Abstract
Background
Cardiac arrest in hospital and out-of-hospital settings is associated with high mortality rates. Therefore, a bedside test that can predict resuscitation outcomes of cardiac arrest patients is of great value. Point-of-care ultrasound (POCUS) has the potential to be used as an effective diagnostic and prognostic tool during cardiac arrest, particularly in observing the presence or absence of cardiac activity. However, it is highly susceptible to “self-fulfilling prophecy” and is associated with prolonged cardiopulmonary resuscitation (CPR), which negatively impacts the survival rates of cardiac arrest patients. As a result, the current systematic review was created to assess the role of POCUS in predicting the clinical outcomes associated with out-of-hospital and in-hospital cardiac arrests.
Methods
The search for scientific articles related to our study was done either through an electronic database search (i.e., PubMed, Medline, ScienceDirect, Embase, and Google Scholar) or manually going through the reference list of the relevant articles. A quality appraisal was also carried out with the Quality Assessment of Diagnostic Accuracy Studies tool (QUADAS-2), and the prognostic test performance (sensitivity and sensitivity) was tabulated.
Results
The search criteria yielded 3984 articles related to our topic, of which only 22 were eligible for inclusion. After reviewing the literature, we noticed a wide variation in the definition of cardiac activity, and the statistical heterogeneity was high; therefore, we could not carry out meta-analyses. The tabulated clinical outcomes based on initial cardiac rhythm and definitions of cardiac activity showed highly inconsistent results.
Conclusion
POCUS has the potential to provide valuable information on the management of cardiac arrest patients; however, it should not be used as the sole predictor for the termination of resuscitation efforts.
Similar content being viewed by others
Introduction
Cardiac arrest, which is characterized by an abrupt loss of cardiac output and contractility, has a very high death rate in both hospital and out-of-hospital settings. Global statistics show that the incidence of out-of-hospital cardiac arrests (OHCA) is approximately 55 per 100,000 adults [1]. Similarly, statistics provided by the American Heart Association show that about 356,000 out-of-hospital cardiac arrests (OHCA) are witnessed in the United States annually, of which 90% are fatal [2]. Therefore, a bedside test that can predict resuscitation outcomes of cardiac arrest patients is of great value.
Point-of-care ultrasound (POCUS) provides emergency physicians with a diagnostic and prognostic tool, especially in cardiac arrest, where physical examination is not always accurate [3, 4]. Historically, POCUS in cardiac arrest was used to identify reversible causes such as cardiac tamponade and right heart strain, which is suggestive of massive pulmonary embolus [5, 6]. However, research shows that it has the potential to be used as an effective diagnostic and prognostic tool during cardiac arrest, particularly in observing the presence or absence of cardiac activity [7]. Despite the literature associating cardiac activity with return of spontaneous circulation (ROSC), POCUS is highly susceptible to bias from “self-fulfilling prophecy” as most physicians are usually not blinded to the outcomes of this test. Furthermore, research suggests that POCUS prolongs Cardiopulmonary resuscitation (CPR), which negatively impacts the survival rates of cardiac arrest patients [8, 9]. Therefore, we conducted an up-to-date prognostic factor systematic review on POCUS during cardiac arrest.
Methods
Protocol and registration
We conducted this systematic review in accordance with the Cochrane Collaboration guiding principles. Our results were reported as per the PRISMA (Preferred Reporting Items for Systematic Review and Meta-Analyses) guiding principles [10].
Search methods
An extensive literature search was carried out on PubMed, Medline, ScienceDirect, Embase, and Google Scholar for all publications between 2000 and December 2022 using the following strategy: (“Point-of-care ultrasound” OR “bedside ultrasound” OR “ultrasonography” OR “ultrasound” OR “POCUS” OR “bedside cardiac ultrasound” OR “echocardiography”) AND (“Cardiac Arrest” OR “Sudden cardiac arrest” OR “Heart attack”) AND (“survival” OR “mortality” OR “return of spontaneous circulation” OR “Cardiac activity”). In addition, reference lists of potential studies were scrutinized for more studies. Grey literature and exact or close duplicates were also eliminated as they would interfere with the scientific purpose of the present research.
