Abstract
Background
Metabolic syndrome (MetS) has been related to the pathogenesis of variety categories of cancers. This meta-analysis aimed to determine the association between MetS and the incidence of lung cancer.
Methods
Relevant cohort studies were identified by search of PubMed, Embase, and Cochrane’s Library databases. Cochrane’s Q test and I2 statistic were used to analyze the heterogeneity. Random-effect model which incorporates the potential heterogeneity was used for the meta-analysis.
Results
Five cohort studies with 188,970 participants were included. A total of 1,295 lung cancer cases occurred during follow-up. Meta-analyses showed that neither MetS defined by the revised NCEP-ATP III criteria (hazard ratio [HR]: 0.94, 95% confidence interval [CI]: 0.84 to 1.05, p = 0.25; I2 = 0) nor the IDF criteria (HR: 0.82, 95% CI: 0.61 to 1.11, p = 0.20; I2 = 0) was associated with an affected risk of lung cancer. Subgroup analyses showed consistent results in women and in men, in studies performed in Asian and non-Asian countries, and in prospective and retrospective cohorts (p all > 0.05). Meta-analysis limited to studies with the adjustment of smoking status also showed similar results (HR: 0.91, 95% CI: 0.80 to 1.05, p = 0.21; I2 = 0). No publication bias was detected based on the Egger regression test (p = 0.32).
Conclusions
Current evidence from cohort studies does not support that MetS is an independent risk factor for the incidence of lung cancer.
Similar content being viewed by others
Background
Metabolic syndrome (MetS) is a cluster of metabolic disorders characterized by the pathophysiological presence of central obesity, insulin resistance, high blood pressure, and dyslipidemia [1]. With the aging of the global population, MetS has become a common health problem in both the developed and the developing countries, with the reported prevalence of 10–30% of the adult populations [2,3,4]. Accumulating evidence confirmed that patients with MetS are at higher risk for the development of many other diseases, such as cardiovascular diseases [5], recurrent stroke [6], venous thromboembolism [7], sleep-disordered breathing [8], and osteoporosis [9].
Lung cancer is one of the most common cancers. In 2012, there were about 1.8 million new lung cancer cases and 1.6 million cases of death, respectively, accounting for about 13% of the total number of cancer diagnosis and 20% of the total number of cancer deaths [10]. Smoking is currently the most important risk factor for lung cancer, but there are still about 25% of lung cancer patients that are non-smokers [11]. Due to the high incidence and mortality related to lung cancer, identify risk factors for the pathogenesis of lung cancer is of important significance. Previous studies showed that MetS may be associated with cancer [12], probably due to their shared pathophysiological mechanisms such as low-grade chronic inflammation [13, 14]. However, subsequent studies showed that the association between MetS and cancer is likely to be site-specific the association between MetS and the incidence of lung cancer has not been fully determined [15]. In a previous meta-analysis [12], by including four cohort studies [16,17,18,19], the authors concluded that presence MetS did not affect the risk of lung cancer. However, besides studies reporting the incidence of lung cancer, they also included a study that reported the lung cancer mortality [16]. Since the outcome of cancer mortality could be affected by many clinical factors such as the anticancer treatments, including studies with mortality data may confound the overall result. Moreover, some subsequently published cohort studies were not included in the previous meta-analysis [20,21,22]. Therefore, we performed an updated meta-analysis to evaluate the association between MetS and subsequent incidence of lung cancer.
Methods
We performed the meta-analysis in accordance with the MOOSE (Meta-analysis of Observational Studies in Epidemiology) [23] and Cochrane’s Handbook [24] guidelines.
Literature search
Databases of PubMed, Embase and Cochrane’s Library were searched for relevant records. As for the search strategy, the combined terms were entered into the above databases as a single search, as ("metabolic syndrome" OR "insulin resistance syndrome" OR "syndrome X") AND ("lung" OR "pulmonary" OR "respiratory") AND ("cancer" OR "neoplasm" OR "carcinoma") AND ("prospective" OR "prospectively" OR "retrospective" OR "retrospectively" OR "followed" OR "follow-up" OR "cohort" OR "cohorts" OR "risk" OR "incidence"). We used this keywords search strategy instead of those searched as "text words" or as "Mesh terms" or “Emtree” to retrieve more comprehensive records. The search was limited to human studies published in English language. The reference lists of original and review articles were also analyzed using a manual approach. The final literature search was performed on April 20, 2020.
Study selection
Articles were included in the meta-analysis if they met all the following criteria: (1) published as full-length article in English; (2) reported as cohort studies (prospective or retrospective, regardless of sample size) with the follow-up duration of at least one year; (3) included adult population (≥ 18 years of age) without lung cancer at baseline; (4) MetS defined according to the criteria of the original articles was identified as exposure of interest at baseline; (5) participants without MetS at baseline was considered as controls; (6) documented the incidences of lung cancer during follow-up; and (7) reported the adjusted hazard ratios (HRs, at least adjusted age and gender) and their corresponding 95% confidence intervals (CIs) for the incidence of lung cancer comparing individuals with MetS at baseline to those without MetS. Reviews, letters, editorials, preclinical studies and non-cohort studies were excluded.
Data extracting and quality evaluation
Two authors independently performed literature searching, data extraction, and quality assessment according to the predefined inclusion criteria. Discrepancies were resolved by consensus. Data that were extracted include: (1) name of first author, year of publication and country where the study was performed; (2) design characteristics (prospective or retrospective); (3) characteristics and numbers of the participants; (4) criteria for the diagnosis of MetS; (5) follow-up period; (6) number of lung cancer cases in each study; and (7) variables adjusted when presenting the results. The quality of each study was evaluated using the Newcastle–Ottawa Scale [25] which ranges from 1 to 9 stars and judges each study regarding three aspects: selection of the study groups; the comparability of the groups; and the ascertainment of the outcome of interest.
Statistical analyses
We used HRs as the general measure for the association between MetS at baseline and the incidence of lung cancer. Data of HRs and their corresponding stand errors (SEs) were calculated from 95% CIs or p values, and were logarithmically transformed to stabilize variance and normalized the distribution [24]. The Cochrane’s Q test and I2 test were used to evaluate the heterogeneity among the include cohort studies [26]. A significant heterogeneity was considered if I2 > 50%. We used a random-effect model to synthesize the HR data because this model is considered as a more generalized method which incorporates of the potential heterogeneity [24]. Sensitivity analyses, by removing individual study one at a time, were performed to test the robustness of the results [27]. Predefined subgroup analyses were performed to evaluate whether the association between MetS and lung cancer incidence was affected by gender of the participants, country of the study, and study design characteristics. Since smoking has been related with increased risk of lung cancer [28], we evaluated whether MetS is associated with lung cancer incidence in studies after adjustment of smoking. Moreover, potential publication bias was assessed by funnel plots with the Egger regression asymmetry test [29]. We used the RevMan (Version 5.1; Cochrane Collaboration, Oxford, UK) and STATA software for the meta-analysis and statistics.
Results
Literature searching
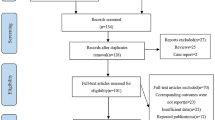
The processes of database searching were presented in Fig. 1. Briefly, 893 articles were found via initial literature searching of the PubMed and Embase databases, and 868 were excluded through screening of the titles and abstracts mainly because they were not relevant to the purpose of the meta-analysis. Subsequently, 25 potential relevant records underwent full-text review. Of these, 20 were further excluded for the reasons listed in Fig. 1. Finally, five cohort studies were included [17,18,19,20,21].
Study characteristics and quality evaluation
The characteristics of the included cohort studies were presented in Table 1. Briefly, our meta-analysis included 188,970 participants from five cohorts. Two studies were performed in Europe [17, 20], and the other three were performed in Asia [18, 19, 21]. Regarding the design, two studies were retrospective [19, 21], whereas the other three were prospective [17, 18, 20]. Four of the studies included general populations [17,18,19, 21], whereas the other one included patients with vascular disease [20]. All of the included cohorts defined MetS according to the criteria of revised National Cholesterol Education Program’s Adults Treatment Panel III (NCEP-ATP III) [30], and two of them also included data of MetS diagnosed with the International Diabetes Federation (IDF) criteria [31]. Diagnosis of MetS according to NCEP ATP III or IDF criteria were based on the baseline measurements of the components of MetS combined with the confirmed treatments such as use of the antihypertensives or glucose-lowering agents among the included studies [17,18,19,20,21]. The incidence of lung cancer cases were mainly confirmed by the local cancer registries and 1,295 lung cancer cases occurred during follow-up. Age and gender were adjusted in all of the included studies when presenting the results, whereas smoking and alcohol intake were adjusted in four cohorts [18,19,20,21] except for one study [17]. The Newcastle–Ottawa scale varied from 7 to 9 in the included cohort studies (Table 2), suggesting the generally good study quality.
Association between the revised NCEP-ATP III defined MetS and lung cancer risk
Five cohort studies [17,18,19,20,21] with 188,970 participants reported the association between MetS diagnosed by revised NCEP-ATP III at baseline and the subsequent risk of lung cancer incidence. Since three of them [18, 19, 21] reported the outcomes separately according to the gender of the participants, a total of eight datasets were included. Result of the meta-analysis did not support a significant association between MetS and the risk of lung cancer (adjusted HR: 0.94, 95% CI: 0.84 to 1.05, p = 0.25; Fig. 2a) with no significant heterogeneity (p for Cochrane’s Q test = 0.72, I2 = 0). Results of sensitivity analyses by excluding one study at a time did not significantly affect the result, suggesting the stability of the main result (Table 3). Specifically, excluding the study [17] in which smoking status was not adjusted showed similar result (adjusted HR: 0.91, 95% CI: 0.80 to 1.05, p = 0.21) with no significant heterogeneity (p for Cochrane’s Q test = 0.65, I2 = 0). Results of subgroup analyses according to the gender of the participants were also similar (for male: adjusted HR: 0.95, 95% CI: 0.80 to 1.12, p = 0.55, I2 = 27%; for female: adjusted HR: 0.84, 95% CI: 0.66 to 1.07, p = 0.15, I2 = 0; Fig. 2b). The difference between the results in male and female participants was not statistically significant (p = 0.40; Fig. 2b). In addition, subgroup analyses showed similar results in studies performed in Asian and non-Asian countries (p > 0.05, Fig. 3a), and in prospective and retrospective cohort studies (p > 0.05, Fig. 3b).
Association between IDF defined MetS and lung cancer risk
Two cohorts [18, 19] with 66,556 participants reported the association between IDF defined MetS and the subsequent risk of lung cancer. Results of the meta-analysis did not show a significant association (adjusted HR: 0.82, 95% CI: 0.61 to 1.11, p = 0.20; I I2 = 0; Fig. 4). Results of subgroup analyses according to the gender of the participants were also similar (for male: adjusted HR: 0.78, 95% CI: 0.44 to 1.39, p = 0.40, I2 = 50%; for female: adjusted HR: 0.81, 95% CI: 0.50 to 1.31, p = 0.39, I2 = 0; Fig. 4). The difference between subgroups was not statistically significant (p = 0.93).
Publication bias
The funnel plot regarding MetS diagnosed by revised NCEP-ATP III at baseline and risk of cognitive decline was shown in Fig. 5. The funnel plot was symmetry on visual inspection. Results of Egger regression test suggested that no significant publication bias was detected (p = 0.32). The publication bias for the meta-analysis of association between IDF defined MetS and the subsequent risk of lung cancer was difficult to estimate since limited cohorts were included.
Discussion
In this meta-analysis, by pooling the results of five cohort studies of 188,970 participants, the result showed that presence of MetS does not significantly influence the subsequent incidence of lung cancer. The results were consistent in male and female participants, in studies performed in Asian and non-Asian countries, in prospective and retrospective cohorts, and in studies in which smoking habit was adjusted in multivariate analyses. Moreover, the association between MetS and lung cancer incidence was not affected by the definitions of MetS (based on the revised NCEP-ATP III or IDF criteria). These results suggested that current evidence from cohort studies does not support that MetS is an independent risk factor for the incidence of lung cancer.
Results of our meta-analysis may reflect the fact that the components of MetS may have different influences on the risk of lung cancer. For the association between obesity and lung cancer risk, current evidence is not consistent. A previous meta-analysis 31 studies showed that obesity is protective factor against lung cancer [32], whereas another study in Chinese patients suggested that the protective effect of obesity against lung cancer may be confounded by the smoking status [33]. Subsequent meta-analysis of six prospective cohort studies indicated that abdominal obesity may be a risk factor for the incidence of lung cancer [34]. Interestingly, a recent meta-analysis with 28 prospective cohort studies suggested a significantly positive relationship between waist circumference, rather than BMI, and lung cancer risk, suggesting there might have an etiological connection between central obesity and lung cancer development [35], rather than overall obesity as evidenced by increased BMI. Moreover, as for the lipids profiles, recent evidence indicated that higher high-density lipoprotein cholesterol level is protective against the lung cancer, whereas higher triglyceride is associated with higher lung cancer incidence [36], and these findings are confirmed by a subsequent case–control study in Chinese patients with and without non-small cell lung cancer [37]. However, a recent prospective cohort study showed that the association between triglyceride and lung cancer risk may be more complicated than expected and presented as a U-shaped association [38]. In addition, results regarding the association between hypertension and lung cancer risk are inconsistent. The result Metabolic Syndrome and Cancer Project indicated a small increased lung cancer risk in men with elevated blood pressure level, but not in women [39]. However, an early study in Korean men showed that hypertension was not an independent risk factor in lung cancer mortality [40]. Similarly, result of a meta-analysis of 14 cohort studies also concluded that diabetes was not associated with lung cancer risk [41]. A recent cohort study showed that the potential impact of diabetes on the risk of lung cancer may be modified by smoking status of the patients, and diabetes may have minimal impact on lung cancer development in the never-smoking population [42]. Taken together, it seems that association between the components of MetS and the risk of lung cancer remain uncertain and may be modified by many factors including smoking status. Additionally, since people with MetS often have unhealthy life styles, such as smoking, alcohol drinking and less exercise, these factors may also confound the association between MetS and lung cancer. Most of the included studies in our meta-analysis have adjusted these factors, which may therefore weaken the association between MetS and Lung cancer incidence. Moreover, accumulating evidence showed that treatments against the components of Mets, such as the use of metformin, may lead to a reduced risk of lung cancer incidence [43]. Whether these factors may confound the association between MetS and Lung cancer risk also deserves further investigation.
The strengths of our study, compared with the previous meta-analysis [12] may include the followings. Firstly, we included only studies with multivariate analyses, which minimized the potential influences of study or participant characteristics on the outcome. Secondly, only studies reporting the incidence of lung cancer were included, rather than the studies that reported the morality of lung cancer. Since lung cancer mortality could also be affected by treatment status after diagnosis, the previous meta-analysis combining the data of lung cancer incidence and mortality may confound the results [12]. Finally, we included five cohort studies with eight datasets, which enabled us to perform multiple stratified analyses to confirm the findings of the main analysis. Despite of this significance, our study also has limitations which should be considered when interpreting the results. Firstly, as a meta-analysis of observational studies, results of our study did not support a sequential association between MetS and lung cancer incidence. Since lung cancers of different histopathological type may have different biological features, the association between MetS and different histopathological type of lung cancer should be analyzed. However, since data according to the histopathological type of lung cancer were not reported in either of the included cohort studies, we were unable to evaluate the outcomes according to the histopathological type of lung cancer. Future studies are warranted in this regard. Secondly, although MetS defined by revised NCEP-ATP III or IDF criteria was not associated with lung cancer incidence, association between MetS defined by other criteria and subsequent lung cancer incidence remains undetermined. Thirdly, although our study combined the data of 188,970 participants and 1295 cases of lung cancer, we could not fully exclude the possibility that the meta-analysis is statistically underpowered for the detection of the association between MetS and lung cancer incidence. Finally, as previous mentioned, although we combine multivariate adjusted data, we could not exclude that there remains residual factors that may confound the result, such as smoking status, dietary factors, and concurrent medications including metformin et al.
In conclusion, results of our meta-analysis showed that current evidence from cohort studies does not support that MetS is an independent risk factor for the incidence of lung cancer. The influences of each component of MetS on pathogenesis of lung cancer should be evaluated in future studies.
Availability of data and materials
The available data and materials section refers to the raw data used in our study are included in manuscript with tables, figures and its supplementary information files. All the authors agreed that the data could be shared if researchers required.
Abbreviations
- MetS:
-
Metabolic syndrome
- MOOSE:
-
Meta-analysis of Observational Studies in Epidemiology
- HRs:
-
Hazard ratios
- SE:
-
Stand error
- NCEP-ATP III:
-
Revised National Cholesterol Education Program’s Adults Treatment Panel III
- IDF:
-
International Diabetes Federation
References
Kumari R, Kumar S, Kant R. An update on metabolic syndrome: metabolic risk markers and adipokines in the development of metabolic syndrome. Diabetes Metab Syndr. 2019;13(4):2409–17. https://doi.org/10.1016/j.dsx.2019.06.005.
He YN, Zhao WH, Zhao LY, Yu DM, Zhang J, Yang XG, Ding GG. Prevalence of metabolic syndrome in Chinese adults in 2010–2012. Zhonghua Liu Xing Bing Xue Za Zhi. 2017;38(2):212–5. https://doi.org/10.3760/cma.j.issn.0254-6450.2017.02.015.
Li R, Li W, Lun Z, Zhang H, Sun Z, Kanu JS, Qiu S, Cheng Y, Liu Y. Prevalence of metabolic syndrome in Mainland China: a meta-analysis of published studies. BMC Public Health. 2016;16:296. https://doi.org/10.1186/s12889-016-2870-y.
Grundy SM. Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol. 2008;28(4):629–36. https://doi.org/10.1161/ATVBAHA.107.151092.
Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, Rinfret S, Schiffrin EL, Eisenberg MJ. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56(14):1113–32. https://doi.org/10.1016/j.jacc.2010.05.034.
Li X, Fang F, Fu X, Lin H, Gao Q. Is Metabolic Syndrome Associated with the Risk of Recurrent Stroke: A Meta-Analysis of Cohort Studies. J Stroke Cerebrovasc Dis. 2017;26(12):2700–5. https://doi.org/10.1016/j.jstrokecerebrovasdis.2017.03.014.
Ageno W, Di Minno MN, Ay C, Jang MJ, Hansen JB, Steffen LM, Vaya A, Rattazzi M, Pabinger I, Oh D, Di Minno G, Braekkan SK, Cushman M, Bonet E, Pauletto P, Squizzato A, Dentali F. Association between the metabolic syndrome, its individual components, and unprovoked venous thromboembolism: results of a patient-level meta-analysis. Arterioscler Thromb Vasc Biol. 2014;34(11):2478–85. https://doi.org/10.1161/ATVBAHA.114.304085.
Xu S, Wan Y, Xu M, Ming J, Xing Y, An F, Ji Q. The association between obstructive sleep apnea and metabolic syndrome: a systematic review and meta-analysis. BMC Pulm Med. 2015;15:105. https://doi.org/10.1186/s12890-015-0102-3.
Zhou J, Zhang Q, Yuan X, Wang J, Li C, Sheng H, Qu S, Li H. Association between metabolic syndrome and osteoporosis: a meta-analysis. Bone. 2013;57(1):30–5. https://doi.org/10.1016/j.bone.2013.07.013.
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J. Jemal A (2015) Global cancer statistics. CA Cancer J Clin. 2012;65(2):87–108. https://doi.org/10.3322/caac.21262.
O'Keeffe LM, Taylor G, Huxley RR, Mitchell P, Woodward M, Peters SAE. Smoking as a risk factor for lung cancer in women and men: a systematic review and meta-analysis. BMJ Open. 2018;8(10):e021611. https://doi.org/10.1136/bmjopen-2018-021611.
Esposito K, Chiodini P, Colao A, Lenzi A, Giugliano D. Metabolic syndrome and risk of cancer: a systematic review and meta-analysis. Diabetes Care. 2012;35(11):2402–11. https://doi.org/10.2337/dc12-0336.
Leisegang K, Henkel R, Agarwal A. Obesity and metabolic syndrome associated with systemic inflammation and the impact on the male reproductive system. Am J Reprod Immunol. 2019;82(5):e13178. https://doi.org/10.1111/aji.13178.
Hursting SD, Hursting MJ. Growth signals, inflammation, and vascular perturbations: mechanistic links between obesity, metabolic syndrome, and cancer. Arterioscler Thromb Vasc Biol. 2012;32(8):1766–70. https://doi.org/10.1161/ATVBAHA.111.241927.
Uzunlulu M, Telci Caklili O, Oguz A. Association between Metabolic Syndrome and Cancer. Ann Nutr Metab. 2016;68(3):173–9. https://doi.org/10.1159/000443743.
Jaggers JR, Sui X, Hooker SP, LaMonte MJ, Matthews CE, Hand GA, Blair SN. Metabolic syndrome and risk of cancer mortality in men. Eur J Cancer. 2009;45(10):1831–8. https://doi.org/10.1016/j.ejca.2009.01.031.
Russo A, Autelitano M, Bisanti L. Metabolic syndrome and cancer risk. Eur J Cancer. 2008;44(2):293–7. .
Inoue M, Noda M, Kurahashi N, Iwasaki M, Sasazuki S, Iso H, Tsugane S. Impact of metabolic factors on subsequent cancer risk: results from a large-scale population-based cohort study in Japan. Eur J Cancer Prev. 2009;18(3):240–7. https://doi.org/10.1097/CEJ.0b013e3283240460.
Osaki Y, Taniguchi S, Tahara A, Okamoto M, Kishimoto T. Metabolic syndrome and incidence of liver and breast cancers in Japan. Cancer Epidemiol. 2012;36(2):141–7. https://doi.org/10.1016/j.canep.2011.03.007.
van Kruijsdijk RC, van der Graaf Y, Peeters PH, Visseren FL. Cancer risk in patients with manifest vascular disease: effects of smoking, obesity, and metabolic syndrome. Cancer Epidemiol Biomarkers Prev. 2013;22(7):1267–77. https://doi.org/10.1158/1055-9965.EPI-13-0090.
Ko S, Yoon SJ, Kim D, Kim AR, Kim EJ, Seo HY. Metabolic Risk Profile and Cancer in Korean Men and Women. J Prev Med Public Health. 2016;49(3):143–52. https://doi.org/10.3961/jpmph.16.021.
Gathirua-Mwangi WG, Monahan PO, Murage MJ, Zhang J. Metabolic syndrome and total cancer mortality in the Third National Health and Nutrition Examination Survey. Cancer Causes Control. 2017;28(2):127–36. https://doi.org/10.1007/s10552-016-0843-1.
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–122.
Higgins J, Green S (2011) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration https://www.cochranehandbook.org
Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P (2010) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. https://doi.org/10.1002/sim.1186.
Patsopoulos NA, Evangelou E, Ioannidis JP. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int J Epidemiol. 2008;37(5):1148–57. https://doi.org/10.1093/ije/dyn065.
Amos CI, Hung R, Bosse Y, Christiani DC, Field JK, Landi MT, Brennan PB, McKay JP (2016) P1.04: Defining the Genetic Architecture of Lung Cancer Etiology: Track: Prevention, Early Detection, Epidemiology and Tobacco Control. J Thorac Oncol 11 (10S):S182. https://doi.org/10.1016/j.jtho.2016.08.026
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
Grundy SM, Cleeman JI, Merz CN, Brewer HB Jr, Clark LT, Hunninghake DB, Pasternak RC, Smith SC Jr, Stone NJ. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110(2):227–39. https://doi.org/10.1161/01.CIR.0000133317.49796.0E.
Alberti KG, Zimmet P, Shaw J. Metabolic syndrome–a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23(5):469–80. https://doi.org/10.1111/j.1464-5491.2006.01858.x.
Yang Y, Dong J, Sun K, Zhao L, Zhao F, Wang L, Jiao Y. Obesity and incidence of lung cancer: a meta-analysis. Int J Cancer. 2013;132(5):1162–9. https://doi.org/10.1002/ijc.27719.
Guo L, Liu S, Zhang S, Chen Q, Zhang M, Quan P, Lu J, Sun X. A meta-analysis of body mass index and the risk of lung cancer in the Chinese population. Zhonghua Yu Fang Yi Xue Za Zhi. 2015;49(7):649–53.
Hidayat K, Du X, Chen G, Shi M, Shi B. Abdominal Obesity and Lung Cancer Risk: Systematic Review and Meta-Analysis of Prospective Studies. Nutrients. 2016;8:12. https://doi.org/10.3390/nu8120810.
Gao J, Lin X, He Y, Fu Y, Wu Y, Liao J, Lian X. The Comparison of Different Obesity Indexes and the Risk of Lung Cancer: A Meta-Analysis of Prospective Cohort Studies. Nutr Cancer. 2019;71(6):908–21. https://doi.org/10.1080/01635581.2019.1595037.
Lin X, Lu L, Liu L, Wei S, He Y, Chang J, Lian X. Blood lipids profile and lung cancer risk in a meta-analysis of prospective cohort studies. J Clin Lipidol. 2017. https://doi.org/10.1016/j.jacl.2017.05.004.
Hao B, Yu M, Sang C, Bi B, Chen J. Dyslipidemia and non-small cell lung cancer risk in Chinese population: a case-control study. Lipids Health Dis. 2018;17(1):278. https://doi.org/10.1186/s12944-018-0925-z.
Lyu Z, Li N, Wang G, Feng X, Chen S, Su K, Li F, Wei L, Li X, Xie S, Guo L, Chen Y, Tan F, Yin J, Cui H, Chen H, Li J, Ren J, Shi J, Wu S, Dai M, He J. Independent and joint associations of blood lipids and lipoproteins with lung cancer risk in Chinese males: A prospective cohort study. Int J Cancer. 2019;144(12):2972–84. https://doi.org/10.1002/ijc.32051.
Stocks T, Van Hemelrijck M, Manjer J, Bjorge T, Ulmer H, Hallmans G, Lindkvist B, Selmer R, Nagel G, Tretli S, Concin H, Engeland A, Jonsson H, Stattin P. Blood pressure and risk of cancer incidence and mortality in the Metabolic Syndrome and Cancer Project. Hypertension. 2012;59(4):802–10. https://doi.org/10.1161/HYPERTENSIONAHA.111.189258.
Lee SY, Kim MT, Jee SH, Im JS. Does hypertension increase mortality risk from lung cancer? A prospective cohort study on smoking, hypertension and lung cancer risk among Korean men. J Hypertens. 2002;20(4):617–22.
Wang Z, Bao C, Su C, Xu W, Luo H, Chen L, Qi X. Association between diabetes or antidiabetic therapy and lung cancer: A meta-analysis. J Diabetes Investig. 2013;4(6):659–66. https://doi.org/10.1111/jdi.12112.
Park HJ, Joh HK, Choi S, Park SM. Type 2 diabetes mellitus does not increase the risk of lung cancer among never-smokers: a nationwide cohort study. Transl Lung Cancer Res. 2019;8(6):1073–7. https://doi.org/10.21037/tlcr.2019.11.01.
Xiao K, Liu F, Liu J, Xu J, Wu Q, Li X. The effect of metformin on lung cancer risk and survival in patients with type 2 diabetes mellitus: a meta-analysis. J Clin Pharm Ther. 2020. https://doi.org/10.1111/jcpt.13167.
Acknowledgement
Not applicable.
Funding
None.
Author information
Authors and Affiliations
Contributions
WL and PH designed the study. LQ, DM, HL and DS performed database search, study inclusion, quality evaluation, and data extraction. MF, YC, FX, ZW, and YW performed statistical analyses and interpreted the data. LQ, DM, HL and WL drafted the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All analyses were based on previous published studies, thus no ethical approval and patient consent are required. All previous published studies were approved by ethics committee respectively.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Qiao, L., Ma, D., Lv, H. et al. Metabolic syndrome and the incidence of lung cancer: a meta-analysis of cohort studies. Diabetol Metab Syndr 12, 95 (2020). https://doi.org/10.1186/s13098-020-00598-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13098-020-00598-0