Abstract
Objectives
To evaluate the effect of Lactobacillus casei 01 on dietary intake, body weight, and glycemic control in patients with T2DM.
Method
Forty patients with T2DM (n = 20 for each group) were assigned into two groups in present trial. The patients in the probiotic group received a daily capsule containing a minimum of 108 CFU of L. casei 01 for 8 week. The placebo group took capsules filled with maltodextrin for the same time period. Dietary intake questionnaires and anthropometric measurements were collected, and the participants were assessed by an endocrinologist at baseline and at the end of the trial.
Results
Lactobacillus casei 01 supplementation significantly decreased total energy, carbohydrate, fat, and protein intake compared with placebo (p = 0.001, p = 0.002, p = 0.009, p = 0.001; respectively). Moreover weight, BMI, and waist circumference were significantly decreased in intervention group compared with placebo group (p < 0.001; p < 0.001; p = 0.029; respectively). In comparison with placebo group serum fetuin-A level, fasting blood sugar, insulin concentration, and insulin resistance were significantly decreased (p = 0.023, p =0.013, p = 0.028; p = 0.007; respectively), and serum SIRT1 level was significantly increased (p = 0.040) in intervention group.
Conclusions
Lactobacillus casei 01 supplementation affected dietary intake and body weight in a way that improved fetuin-A and SIRT1 levels and glycemic response in subjects with T2DM. Affecting the fetuin-A and SIRT1 levels introduces a new known mechanism of probiotic action in diabetes management.
Similar content being viewed by others
Introduction
The increase in obesity levels, because of unhealthy lifestyle, is predicted to significantly augment the incidence of type 2 diabetes. Type 2 diabetes mellitus (T2DM), a metabolic disorder known by high blood glucose, is among the top ten causes of death globally. The global prevalence of diabetes in adults of 20–79 years is now 7.3% (4.8–11.9%) that is estimated to reach to 8.3% (5.6%–13.9%) by 2045 [1]. There is strong and consistent evidence that obesity management can be beneficial in the treatment of type 2 diabetes [2, 3]. In overweight and obese patients with type 2 diabetes, modest weight loss has been shown to improve glycemic control and to reduce the need for glucose-lowering medications [4]. Small studies have demonstrated that in obese patients with type 2 diabetes dietary energy restriction can reduce A1C and fasting glucose in the absence of pharmacologic therapy or ongoing procedures [5].
Common current medications approved for the treatment of T2DM has a number of limitations, such as side effects and secondary failure; so, much effort has been focused on natural products as complementary or alternative diabetic treatments without side effects or toxicity [6, 7]. In recent years, it has been reported that probiotics, especially lactic acid bacteria, have efficacy relating to the management of diabetes [8]. Probiotics, the live microorganisms, present health benefits to the host, especially when administered in sufficient amounts [9].
The intestinal microbiota could affect the host by influencing bile acid metabolism, body weight, pro-inflammatory status and insulin resistance, and modulating the gut hormones. Modulating gut microbiota by consumption of probiotics could have beneficial effects on glucose metabolism through several mechanisms [10]. One of the key therapeutic goal in both the prevention and management of T2DM is weight reduction [11]. Physiologic functions of probiotics would contribute to the health of gut microbiota and can affect appetite and food intake, body weight and composition and metabolic functions through gastrointestinal pathways and modulate the gut bacterial community [12].
Considering the potential of probiotic bacteria the aim of the present randomized clinical trial was to investigate the effects of Lactobacillus casei 01 (L. casei 01) supplementation on dietary intake, body weight, and glycemic control in patients with T2DM.
Materials and methods
Subjects
An 8 week, parallel-group, randomized controlled trial was conducted in the Sheykholrayis Polyclinic of Tabriz University of Medical Sciences, Tabriz, Iran. The recruitment process of participants began in September 2016, and the intervention was carried out in January 2017.