Selection criteria, outcomes and definitions
Two review authors used the PICOST (Population, Intervention, Comparator, Outcome, Study design, Time frame) framework to formulate study questions used to include articles in the current review. The proposed framework was as follows; adult patients with IHCA or OHCA (P); point-of-care ultrasound or echocardiography during cardiopulmonary resuscitation (CPR) (I); absence of finding or different finding on POCUS during CPR (C); prognostic clinical outcomes, i.e., return of spontaneous circulation (ROSC), survival to hospital admission (SHA) and survival to hospital discharge (SHD) (O); human randomized controlled studies and observational studies (prospective, cross-sectional and retrospective) (S); studies published from the year 2000 and written in English (T). Studies that included pediatric patients, animal studies, letters to the editor, case reports and series, systematic reviews and meta-analysis, guidelines, and abstracts without full articles were also excluded.
The main outcomes analyzed in this review article were: ROSC, SHD and SHA. These outcomes were analyzed according to the initial cardiac arrest rhythm, definition of cardiac activity and level of POCUS training.
The definition of “cardiac activity” is very heterogeneous as it varies from study to study; however, in the current review it was defined a priori as any visible movement of the myocardium or valvular contraction. In addition, the level of training was classified as either experienced or inexperienced. Inexperience referred to POCUS operators who had to undergo hands-on and theoretical training before carrying out POCUS examinations, while experienced referred to operators who had undergone the training and had at least 2 years of experience with POCUS.
Quality assessment
The risk of bias assessment was independently carried out by two reviewers using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool embedded in the Review Manager software (RevMan 5.4.1) (Kappa 0.81). This tool generally comprises 4 bias assessment domains, including participants/patient selection, index test, reference standard, and flow and timing, and 3 applicability domains, including patient selection, index test, and reference standard.
Data extraction
The process of extracting relevant data was entrusted to two reviewers, who collated and summarized the pertinent study data, demographic data, initial cardiac rhythm, cardiac arrest setting, POCUS operator, and study outcomes (Table 1). The reviewers discussed any disagreements that occurred throughout the data extraction process or sought advice from a third reviewer who functioned as the arbiter.
Data synthesis
The present systematic review was constructed for prognosis rather than diagnostic test accuracy; therefore, the specificity and sensitivity of cardiac activity were used to predict the resuscitation outcomes (i.e., ROSC, SHA, and SHD) of cardiac arrest using POCUS. Initially, we planned to carry out meta-analyses of the clinical outcomes; however, after analyzing the studies we found that the pooled data had substantial statistical heterogeneity (I2 > 50%), thus all meta-analyses were eliminated. In the ROSC sensitivity and specificity analysis, we defined true positive as the number of individuals achieving ROSC with cardiac activity on POCUS, while false negative defined patients achieving ROSC without cardiac activity. On the other hand, true negative defined non-ROSC without cardiac activity, and false positive defined non-ROSC with cardiac activity. Similarly, in the analysis of SHA and SHD, true positives, false positives, true negatives, and false negatives referred to patients with cardiac motion and surviving to admission or discharge, patients with cardiac activity but not surviving to admission or discharge, patients without cardiac activity and not surviving to admission or discharge, and patients without cardiac activity but surviving to admission or discharge, respectively.
Results
Study selection
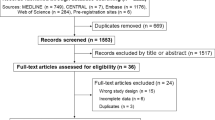
The thorough database search accumulated 3984 articles of which 2896 were excluded based on the duplicate check and screening criteria. After employing the eligibility criteria on 115 articles, 93 were excluded and 22 scientific journals were included for review. The full selection criteria are outlined in the PRISMA diagram below (Fig. 1).
Bias assessment
The results of the bias assessment performed using the QUADAS-2 tool are provided in Fig. 2 below. Our assessment revealed that all studies had a high risk of flow and timing bias since physicians were not blinded to the POCUS results. This lack of blinding may have influenced the decision to terminate resuscitation efforts, creating a self-fulfilling prophecy. Moreover, our assessment revealed that some studies had patient selection concerns due to patient exclusions, convenience sampling, and single-center designs. Unclear and high risk of bias was also seen in the index test due to the lack of pre-defined cut-offs and the fact that some physicians had access to electrocardiogram results before performing the POCUS exams. Overall, the risk of bias across the studies was high, thus, lowering the certainty of evidence from these studies (Table 1).
Does the presence of cardiac activity on pre-hospital POCUS predict resuscitation outcomes of cardiac arrest patients?
Two observational studies [11, 15] of 272 OHCA patients reported the role of pre-hospital POCUS in predicting resuscitation outcomes of cardiac arrest patients (Table 2). The outcomes from these studies were further stratified according to the initial cardiac rhythm and the definition of cardiac activity. The review of data from these studies demonstrated a sensitivity range of 0.95–1.00 and a specificity range of 0.41–0.70 for SHA in patients presenting PEA as the initial rhythm and ranges of sensitivity (0.50–0.69) and specificity (0.83–1.00) in asystole patients. On the other hand, only one study reported resuscitation outcomes for patients with VF/VT rhythms, of which cardiac activity predicted SHA with a 1.00 (95% CI 0.15–1.00) sensitivity and 0.67 (95% CI 0.30–0.93) specificity [11].
When the outcomes were stratified according to the definition of cardiac activity, one observational study with unspecified cardiac activity reported a sensitivity of 0.86 (95% CI 0.70–0.94) and specificity of 0.60 (95% CI 0.47–0.725) for SHA [15]. The other study where cardiac activity was defined as any movement of the myocardium reported sensitivity (0.80; 95% CI 0.31–0.97) and specificity (0.84; 95% CI 0.68–0.93) for SHA [11].
Considering the findings in these studies, it is evident that cardiac activity on pre-hospital POCUS has an inconsistent prognostic value. Therefore, for patients with OHCA, resuscitation is still the main priority and should be managed according to the advanced life support (ALS) guidelines [33].
Does the presence of cardiac activity on in-hospital (ICU & ED) POCUS predict resuscitation outcomes of cardiac arrest patients?
In patients with PEA as the initial cardiac arrest rhythm, the presence of cardiac activity predicts the possibility of ROSC with a sensitivity range of 0.43–1.00 and a specificity range of 0.33–0.93, while it predicts the likelihood of SHA with a sensitivity range of 0.67–1.00 and specificity range of 0.50–0.93 [13, 16,17,18,19, 23, 27, 31]. On the other hand, for patients with Asystole as the initial rhythm, the presence of cardiac activity predicts the possibility of ROSC with a sensitivity range of 0.0–0.11 and specificity range of 0.97–1.00 while it predicts the likelihood of SHA with a sensitivity range (0.0–1.00) and specificity range (0.85–1.00) [13, 16, 18, 23, 27, 28, 31]. Similarly, the presence of cardiac activity for patients with VF/VT as the initial rhythm can estimate the possibility of ROSC with ranges of sensitivity (0.0–1.00) and specificity (0.0–1.00) and the possibility of SHA with a range of sensitivity (0.94–1.00) and specificity (0.50–1.00) [13, 16, 23, 27, 31] (Table 2).
After stratifying the outcomes based on the definition of cardiac activity, we observed that the presence of unspecified cardiac activity had sensitivity ranges of 0.62–0.73 and 0.72–0.94 and specificity ranges of 0.92–0.98 and 0.60–0.98 for ROSC and SHD, respectively [14,15,16, 18, 24]. One observational study also reported that presence of unspecified cardiac activity had a sensitivity of 0.48 (95% CI 0.28–0.69) and specificity of 0.77 (95% CI 0.69–0.83) for SHD [24] (Table 2).
On the other hand, 5 observational studies [11, 13, 21, 30, 32] with 1267 OHCA and IHCA patients defined cardiac activity as the presence of any movement of the myocardium. Outcomes from two of these studies reported a sensitivity range of 0.52–0.64 and a specificity range of 0.78–0.95 for ROSC [21, 30]. Conversely, 4 of the 5 observational studies reported ranges of sensitivity (0.31–0.98) and specificity (0.73–0.95) for SHA [11, 13, 21, 30], while 3 reported ranges of sensitivity (0.40–0.77) and specificity (0.68–0.83) for SHD [21, 30, 32] (Table 2).
In addition, 8 observational studies [17, 22, 23, 25, 26, 28, 29, 31] with 717 IHCA and OHCA patients defined cardiac activity as any atrial, valvular, or ventricular motion. 7 of these studies reported sensitivity ranges of 0.25–0.95, and specificity ranges of 0.33–0.95 for ROSC [17, 22, 23, 25, 26, 28, 31], while other 3 articles reported ranges of sensitivity (0.75–0.98) and specificity (0.60–0.79) for SHA [22, 25, 26]. Conversely, two studies reported sensitivity ranges of 0.85 to 1.00 and specificity ranges of 0.62–0.74 for SHD[25, 29] (Table 2).
Finally, organized cardiac activity was reported in 3 observational studies [12, 19, 20]. Outcomes from two of these articles showed that organized cardiac activity predicted the possibility of ROSC with a sensitivity range of 0.34 to 0.53 and a specificity range of 0.38–0.96 [12, 20]. Similarly, organized cardiac activity predicted the likelihood of SHA and SHD with sensitivity ranges of 0.39–0.86 and 0.67–1.00 and specificity ranges of 0.38–0.96 and 0.91, respectively (Table 2).
Does the level of POCUS training influence the ability to predict clinical outcomes of cardiac arrest?
The current review shows that in cardiac arrest patients where inexperienced sonographers perform POCUS exams, cardiac activity predicts the possibility of ROSC with a sensitivity range of 0.26–0.62 and a specificity range of 0.89–0.98 [24, 30, 31], while it predicts the possibility of SHA with a sensitivity range of 0.31–0.98 and specificity range of 0.67–0.91 [11, 13, 18, 28, 30]. Additionally, four observational studies reported a sensitivity ranges of 0.40–1.00 and specificity ranges of 0.62–0.83 for SHD [24, 29, 30, 32] (Table 2).
On the other hand, cardiac activity observed by experienced sonographers, had a predictive sensitivity range of 0.25–0.95 and a specificity range of 0.70–0.96 for ROSC [12, 21,22,23, 25, 26] and sensitivity range of 0.39–0.94 and specificity range of 0.64 to 0.98 for SHA [12, 16, 19, 21, 22, 25]. Furthermore, outcomes from 4 studies reported ranges of sensitivity (0.67–1.00) and specificity (0.51–0.89) for SHD [12, 20, 21, 25] (Table 2).
Considering the evidence in these studies, POCUS performed by relatively inexperienced physicians (i.e., those with less than 2 year experience) seems to have a similar prognostic value as that performed by experienced physicians. However, more high-quality randomized trials are required to support this finding. Furthermore, we noticed a wide variation in sensitivity and specificity values when POCUS was performed by experienced sonographers. Although, the definitive cause of this variation is not well known, it can be attributed to factors such as different definitions of cardiac activity, the initial cardiac rhythm, and number of echocardiography findings.
Discussion
The current study was designed to evaluate the ability of POCUS to predict resuscitation outcomes in adult cardiac arrest patients in any setting. Unfortunately, we could not pool clinical outcomes in meta-analyses due to the high risk of bias and statistical heterogeneity between studies.
The main goal of using POCUS in cardiac arrest is to improve resuscitation outcomes by identifying cardiac activity [34]. However, after reviewing articles in the current study, we noticed a wide variation in the definition of cardiac activity. This finding is consistent with a previous systematic review by the Advanced Life Support Task Force of the International Liaison Committee on resuscitation [35]. Moreover, the present study has shown that irrespective of the POCUS setting and definition of cardiac motion, the sensitivity and specificity values are highly inconsistent, with values as low as 25% [23] and 33% [20] recorded in patients presenting any atrial, valvular, or ventricular movement. This evidence suggests that the presence or absence of cardiac activity is insufficient to inform the decision to terminate resuscitation efforts. Therefore, resuscitation efforts should be continued until they prove futile rather than terminating based on the initial sonographic findings. Moreover, we would recommend that a uniform definition of cardiac activity be generated to assist in interpreting future outcomes.
Although the current study implied that cardiac motion does not inform the decision to terminate resuscitation efforts, there is a high risk of cardiac motion on POCUS being used as a self-fulfilling prophecy. A recent questionnaire about termination of resuscitation revealed that about 19% of physicians and 40% of nurses were comfortable with terminating resuscitation efforts after observing cardiac standstill on the echocardiography [36]. However, it is worth noting that even patients with cardiac standstill on the initial ultrasonographic findings may gain cardiac activity after some time. For instance, Gaspari and colleagues reported that the rate of SHD was approximately 3 to 4 times with cardiac activity on initial ultrasonographic findings. However, patients without cardiac activity had longer resuscitation attempts, of which 11% regained cardiac activity during the resuscitation attempts meaning that the sonographic findings are not static [21]. Therefore, cardiac sonographic findings must be cautiously interpreted [37, 38].
Successful resuscitation of patients with PEA or Asystole requires considerable time and effort. Therefore, POCUS is used to identify cardiac motion in these patients and improve resuscitation outcomes. Our review suggests that in patients with PEA or VT/VF as the initial rhythm, cardiac activity tends to have higher sensitivity for predicting ROSC and SHA compared to patients with Asystole. However, the evidence provided in these studies has a high risk of bias; thus, the certainty of the evidence is subjective. In addition, POCUS can be used to identify reversible causes of PEA and asystole, such as hypovolemia, pulmonary embolism, and pericardial effusion. However, detecting pulmonary embolism during resuscitation is modest at best since the right ventricle is usually dilated [37, 38].
POCUS is also a useful tool in differentiating between true and pseudo-PEA. Pseudo-PEA is described as the presence of myocardial electrical activity without a detectable pulse but with coordinated cardiac activity, while true PEA usually refers to the condition where a patient has myocardial electrical activity without a palpable pulse and cardiac activity [39]. Tomruk and colleagues reported that sonography identified 34.4% cases of pseudo-PEA, of which 68.2% were successfully resuscitated. The study also shows that true PEA was detected in 42 out of 64 patients, of which only 20 went on to have successful resuscitation. Similarly, Breitkreutz et al. [15] reported that bedside ultrasound could detect pseudo-PEA in 38 patients, of which 21 survived until hospital admission while 17 died on the scene. True PEA was also diagnosed in 13 patients, of which only one survived to hospital admission while 12 died on the scene. This distinction between true and pseudo-PEA is important because standard cardiac arrest treatments may result in harmful outcomes among patients with pseudo-PEA.
Interestingly, evidence shows that POCUS can be used to make an actual rhythm diagnosis during resuscitation. Thandar and colleagues reported that three patients initially assessed to be in asystole rhythm were diagnosed with ventricular fibrillation after sonographic exams [30]. This accurate diagnosis was essential in making critical decisions and prompting defibrillation. Similarly, two previous case reports reported that VF mimicking asystole was only diagnosed using ultrasound [40, 41]. This misdiagnosis can be explained by the fact that during resuscitation, electrocardiogram leads may be displaced, causing the monitor to show an asystole rhythm.
Research shows that POCUS carried out by trained physicians allows for better evaluation of quality compressions and quick diagnosis of reversible causes of cardiac arrest [42]. However, there is no evidence to suggest that the level of training might affect resuscitation outcomes of cardiac arrest. Our review found that POCUS carried out by relatively inexperienced physicians has almost similar sensitivity and specificity for predicting resuscitation outcomes as POCUS performed by experienced sonographers. This means that physicians with brief and specific POCUS training can accurately identify cardiac motion during resuscitation. However, more high-quality randomized studies are required to support our findings.
Although the current review only evaluated the role of POCUS in clinical outcomes (SHA, ROSC, and SHD), evidence suggests that POCUS can influence the resuscitation time and the intervention used on cardiac arrest patients. Atkinson and colleagues reported longer resuscitation durations in patients showing cardiac activity on POCUS than patients without cardiac activity (27.33 min (95% CI 17.7–37.0) vs. 11.51 min (95% CI 10.2–12. 8), respectively) [12]. Furthermore, patients who did not undergo the POCUS exam had a significantly lower resuscitation duration (14.36 min; 95% CI 9.89–18.8; p = 0.001). This increased resuscitation duration among patients with cardiac activity suggests that emergency physicians and team provided increased resuscitation efforts when cardiac activity was observed and stopped resuscitation earlier for patients not undergoing POCUS or in those without evidence of cardiac activity. This finding in addition to improved ROSC, SHA and SHD rates in that study suggests that the use of POCUS during cardiac arrest may have a direct impact on clinical outcomes. However, more randomized trials are required to establish the role of POCUS in cardiac arrest.
The same trend was also noticed for the interventions used, of which the rate of endotracheal intubation was significantly higher for patients with cardiac activity than those without and those who did not undergo POCUS exam (95.23% (95% CI 86.13–104.35) vs. 46.54% (95% CI 38.79–54.29) vs. 65.11% (95% CI 50.87–79.36), respectively; p < 0.001) [12]. The study also showed that epinephrine was given to a larger proportion of patients with cardiac activity than those without or those who did not undergo POCUS (100%; 100–100 vs. 82.39%; 76.5–88.3 vs. 81.39%; 69.76–93.03, respectively; < 0.001). Gaspari and colleagues also showed that the duration for resuscitation was longer when cardiac activity was recorded on the POCUS than when the POCUS showed cardiac standstill (18 min (IQR 10–30) vs. 12 min, (IQR 8–17), p < 0.05) [21]. However, this study reported no difference in the time between the doses of epinephrine. Similarly, a 2017 retrospective study reported that when patients with organized cardiac activity recorded in POCUS were treated with epinephrine, the ROSC and SHA rates were higher (54.7 and 37.7%). The study further reports that patients that recorded disorganized cardiac activity on POCUS and were treated with the standard ACLS interventions had significantly lower ROSC and SHA rates (37.2 and 17.9%, respectively; p < 0.005) [43].
Limitations
The analysis made in the current study should be interpreted with consideration of the following weaknesses. First, it should be noted that the eligibility criteria of the present study allowed the inclusion of scientific journals published in English only, which might have introduced selection bias in our analysis. Additionally, the eligibility criteria only included studies from the year 2000 because we wanted to have more recent information on the prognostic value of POCUS; thus, other studies relevant to our topic may have been omitted, thus increasing the selection bias of our study. Secondly, all the studies included in this review were designed as observational studies meaning that the resuscitators were not blinded to the POCUS results. This might have influenced the decision of the resuscitators to stop resuscitation efforts after seeing cardiac standstill in the POCUS results. To minimize this bias, it is essential that all the resuscitative efforts are done for a specified period, irrespective of the POCUS results. Thirdly, for studies where cardiac activity was not defined, we opted assign them “unspecified” without contacting the authors meaning that our analysis may have had some reporting bias. Lastly, we could not perform any meta-analyses on clinical outcomes due to the high risk of bias and heterogeneity; therefore, only quantitative information was provided to analyze the role of POCUS in predicting resuscitation outcomes of cardiac arrest patients.
Conclusion
POCUS has inconsistent prognostic value; hence, should not be used as the sole predictor in determining the termination of resuscitation efforts in cardiac arrest patients. