels were above the target range (%TAR = > 140 mg/dl), (3) the percentage of time in which glucose levels were below the target range (%TBR = < 70 mg/dl), (4) side-effects of d-allulose, and (5) hypoglycemia or hyperglycemia symptoms reported by the participants.
Statistical analyses
The trapezoidal method was employed to calculate the glucose increment area under the curve (iAUC). The average glucose TIR, TAR, and TBR percentages were analyzed within 180 min after iftar. Meanwhile, all statistical analyses were undertaken using SPSS version 25 (IBM Corp, New York, USA) software. Results were presented in graphs developed using Microsoft Excel. Frequencies and percentages were used to express the categorical variables, whereas normally distributed data (continuous variables) were summarized using means and standard deviations (SD). Glucose levels between the control and consumption periods and differences within repeated days were assessed using the one-way repeated measure ANOVA with Bonferroni post hoc test. The paired sample t-test was used to compare the average glucose TIR, TAR, and TBR percentages. A p-value less than 0.05 was considered for statistical significance in all the tests conducted.
Results
Recruitment and participant characteristics
A total of 107 T2DM patients who were eligible were recruited in this study. Of these, 21 provided their consent but only 12 participants completed the final analysis (Fig. 2). Overall, equal numbers of males and females were obtained in this study, with a mean age of 55.2 ± 6.83 years. Meanwhile, the participants’ HbA1c pre-Ramadan, duration of diabetes, and BMI were 6.7 ± 0.41%, 6.6 ± 6.3 years, and 32.2 ± 7.6 kg/m2, respectively. More than half (66.7%) of the participants received a combination of two OHAs for diabetes treatment (Table 1).
Effect of d-allulose on postprandial peak glucose
Figure 3a and b show the mean postprandial glucose values and the glucose iAUC after iftar at 0–180 min, respectively. The participants’ postprandial glucose values were significantly lower at each time point (after iftar at 0–180) in the consumption period compared to the control period (p < 0.01) (Fig. 3a). Likewise, the postprandial glucose iAUC was significantly attenuated during the consumption period (22,474.1 ± 5576.8 mg∙min/dl) compared to the control period (26,792.3 ± 6411.9 mg∙min/dl; p < 0 0.01) (Fig. 3b).
The comparison of postprandial glucose at control and consumption (D-allulose) periods. Figure 3a shows postprandial glucose at 0 (pre) to 180 min after iftar and Fig. 3b shows the average glucose incremental area under the curve (iAUC) (3b) within 180 min after iftar. Data reported as mean ± SD of glucose values of 5 days of the control period and 5 days of the consumption period. Postprandial glucose levels were significantly different between both periods (*p < 0.05; **p < 0.01)
Percentage of time: glucose in range
Figure 4 shows the comparison of %TIR, %TBR, and %TAR within 180 min after iftar between the control and consumption periods. During the consumption period, the %TIR was significantly higher (62 ± 25.99% vs. 44 ± 28.48%; p = 0.0032) and %TAR was significantly lower (34 ± 29.84% vs. 54 ± 31.08%; p = 0.0015) than in the control period. No significant difference was observed in the %TBR. Figure 5 shows the 24-h glucose changes throughout the study period. The average daily glucose was lower in the consumption period than in the control period, with 92.1 mg/dL and 101.1 mg/dL, respectively. The lower values in the consumption period were also reported for %TIR and %TAR. No significant difference in glucose changes was observed between the control and consumption periods.
The effect of D-allulose during the consumption period compared to control period on the percentage of time postprandial glucose in-target range (%TIR), percentage of time glucose above-target range (%TAR) and percentage of time glucose below-target range (%TBR). Percentages of TIR, TAR and TBR were analyzed by obtaining the average of frequency glucose in TIR, TAR and TBR for 5 days (control and consumption periods) at 15 to 180 min after iftar. The average then divided by 12 times point and multiple with 100. Data reported as mean ± SD. There were significant differences of postprandial glucose between both periods (**p < 0.01)
Side-effects of d-allulose
Regarding the side effects of d-allulose, four events of minimally loose stool were reported among two participants. One participant experienced one instance in the early morning after consuming d-allulose on days 3, 4, and 5, whereas the other participant reported a similar event at night on the last day of administration. Nevertheless, no other side effects were observed. Symptomatic hypoglycemia and hyperglycemia were also not reported by participants.
Discussion
This pilot study was conducted during Ramadan when Muslims fast in the daytime from sunrise to sunset. A small number of participants completed this study. The participants consumed 8.5 g of d-allulose before the iftar meal for five consecutive days. Resultantly, the postprandial glucose values and the iAUC were significantly lower during the d-allulose period. The %TIR also increased while the %TAR decreased in comparison to the control period. Hence, we accepted our hypothesis.
Previous studies have shown that supplementation of d-allulose with carbohydrate intake improved postprandial glucose metabolism [29, 30]. Clinical studies in pre-diabetes and T2DM patients revealed a significant reduction in postprandial glucose levels, glucose iAUCs, and insulin levels following d-allulose supplementation [19, 31]. Furthermore, compared to healthy individuals, administration of d-allulose led to significantly lower blood glucose levels among pre-diabetes [19] and diabetes patients [31].
In the present study, 8.5 g of d-allulose was effective in suppressing glucose elevation before carbohydrate intake in diabetic patients in Malaysia. The amount of d-allulose used in this study was calculated based on the average carbohydrate intake of Malaysians [19, 32], and it has not been tested in previous studies. Prior clinical research reported that a minimum of 5 g to 10 g of d-allulose before carbohydrate loading is sufficient to reduce postprandial blood glucose levels, insulin levels, and glucose iAUCs among pre-diabetes individuals [19] and diabetic patients [31]. Nonetheless, the outcomes in healthy subjects may vary. Postprandial blood insulin and glucose levels were significantly suppressed in Japanese subjects administered 5 g and 7.5 g of d-allulose on 75 g maltodextrin [18] and 5 g of d-allulose in response to standardized meals [19, 24]. However, a recent study in the United States showed insulin levels were only significantly reduced with 10 g of d-allulose [23]. A Canadian study failed to demonstrate a suppressive effect of d-allulose [33]. The researchers suggested that the wide inter-individual variation in participants’ glucose responses might explain the results. Additionally, no effect was detected in blood glucose and insulin levels following a single administration of 7.5 g d-allulose in healthy participants, thus reflecting that hypoglycemia is not induced by d-allulose [18]. Overall, d-allulose showed better suppression of postprandial glucose elevation among people with impaired glucose tolerance but studies on diabetes patients are still limited.
Insulin levels were not measured in this study due to the limitations in the function of the FCGM devices. This is the first attempt to investigate participants’ real-carbohydrate intake. Nevertheless, the participants’ carbohydrate and calorie intake were not analyzed but they were instructed to record the quantities of their iftar meals in their food diaries as supporting information. Overall, the menu differed among the participants but the nutritional intake or changes in the menu were not significantly different in each participant during control and d-allulose periods. Most of them consumed 150 g of rice on average apart from other side dishes and desserts. In Muslim societies, including Malaysia, it has become a tradition where iftar is celebrated as a mini feast to mark the break of fast on that day with family members or friends. In general, Malaysian dietary patterns contain high sugar and fat intakes, and compared to other ethnic groups, Malays consume high-calorie meals during daily intake [34]. Hence, maintaining a healthy diet during Ramadan is a challenge for individuals with diabetes [35].
The possible underlying mechanisms explaining the role of d-allulose in suppressing postprandial glucose levels and enhanced insulin regulation following the consumption of carbohydrate foods have been demonstrated in previous animal studies. One of the underpinning events is the inhibition of carbohydrate-digesting enzymes [36,37,38] or competition with glucose transporters in small intestine [39, 40], thus reducing intestinal glucose absorption. Findings suggested that d-allulose shares the same sugar transporters of d-glucose and d-fructose in the small intestine [39, 40]. Competition for the transportation of d-glucose and d-fructose was elicited due to the presence of d-allulose, thereby reducing the permeation rate of both sugars These were evidenced by an in vitro study conducted in Caco-2 monolayer cell lines. The glucose permeability was reduced by 60% when the addition of 30 mM allulose to 30 mM glucose was combined [40]. Another study on rats showed Tracer d-[14C]-fructose uptake was reduced to 54.8% in 50 mM d-allulose [39]. Furthermore, rats’ studies revealed that d-allulose stimulated glucagon-like peptide-1 secretion and enhanced insulin release, as a result improved glucose tolerance [41, 42] and provided a therapeutic effect on pancreatic β-cell function [37]. Immunohistochemical analyses demonstrated healthy pancreatic β-cell were clearly observed in both T2DM rat models and normal rats treated with d-allulose over 60 weeks; meanwhile, hyperplastic changes and extensive fibrosis were seen in control T2DM rat models [37]. These findings suggest supplemental d-allulose has potential effects on diabetes prevention and treatment.
This is the first study in which FCGM was used to measure glucose levels following d-allulose supplementation. Previous studies had primarily used antecubital veins or fingertips for blood glucose measurements. Within 180 min after iftar, the %TAR decreased and %TIR increased significantly compared to the control period. This indicated that d-allulose shortened the hyperglycemic phase and maintained the glucose level in the normal target range for a longer time compared to the control period. A critical phase occurs after a meal in which glucose is expected to reach the target within two hours of food ingestion to improve postprandial hyperglycemia, thus preventing the progression of microvascular and macrovascular complications in T2DM patients [15]. Nevertheless, glycaemic targets may differ depending on the severity of diabetes.
Two participants reported an instance of a minimally loose stool after d-allulose consumption, and these were not considered diarrhea. Studies have shown that d-allulose is safe for consumption in healthy individuals [19, 43] and T2DM patients [44]. A gastrointestinal tolerance test on healthy participants [43] reported that severe diarrhea was noted only at a dose of 0.5 g/kg of body weight. Two long-term safety evaluation studies reported no significant clinical concerns after taking 5 g of d-allulose thrice daily [19, 44]. The present pilot study had some limitations. Given that the study was conducted during the COVID-19 pandemic under the restricted movement order implementation by the Malaysian government, the outpatient clinic had to adhere to strict standard operating procedures. As a result, the number of appointments in a given period was reduced and only a few patients could participate in this study. These limitations contributed to the lack of a high-quality study design for more precise outcomes in this research. The FCGM is relatively new in Malaysia and its use during Ramadan was frowned upon by some patients who wished to have Ramadan free of intrusions due to religious and cultural commitments. Furthermore, this study only involved Malay patients at a single medical institution; thus, the findings cannot be generalized to other patients at other medical institutions. The outcomes could be better clarified in future studies by enrolling a larger sample size.
Conclusions
Our findings suggest that supplementation with 8.5 g of d-allulose before iftar meal revealed a significant suppressive effect on postprandial glucose elevation. This study also confirmed that no severe side effects of d-allulose or symptomatic hypoglycemia or hyperglycemia were reported throughout the study period. We believe d-allulose has its potential role in improving glycemic control in T2DM patients, however it cannot be applied clinically immediately. These findings will support the need for future clinical studies with adequate power and considering other parameters that might affect glycemic changes during Ramadan fasting.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ANOVA:
-
Analysis of variance
- BMI:
-
Body mass index
- DBP:
-
Diastolic blood pressure
- HbA1c:
-
Glycated haemoglobin
- iAUC:
-
Increment area under the curve
- SBP:
-
Systolic blood pressure
- TAR:
-
Above-target range
- TBR:
-
Below-target range
- TIR:
-
In-target range
- T2DM:
-
Type 2 diabetes mellitus.
- WC:
-
Waist circumference
- OHAs:
-
Oral hypoglycemic agents
References
Cho NH, Shaw JE, Karuranga S, et al. IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–81. https://doi.org/10.1016/j.diabres.2018.02.023.
Jabbar A. Epidemiology of diabetes and Ramadan fasting in diabetes and Ramadan: Practical Guideline. International Diabetes Federation (IDF), in collaboration with the Diabetes and Ramadan (DAR) International Alliance 2016; 17–28.
Ministry of Health Malaysia. National health and morbidity survey 2019: key findings, National Institutes of Health (NIH); 2020.
Abdullah N, Murad NAA, Attia J, et al. Differing contributions of classical risk factors to type 2 diabetes in multi-ethnic Malaysian populations. Int J Environ Res Public Health. 2018;15:1–15. https://doi.org/10.3390/ijerph15122813.
Tee E-S, Yap RWK. Type 2 diabetes mellitus in Malaysia: current trends and risk factors. Eur J Clin Nutr. 2017;71:844–9. https://doi.org/10.1038/ejcn.2017.4.
Sadikot S, Jothydev K, Zargar AH, Ahmad J, Arvind SR, Saboo B. Clinical practice points for diabetes management during Ramadan fast. Diabetes Metab Syndr Clin Res Rev. 2017;11:S811–9. https://doi.org/10.1016/j.dsx.2017.06.003.
Rouhani MH, Azadbakht L. Is Ramadan fasting related to health outcomes? A review on the related evidence. J Res Med Sci. 2014;19:987–92.
Almansour HA, Chaar B, Saini B. Fasting, diabetes, and optimizing health outcomes for Ramadan observers: a literature review. Diabetes Ther. 2017;8:227–49. https://doi.org/10.1007/s13300-017-0233-z.
Hassanein M, Hussein Z, Shaltout I, et al. The DAR 2020 global survey: Ramadan fasting during COVID 19 pandemic and the impact of older age on fasting among adults with type 2 diabetes. Diabetes Res Clin Pract. 2021;173: 108674. https://doi.org/10.1016/j.diabres.2021.108674.
Zainudin SB, Abu Bakar KN, Abdullah S, Hussain A. Diabetes education and medication adjustment in Ramadan (DEAR) program prepares for self-management during fasting with tele-health support from pre-Ramadan to post-Ramadan. Ther Adv Endocrinol Metab. 2018;9:231–40. https://doi.org/10.1177/2042018818781669.
Alfadhli EM. Higher rate of hyperglycemia than hypoglycemia during Ramadan fasting in patients with uncontrolled type 1 diabetes: insight from continuous glucose monitoring system. Saudi Pharm J. 2018;26:965–9. https://doi.org/10.1016/j.jsps.2018.05.006.
The Holy Koran. Al-Bakarah. Sura 2: verses 183–5.
Lee JY, Wong CP, Tan CSS, Nasir NH, Lee SWH. Telemonitoring in fasting individuals with type 2 diabetes mellitus during Ramadan: a prospective, randomised controlled study. Sci Rep. 2017;7:1–9. https://doi.org/10.1038/s41598-017-10564-y.
Mansour AA. Attitude of patients with diabetes mellitus toward fasting Ramadan in Basrah. Iraq Endocrinol Int J. 2018;6:4–7. https://doi.org/10.15406/emij.2018.06.00146.
Hiyoshi T, Fujiwara M, Yao Z. Postprandial hyperglycemia and postprandial hypertriglyceridemia in type 2 diabetes. J Biomed Res. 2019;33:1–16. https://doi.org/10.7555/JBR.31.20160164.
Lessan N, Hannoun Z, Barakat Hasan H, MT. Glucose excursions and glycaemic control during Ramadan fasting in diabetic patients: insights from continuous glucose monitoring (CGM). Diabetes Metab. 2015;41:28–36. https://doi.org/10.1016/j.diabet.2014.11.004.
Jan H, Mohamed J, Nazri NH, Loy S. Ramadan bazaar and Ramadan buffets: the possible influence on eating behavior and health among Malaysian Muslims. J Fasting Health. 2013;1:43–5.
Iida T, Kishimoto Y, Yoshikawa Y, et al. Acute d-psicose administration decreases the glycemic responses to an oral maltodextrin tolerance test in normal adults. J Nutr Sci Vitaminol. 2008;54:511–4. https://doi.org/10.3177/jnsv.54.511.
Hayashi N, Iida T, Yamada T, et al. Study on the postprandial blood glucose suppression effect of d-psicose in borderline diabetes and the safety of long-term ingestion by normal human subjects. Biosci Biotechnol Biochem. 2010;74:510–9. https://doi.org/10.1271/bbb.90707.
Hayashi N, Yamada T, Takamine S, Iida T, Okuma K, Tokuda M. Weight reducing effect and safety evaluation of rare sugar syrup by a randomized double-blind, parallel-group study in human. J Funct Foods. 2014;11:152–9. https://doi.org/10.1016/j.jff.2014.09.020.
Chung MY, Oh DK, Lee KW. Hypoglycemic health benefits of D-psicose. J Agric Food Chem. 2012;60:863–9. https://doi.org/10.1021/jf204050w.
U.S. Food & Drug Administration. GRAS Notices [Internet]. https://www.accessdata.fda.gov/scripts/fdcc/?set=GRASNotices&id=498 Assessed 14 Dec 2020
Franchi F, Yaranov DM, Rollini F, et al. Effects of d- allulose on glucose tolerance and insulin response to a standard oral sucrose load: results of a prospective, randomized, crossover study. BMJ Open Diabetes Res Care. 2021;9: e001939. https://doi.org/10.1136/bmjdrc-2020-001939.
Kimura T, Kanasaki A, Hayashi N, Yamada T, Iida T, Nagata Y. D-Allulose enhances postprandial fat oxidation in healthy humans. Nutrition. 2017;43–44:16–20. https://doi.org/10.1016/j.nut.2017.06.007.
Ministry of Health Malaysia. Practical guide to diabetes management in Ramadan. Malaysia: Putrajaya; 2015.
Faul F, Erdfelder E, Buchner A, Lang A-G. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41:1149–60. https://doi.org/10.3758/BRM.41.4.1149.
Malaysian Dietitians’ Association. Medical nutrition therapy guidelines for Type 2 Diabetes Mellitus. Malaysian Dietitians’. 2nd ed. Putrajaya: Association and Ministry of Health; 2013.
Bailey T, Bode BW, Christiansen MP, Klaff LJ, Alva S. The performance and usability of a factory-calibrated flash glucose monitoring system. Diabetes Technol Ther. 2015;17:787–94. https://doi.org/10.1089/dia.2014.0378.
Braunstein CR, Noronha JC, Khan TA, et al. Effect of fructose and its epimers on postprandial carbohydrate metabolism: a systematic review and meta-analysis. Clin Nutr. 2020;39:3308–18. https://doi.org/10.1016/j.clnu.2020.03.002.
Hossain A, Yamaguchi F, Matsuo T, et al. Rare sugar D-allulose: potential role and therapeutic monitoring in maintaining obesity and type 2 diabetes mellitus. Pharmacol Ther. 2015;155:49–59. https://doi.org/10.1016/j.pharmthera.2015.08.004.
Noronha JC, Braunstein CR, Glenn AJ, Khan TA, Viguiliouk E, Noseworthy R, et al. The effect of small doses of fructose and allulose on postprandial glucose metabolism in type 2 diabetes: a double-blind, randomized, controlled, acute feeding, equivalence trial. Diabetes Obes Metab. 2018;20:2361–70. https://doi.org/10.1111/dom.13374.
Lee YY, Muda WAMW. Dietary intakes and obesity of Malaysian adults. Nutr Res Pract. 2019;13:159–68. https://doi.org/10.4162/nrp.2019.13.2.159.
Braunstein CR, Noronha JC, Glenn AJ, et al. A double-blind, randomized controlled, acute feeding equivalence trial of small, catalytic doses of fructose and allulose on postprandial blood glucose metabolism in healthy participants: the fructose and allulose catalytic effects (FACE) trial. Nutrients. 2018;10:750. https://doi.org/10.3390/nu10060750.
Ali A, Margetts BM, Zainuddin AA. Exploration of the principal component analysis (PCA) approach in synthesizing the diet quality of the Malaysian population. Nutrients. 2021;13:70. https://doi.org/10.3390/nu13010070.
Lee JY, Wong CP, Tan CSS, Nasir NH, Lee SWH. Type 2 diabetes patient’s perspective on Ramadan fasting: a qualitative study. BMJ Open Diabetes Res Care. 2017;5: e000365. https://doi.org/10.1136/bmjdrc-2016-000365.
Matsuo T. Dietary D-psicose, a C-3 epimer of D-fructose, suppresses the activity of hepatic lipogenic enzymes in rats. Asia Pac J Clin Nutr. 2001;10:233–7. https://doi.org/10.1046/j.1440-6047.2001.00246.x.
Hossain A, Yamaguchi F, Hirose K, et al. Rare sugar D-psicose prevents progression and development of diabetes in T2DM model Otsuka Long-Evans Tokushima fatty rats. Drug Des Devel Ther. 2015;9:525–35. https://doi.org/10.2147/DDDT.S71289.
Shintani T, Yamada T, Hayashi N, et al. Rare sugar syrup containing d-allulose but not high-fructose corn syrup maintains glucose tolerance and insulin sensitivity partly via hepatic glucokinase translocation in Wistar rats. J Agric Food Chem. 2017;65:2888–94. https://doi.org/10.1021/acs.jafc.6b05627.
Kishida K, Martinez G, Iida T, Yamada T, Ferraris RP, Toyoda Y. D-Allulose is a Substrate of Glucose Transporter Type 5 (GLUT5) in the small intestine. Food Chem. 2019;277:604–8. https://doi.org/10.1016/j.foodchem.2018.11.003.
Hishiike T, Ogawa M, Hayakawa S, et al. Transepithelial transports of rare sugar. J Agric Food Chem. 2013;61:7381–6. https://doi.org/10.1021/jf401449m.
Hayakawa M, Hira T, Nakamura M, Iida T, Kishimoto Y, Hara H. Secretion of GLP-1 but not GIP is potently stimulated by luminal d-Allulose (d-Psicose) in rats. Biochem Biophys Res Commun. 2018;496:898–903. https://doi.org/10.1016/j.bbrc.2018.01.128.
Iwasaki Y, Sendo M, Dezaki K, et al. GLP-1 release and vagal afferent activation mediate the beneficial metabolic and chronotherapeutic effects of D-allulose. Nat Commun. 2018;9:113–29. https://doi.org/10.1038/s41467-017-02488-y.
Han Y, Choi BR, Kim SY, et al. Gastrointestinal tolerance of d-allulose in healthy and young adults A non-randomized controlled trial. Nutrients. 2018;10:2010. https://doi.org/10.3390/nu10122010.
Tanaka M, Hayashi N, Iida T. Safety evaluation of 12-week continuous ingestion of D-allulose in borderline diabetes and type 2 diabetes. Fundam Toxicol Sci. 2019;6:225–34. https://doi.org/10.2131/fts.6.225.
Acknowledgements
Thank you to the Director General of Health Malaysia for the permission to publish this paper, and to the International Institute of Rare Sugar Research and Education, Kagawa University for the supervision.
Funding
This study was funded by Matsutani Chemical Industry Co., Ltd., Hyogo, Japan.
Author information
Authors and Affiliations
Contributions
Conceptualization and methodology, KM, SJ, KF, TK, HI, SS, TS, TI, and TY; formal analysis, KM, SJ, KF, TK, HI, SS, TS, TI, and TY; investigation, SJ, KLS, SLO, and ZM; writing—original draft preparation, SJ, KM.; writing—review and editing, SJ, KM, KF, TK, HI, SS, TS, TI, and TY; funding acquisition, KM. and KF. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Medical Research Ethics Committee of Malaysia (approval No. KKM/NIHSEC/P19-2734). Written informed consent was obtained from all subjects involved in the study.
Consent for publication
The permission to publish this paper was obtained from Director General of Health Malaysia.
Competing interests
The authors have no competing interest to report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Japar, S., Fukunaga, K., Kobayashi, T. et al. A pilot study on the effect of d-allulose on postprandial glucose levels in patients with type 2 diabetes mellitus during Ramadan fasting. Diabetol Metab Syndr 14, 86 (2022). https://doi.org/10.1186/s13098-022-00856-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13098-022-00856-3