The target population of the present study was patients with T2DM. Subjects were contacted a day before commencing the supplementation, and the study was thoroughly explained to them. Volunteers were composed of 44 patients with T2DM, 30–50 years of age, and body mass index (BMI) lower than 35 kg/m2. All patients had been diagnosed with T2DM for at least 1 year. Exclusion criteria were smoking, the presence of kidney, liver, and/or inflammatory intestinal disease, thyroid disorders, immunodeficiency diseases, required insulin injections, use of nutritional supplements within the previous 3 weeks of testing, use of estrogen or progesterone, pregnancy or breast-feeding, consuming any type of antibiotics, and consuming any other probiotic products within the previous 2 months of testing. Primary endpoints were the promotion of SIRT1, reduction of fetuin-A levels, and control of glycemic response, and secondary endpoint was the management of dietary intake and body weight.
The sample size for the current study was calculated on the basis of FBS results reported by Ostadrahimi et al. [13] with a confidence level of 95% and a power of 80%, which was found to be 18 patients. Taking into account the probable dropout of patients during the intervention course as well as the patients who may not adhere to the study protocol, 22 patients with T2DM were recruited for each group.
Study design and measurements
Of 44 patients who had met the inclusion criteria, 4 were excluded because of their unwillingness to participate in the study. Subjects were randomly assigned to the probiotic (n = 20) or placebo (n = 20) group, using a block randomization procedure with stratified subjects in each block based on sex and age. The allocation of the intervention or placebo group was concealed from the researchers, and the probiotic and placebo capsules had both an identical appearance and labeled information. Therefore, neither the subjects nor the investigators were aware of the treatment assignments in this double-blinded study. Over 8 weeks, both groups consumed probiotic capsules containing 108 cfu L. casei 01 (Chr. Hansen, Denmark) or placebo capsules. Considering the buffering capacity of the food on the survival of probiotic microbes during gastrointestinal transit [14], the patients were asked to take the capsules with or just prior to a meal containing some fats. All patients were asked, throughout the 8-week trial, to maintain their usual dietary habits and lifestyle. The patients were instructed to keep the capsules under refrigeration and to avoid any changes in medication, if possible.
Arrangements were made so that the patients would receive the 8-week supply of their probiotic or placebo capsules at the beginning of the trial and were asked to take a capsule daily. Compliance with the capsule consumption guidelines was monitored by telephone interviews once a week. Information on demographic and anthropometric measurements and fasting blood samples were collected at the beginning and at the end of the trial. Nutrient intakes during 3 days were estimated using a 24-h dietary recall at the beginning, in the middle, and at the end of the study. Three-day averages of macro- and micro-nutrient intakes were analyzed by Nutritionist 4 software (First Databank, Hearst Corp, San Bruno, CA, USA). International Physical Activity Questionnaire (IPAQ) [15] was completed for participants to assess physical activity level.
Anthropometric measurements were recorded by trained personnel. A blood sample was drawn for each patient after an overnight fasting. All whole blood and serum samples were collected and kept at − 70 °C until the assay. Blood samples were analyzed at the Drug Applied Research Center (Tabriz University of Medical Sciences, Tabriz, Iran).
Fasting blood glucose was measured using the standard enzymatic method with the Pars Azmun kit (Karaj, Iran). Glycated hemoglobin (HbA1c) was measured in the whole blood by cation exchange chromatography with the NycoCard HbA1c kit (Oslo, Norway). Insulin concentration was determined by a chemiluminescent immunoassay using a Liaison analyzer (Diameter, Italy). To measure insulin resistance, insulin resistance index, HOMA-IR (Homeostatic Model Assessment of Insulin Resistance), was used: HOMA1-IR = FPI (mg/dl) × FPG (mg/dl))/22.5. Serum fetuin-A and SIRT1 concentrations were measured by human ELISA kits (Diameter, Italy and Bioassay Technology Laboratory, China).
The present study was conducted in accordance with the guidelines laid down in the Declaration of Helsinki, and all procedures were approved by the Ethics Committee of Tabriz University of Medical Sciences (no. IR.TBZMED.REC.1395.402). A written informed consent was obtained from each patient.
Intervention procedure
Hard yellow gelatin capsules were used as delivery vehicle in the present study. L. casei 01 was the active agent of the probiotic capsules, and maltodextrin was used as the excipient. The capsules were prepared using a capsule filling device under aseptic condition. To check the quality of probiotic capsules and ensure that an adequate dose of the probiotic was consumed by the experiment group (at least 108 CFU/day), the bacterial count of the capsules was checked by a food technologist at the baseline, in the middle, and at the end of the trial period, by culturing the contents of three capsules at each. The capsules were cultured with the use of MRS agar (De Man, Rogosa and Sharpe agar) via serial dilution and the pour plate technique. Bacterial enumeration of the capsules showed that the capsules contained a minimum of 108 colony-forming units of L. casei 01 during the study period The placebo capsules contained only maltodextrin. Since the bacterial count of the excipient could confound the outcomes of the study, the powder was cultured to ensure it was free of pathogens. Capsule count was performed by the researcher at the end of the study to evaluate compliance.
Statistical analyses
Statistical analysis was performed by SPSS software (ver. 17; SPSS Inc. IL, Chicago, USA). Normality of the numeric variables was checked by Kolmogorov–Smirnov test [16]. Data were presented using mean (SD), median (min–max) for the numeric normal, and non-normal variables, respectively as well as the percentage of frequency for categorical variables. The between-group comparisons of baseline measures and demographic variables were conducted with independent t-test and/or Chi square test where appropriate. For within-group comparisons, paired t-tests were used, where before and after intervention measurements were taken. To assess the effect of intervention, the analysis of covariance (ANCOVA) was used to control baseline measurements and confounders. In all analyses, p values less than 0.05 were considered statistically as significant.
Results
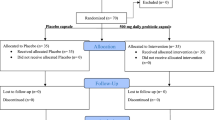
As revealed in the study flow diagram (Fig. 1), 40 patients with T2DM [probiotic (n = 20) and placebo (n = 20)] completed the trial. Capsule counts showed good compliance on the part of the participants who completed the study, and no adverse effects were reported.
Baseline characteristics of the patients are presented in Table 1; there were no significant differences between the two groups with regard to any of the baseline characteristics (p > 0.05).
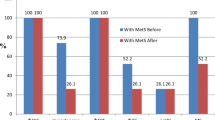
The analysis of dietary questionnaires, which is presented in Fig. 2, revealed that the two groups had no significant differences for TEI and the intake of carbohydrate, fat, and protein at baseline [− 54.32 (− 298.83 to 190.29), 0.656; − 10.15 (− 43.24 to 22.94), 0.387; − 2.02 (− 10.20 to 6.15), 0.619; -2.22 (− 11.41 to 6.96), 0.627; respectively]. Taking a look to Fig. 2 we can find that the TEI and the intake of carbohydrate, fat, and protein was significantly reduced during the intervention period in the probiotic group compared with placebo group [− 35.80 (− 55.47 to − 16.13), 0.001; − 8.67 (− 13.82 to − 3.52), 0.002; − 4.29 (− 7.45 to − 1.13), 0.009; − 1.35 (− 1.89 to − 0.81), 0.001; respectively]. Moreover, he within group changes were significant [p = 0.003, p < 0.001, p < 0.001, p = 0.001; respectively].
The effect of consumption of L. casei 01 on anthropometric variables is presented in Fig. 3. The anthropometric variables including weight, BMI, waist circumference, and WHR were not significantly different at baseline between the two groups [− 6.30 (− 15.74 to 3.14), 0.185; − 2.43 (− 5.47 to 0.60), 0.113; − 5.40 (− 11.54 to 0.74), 0.083; − 0.01 (− 0.05 to 0.03), 0.647; respectively]. As shown in Fig. 3 consumption of L. casei 01 for 2 months significantly decreased weight, BMI, and waist circumference in diabetic patients compared with placebo group [− 1.52 (− 2.31 to − 0.76), < 0.001; − 0.84 (− 1.26 to − 0.41), < 0.001; − 1.77 (− 3.36 to − 0.18), 0.029; respectively]. Moreover the within group changes were significant in probiotic group [− 1.20 (− 1.81 to − 0.58), 0.001; − 0.485 (− 0.73 to − 0.23), 0.001; − 2.15 (3.30 to − 0.99), 0.001; respectively]. Although between-group change for WHR was not significant [− 0.01 (− 0.02 to 0.00), 0.052)] the within-group change was statistically significant [− 0.020 (− 0.031 to − 0.009), 0.001].
The effect of L. casei 01 supplementation on biochemical parameters is shown in Fig. 4. The serum fetuin-A level was significantly decreased and level of SIRT1 significantly increased after 2-month intervention in probiotic group in comparison with placebo group [− 17.56 (− 32.54 to − 2.58), 0.023; 0.52 (0.026 to 1.02), 0.040; respectively]. The within group changes were statistically significant [− 11.90 (− 20.29 to − 3.51), 0.008; 0.52 (0.17 to 0.87), 0.006; respectively]. After the 2-month intervention, FBS, serum fasting insulin level, and HOMA.IR index significantly reduced in the intervention group compared with placebo group [− 28.32 (− 50.23 to − 6.41), 0.013; − 3.12 (− 5.90 to − 0.35), 0.028; − 32.31 (− 55.09 to − 9.54), 0.007; respectively]. The within-group differences for the mentioned glycemic response parameters were significant [− 28.35 (− 45.39 to − 11.31), 0.002; − 2.33 (− 4.48 to − 0.18), 0.035; − 29.72 (− 45.62 to − 13.82), 0.001; respectively]. Evaluation of HbA1c after 2-month supplementation showed no significant reduction in probiotic group in comparison with placebo group [− 0.45 (− 0.96 to 0.05), 0.077]; moreover the within group reduction was not significant [− 0.24 (− 0.60 to 0.12), 0.190].
Discussion
Management of diabetes without any side effects by natural food is a challenge for medical nutrition therapy of diabetes. To the best of our knowledge, this is the first study evaluating the effect of L. casei 01 supplementation on dietary intake and anthropometric parameters in patients with T2DM. L. casei 01 supplementation for 8 weeks significantly affected dietary intake and anthropometric indexes, including weight, BMI, and waist circumference. Moreover, the outcomes showed that, compared with placebo, L. casei 01 supplementation decreased fetuin-A and increased SIRT1 level and improved glycemic response in patients with T2DM.
Probiotics have physiologic functions that contribute to the health of gut microbiota, can affect food intake and appetite, body weight and composition and metabolic functions through gastrointestinal pathways and modulation of the gut bacterial community [10, 12]. By modulating the gut microbiota, probiotics can affect the energy balance and/or metabolism of the host. Limited evidence exists on the effect of probiotic consumption on weight management in humans. The findings of present trial were in accordance with the study conducted by Kadooka et al. in which they reported that a supplementation of fermented milk with Lactobacillus gasseri SBT2055 for 12 weeks induces significant weight loss and a decrease in BMI, waist and hip circumferences, and body fat mass [17]. Omar et al. showed that the consumption of yogurt supplemented with Lactobacillus leads to a decrease in total body fat mass [18].
A possible way for manipulating the mammalian eating behavior and body weight by probiotic bacteria is appetite-regulating hormones. Supplementation with VSL#3, containing Lactobacillus strains, in mice reduced appetite-inducing hormones and neuropeptide Y in the hypothalamus [19, 20]. Moreover the levels of cholecystokinin, leptin, and other satiety peptides, which regulate food intake and hunger by affecting vagus nerve signaling, were improved [21]. Moreover; probiotics can modulate energy intake and metabolism by the production of short chain fatty acids (SCFAs) from indigestible polysaccharides [12]. SCFAs such as acetate, butyrate and propionate, produced by bacterial fermentation function as energy substrates, regulates satiety and food intake [22]. By activating the G-protein-coupled receptors GPR41 and GPR43 on intestinal epithelial cells, SCFAs stimulate peptide YY (PYY) and glucagon-like peptide (GLP)-1 secretion [12].
According to the results shown in Fig. 4 the level of fetuin-A and SIRT1 was significantly affected by probiotic consumption. The effect of probiotic supplementation on fetuin-A and/or SIRT1 was not evaluated in previous studies. Fetuin-A, a circulating glycoprotein that is secreted by the liver and adipose tissues, inhibits insulin receptor tyrosine kinase activity in animal studies [23]. Fetuin-A knockout mice have enhanced glucose sensitivity, resistance to weight gain and lower serum-free fatty acid levels [24]. In humans, the liver-secreted fetuin-A is associated with atherosclerosis, insulin resistance, T2DM, and metabolic syndrome [25]. In cross-sectional analyses, Ismail et al. [26] showed that fetuin-A levels were higher in adults and children with obesity and metabolic syndrome. Sirtuins (SIRTs), ubiquitous deacetylase, are main regulators of energy homeostasis and metabolism [27]. SIRT1 has a positive impact on obesity, diabetes mellitus, liver steatosis, and other metabolic disorders [28]. Due to its deacetylation activity, SIRT1 influences many steps of glucose metabolism in liver, pancreas, muscle and adipose tissue and regulates insulin secretion [29]. It has been demonstrated that lean subjects have higher expression of SIRT1 in the adipose tissue compared to obese.
It has been reported that weight loss and caloric restriction (CR) can affect both fetuin-A and SIRT1 levels [25, 26]. Haukeland et al. [30] showed that substantial weight loss in children led to a significant decrease in fetuin-A concentrations. Brix et al. [31] reported that elevated fetuin-A levels in morbid obesity decreased after bariatric surgery. Choi et al. [25] found that CR significantly decreases hepatic fetuin-A expression and its circulating levels in overweight rats and humans with T2DM. Mariani et al. [32] found that the reduction of body fat mass was associated with increased plasma SIRT1; moreover they showed that, in addition to the tissue levels, the circulating SIRT1 could be increased by a negative caloric balance. Calorie restriction (CR) has been reported to increase SIRT1 protein levels and activity in mice, rats, and humans [33, 34].
Considering the effect of calorie restriction and weight loss on fetuin-A and SIRT1 levels it can be understood that by reducing the appetite and dietary intake and body weight, L. casei 01 could affect the plasma level of fetuin-A and SIRT1 in patients with T2DM in present trial.
Improvements in glycemic control by probiotic bacteria, as seen in this study, were in accordance with other similar studies conducted previously [35,36,37,38]. The antidiabetic property of Bifidobacteria and Lactobacillus has been evaluated in human and animal studies [8, 36, 39,40,41]. Ejtahed et al. [42] declared that probiotic yogurt, containing Lactobacillus acidophilus La5 and Bifidobacterium lactis Bb12, decreased fasting blood glucose and HbA1c in patients with T2DM. Ostadrahimi et al. [13] revealed that consumption of probiotic fermented milk, containing L. acidophilus, L. casei, and Bifidobacteria decreased fasting blood glucose and HbA1c compared with control group. The results of a meta-analysis, conducted by Yao et al. [43], demonstrated that probiotics supplementation was associated with significant improvement in fasting insulin level in patients with T2DM. Andreasen et al. showed that the intake of L. acidophilus NCFM for 4 weeks preserved insulin sensitivity compared with placebo [36]. Similarly Kobyliak et al. found that supplementation with alive multi-probiotic for 8 weeks was associated with significant reduction of HOMA-IR [44]. Several possible mechanisms of hypoglycemic effect of probiotics are discussed. Probiotics can affect gut bacteria to produce insulin-tropic polypeptides and GLP-1 (glucagon-like peptide-l) and glucose-dependent insulinotropic polypeptide [GIP]; so, increase glucose uptake by muscle, stimulates the liver absorption of blood glucose, and increase in the amount of insulin released from the β cells of the islets [43]. By modulation of intestinal microbiota composition probiotics can improve intestinal barrier function and diminish the translocation of micro-organisms and their derivatives [44], from the gut to the systemic circulation, thereby reducing the concomitant release of pro-inflammatory cytokines. Moreover, antioxidant properties of lactic acid bacteria have been shown in previous studies [24]. Modulating inflammation and oxidative stress has been announced as the possible mechanisms of probiotics’ action in improvement of glycemic response in previous researches.
Considering the results of present trial L. casei 01 could control glycemic response by controlling dietary intake and body weight and then manipulating the level of fetuin-A and SIRT1 in patients with T2DM. Affecting fetuin-A and SIRT1 levels could be introduce as a new-known mechanism of lactic acid bacteria’s action in diabetes management. Further studies on the effects of other probiotic strains on dietary intake, anthropometric indexes, and serum fetuin-A and SIRT1 levels in diabetic patients would be useful.
Evaluating the appetite hormones is suggested to be done in future researches which was the limitation of present trial.
Conclusion
According to the results of present trial, probiotics affected patients’ weight, BMI, and waist circumference by influencing dietary intake. The beneficial effects of probiotics on body weight could be translated into favorable metabolic effects, i.e. improvements in fetuin-A and SIRT1 levels, insulin resistance/glycemic control, and exert beneficial effects on glucose homeostasis. Taking into account the metabolic impacts of SIRT1 and fetuin-A, management of their levels could be effective in diabetes control. The results of present trial helped us to reveal a new mechanism of probiotics action in diabetes and its related metabolic disorders’ control.
Change history
26 May 2023
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1186/s13098-023-01094-x
Abbreviations
- T2DM:
-
type 2 diabetes mellitus
- SIRTs:
-
sirtuins
- NCDs:
-
non-communicable diseases
- BMI:
-
body mass index
- IPAQ:
-
International Physical Activity Questionnaire
- HbA1c:
-
glycated hemoglobin
- HOMA-IR:
-
homeostatic model assessment of insulin resistance
- FPI:
-
fasting plasma insulin
- FPG:
-
fasting plasma glucose
- WHR:
-
waist to heap ratio
- GIP:
-
glucose-dependent insulinotropic polypeptide
- GLP:
-
glucagon-like peptide
- TLR:
-
toll-like receptor
- CR:
-
calorie restriction
- SCFA:
-
short chain fatty acids
- GPR:
-
G-protein-coupled receptor
- PYY:
-
peptide YY
References
International Diabetes Federation. The global picture. 8th ed. Brussels: International Diabetes Federation; 2017.
Rothberg AE, McEwen LN, Kraftson AT, Fowler CE, Herman WH. Very-low-energy diet for type 2 diabetes: an underutilized therapy? J Diabetes Complications. 2014;28(4):506–10.
Association AD. 7. Obesity management for the treatment of type 2 diabetes: standards of medical care in diabetes—2018. Diabetes Care. 2018;41(Supplement 1):S65–72.
Pastors JG, Warshaw H, Daly A, Franz M, Kulkarni K. The evidence for the effectiveness of medical nutrition therapy in diabetes management. Diabetes Care. 2002;25(3):608–13.
Steven S, Hollingsworth KG, Al-Mrabeh A, Avery L, Aribisala B, Caslake M, et al. Very-low-calorie diet and 6 months of weight stability in type 2 diabetes: pathophysiologic changes in responders and nonresponders. Diabetes Care. 2016. https://doi.org/10.2337/dc15-1942
Mang B, Wolters M, Schmitt B, Kelb K, Lichtinghagen R, Stichtenoth D, et al. Effects of a cinnamon extract on plasma glucose, HbA1c, and serum lipids in diabetes mellitus type 2. Eur J Clin Invest. 2006;36(5):340–4.
Lee Y-S, Park M-J, Choi J-E, Kim J-Y, Nam M-S, Jeong Y-H. Effects of silk protein hydrolysates on blood glucose level, serum insulin and leptin secretion in OLETF rats. J Korean Soc Food Sci Nutr. 2007;36(6):703–7.
Yun S, Park H, Kang J. Effect of Lactobacillus gasseri BNR17 on blood glucose levels and body weight in a mouse model of type 2 diabetes. J Appl Microbiol. 2009;107(5):1681–6.
Bastani P, Akbarzadeh F, Homayouni A, Javadi M, Khalili L. Health benefits of probiotic consumption. Microbes in food and health. Beriln: Springer; 2016. p. 163–83.
Rad AH, Abbasalizadeh S, Vazifekhah S, Abbasalizadeh F, Hassanalilou T, Bastani P, Ejtahed HS, et al. The future of diabetes management by healthy probiotic microorganisms. Curr. Diabetes Rev. 2017;13(6):582–9.
Van Gaal L, Scheen A. Weight management in type 2 diabetes: current and emerging approaches to treatment. Diabetes Care. 2015;38(6):1161–72.
Kobyliak N, Conte C, Cammarota G, Haley AP, Styriak I, Gaspar L, et al. Probiotics in prevention and treatment of obesity: a critical view. Nutr Metab. 2016;13(1):14.
Ostadrahimi A, Taghizadeh A, Mobasseri M, Farrin N, Payahoo L, Gheshlaghi ZB, et al. Effect of probiotic fermented milk (kefir) on glycemic control and lipid profile in type 2 diabetic patients: a randomized double-blind placebo-controlled clinical trial. Iranian J Public Health. 2015;44(2):228.
Tompkins T, Mainville I, Arcand Y. The impact of meals on a probiotic during transit through a model of the human upper gastrointestinal tract. Benef Microb. 2011;2(4):295–303.
Moghaddam MB, Aghdam FB, Jafarabadi MA, Allahverdipour H, Nikookheslat SD, Safarpour S. The Iranian Version of International Physical Activity Questionnaire (IPAQ) in Iran: content and construct validity, factor structure, internal consistency and stability. World Appl Sci. 2012;18(8):1073–80.
Asghari Jafarabadi M, Mohammadi S. Statistical series: introduction to statistical inference (point estimation, confidence interval and hypothesis testing). J Diabetes Lipid Disorders. 2013;12(3):173–92.
Kadooka Y, Sato M, Imaizumi K, Ogawa A, Ikuyama K, Akai Y, et al. Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur J Clin Nutr. 2010;64(6):636.
Omar JM, Chan Y-M, Jones ML, Prakash S, Jones PJ. Lactobacillus fermentum and Lactobacillus amylovorus as probiotics alter body adiposity and gut microflora in healthy persons. J Funct Foods. 2013;5(1):116–23.
Yadav H, Lee J-H, Lloyd J, Walter P, Rane SG. Beneficial metabolic effects of a probiotic via butyrate-induced GLP-1 hormone secretion. J Biol Chem. 2013;288(35):25088–97.
Homayouni-Rad A, Soroush A-R, Khalili L, Norouzi-Panahi L, Kasaie Z, Ejtahed H-S. Diabetes management by probiotics: current knowledge and future pespective. Int J Vitam Nutr Res. 2017;1(1):1–13.
Duca FA, Swartz TD, Sakar Y, Covasa M. Increased oral detection, but decreased intestinal signaling for fats in mice lacking gut microbiota. PLoS ONE. 2012;7(6):e39748.
Shen J, Obin MS, Zhao L. The gut microbiota, obesity and insulin resistance. Mol Aspects Med. 2013;34(1):39–58.
Srinivas P, Wagner AS, Reddy LV, Deutsch D, Leon MA, Goustin AS, et al. Serum alpha 2-HS-glycoprotein is an inhibitor of the human insulin receptor at the tyrosine kinase level. Mol Endocrinol. 1993;7(11):1445–55.
Mathews ST, Singh GP, Ranalletta M, Cintron VJ, Qiang X, Goustin AS, et al. Improved insulin sensitivity and resistance to weight gain in mice null for the Ahsg gene. Diabetes. 2002;51(8):2450–8.
Choi KM, Han KA, Ahn HJ, Lee SY, Hwang SY, Kim BH, et al. The effects of caloric restriction on F etuin-A and cardiovascular risk factors in rats and humans: a randomized controlled trial. Clin Endocrinol. 2013;79(3):356–63.
Ismail NA, Ragab S, El Dayem SMA, ElBaky AA, Salah N, Hamed M, et al. Fetuin-A levels in obesity: differences in relation to metabolic syndrome and correlation with clinical and laboratory variables. Arch Med Sci AMS. 2012;8(5):826.
Uskova M, Kravchenko L. Antioxidant properties of lactic acid bacteria—probiotic and yogurt strains. Vopr Pitan. 2009;78(2):18–23.
Herranz D, Serrano M. SIRT1: recent lessons from mouse models. Nat Rev Cancer. 2010;10(12):819.
Turkmen K, Karagoz A, Kucuk A. Sirtuins as novel players in the pathogenesis of diabetes mellitus. World J Diabetes. 2014;5(6):894.
Haukeland JW, Dahl TB, Yndestad A, Gladhaug IP, Løberg EM, Haaland T, et al. Fetuin A in nonalcoholic fatty liver disease: in vivo and in vitro studies. Eur J Endocrinol. 2012;166(3):503–10.
Brix JM, Stingl H, Höllerl F, Schernthaner GH, Kopp H-P, Schernthaner G. Elevated Fetuin-A concentrations in morbid obesity decrease after dramatic weight loss. J Clin Endocrinol Metab. 2010;95(11):4877–81.
Mariani S, Fiore D, Persichetti A, Basciani S, Lubrano C, Poggiogalle E, et al. Circulating SIRT1 increases after intragastric balloon fat loss in obese patients. Obes Surg. 2016;26(6):1215–20.
Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell. 2006;127(6):1109–22.
Cantó C, Auwerx J. Caloric restriction, SIRT1 and longevity. Trends Endocrinol Metab. 2009;20(7):325–31.
Laitinen K, Poussa T, Isolauri E. Probiotics and dietary counselling contribute to glucose regulation during and after pregnancy: a randomised controlled trial. Br J Nutr. 2008;101(11):1679–87.
Andreasen AS, Larsen N, Pedersen-Skovsgaard T, Berg RM, Møller K, Svendsen KD, et al. Effects of Lactobacillus acidophilus NCFM on insulin sensitivity and the systemic inflammatory response in human subjects. Br J Nutr. 2010;104(12):1831–8.
Ejtahed HS, Mohtadi Nia J, Homayouni Rad A, Niafar M, Asghari Jafarabadi M, Mofid V. The effects of probiotic and conventional yoghurt on diabetes markers and insulin resistance in type 2 diabetic patients: a randomized controlled clinical trial. Iranian J Endocrinol Metab. 2011;13(1):1–8.
Asemi Z, Zare Z, Shakeri H, Sabihi S-S, Esmaillzadeh A. Effect of multispecies probiotic supplements on metabolic profiles, hs-CRP, and oxidative stress in patients with type 2 diabetes. Ann Nutr Metab. 2013;63(1–2):1–9.
Yadav H, Jain S, Sinha P. Antidiabetic effect of probiotic dahi containing Lactobacillus acidophilus and Lactobacillus casei in high fructose fed rats. Nutrition. 2007;23(1):62–8.
Moroti C, Magri LFS, de Rezende Costa M, Cavallini DC, Sivieri K. Effect of the consumption of a new symbiotic shake on glycemia and cholesterol levels in elderly people with type 2 diabetes mellitus. Lipids Health Dis. 2012;11(1):29.
Lin C-H, Lin C-C, Shibu MA, Liu C-S, Kuo C-H, Tsai F-J, et al. Oral Lactobacillus reuteri GMN-32 treatment reduces blood glucose concentrations and promotes cardiac function in rats with streptozotocin-induced diabetes mellitus. Br J Nutr. 2014;111(4):598–605.
Ejtahed HS, Mohtadi-Nia J, Homayouni-Rad A, Niafar M, Asghari-Jafarabadi M, Mofid V. Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition. 2012;28(5):539–43.
Yao K, Zeng L, He Q, Wang W, Lei J, Zou X. Effect of probiotics on glucose and lipid metabolism in type 2 diabetes mellitus: a meta-analysis of 12 randomized controlled trials. Med Sci Monit Int Med J Exp Clin Res. 2017;23:3044.
Kobyliak N, Falalyeyeva T, Mykhalchyshyn G, Kyriienko D, Komissarenko I. Effect of alive probiotic on insulin resistance in type 2 diabetes patients: randomized clinical trial. Diabetes Metab. Syndr. 2018;12:617–24.
Authors’ contributions
LK and BA conceived the idea, participated in study design, supervised the entire work and helped with drafting of manuscript. LK and IF participated in the design of the study and performed the experiments. TH participated in the performance of the experiments. MAJ conducted the statistical analysis. MMA conducted the biochemical analysis. All authors read and approved the final manuscript.
Acknowledgements
The authors are grateful to the financial support of the Vice Chancellor for Research of Tabriz University of Medical Sciences, Tabriz, Iran.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Data are all contained within the paper.
Consent for publication
Not applicable.
Ethics approval and consent to participate
All procedures followed were in accordance with the ethical standards of the Ethical Committee of Tabriz University of Medical Sciences. Informed consent was obtained from all individual participants included in the study (No. IR.TBZMED.REC.1395.402).
Funding
The present study was funded by the Vice Chancellor for Research of Tabriz University of Medical Sciences, Tabriz, Iran.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1186/s13098-023-01094-x
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Khalili, L., Alipour, B., Asghari Jafarabadi, M. et al. RETRACTED ARTICLE: Probiotic assisted weight management as a main factor for glycemic control in patients with type 2 diabetes: a randomized controlled trial. Diabetol Metab Syndr 11, 5 (2019). https://doi.org/10.1186/s13098-019-0400-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13098-019-0400-7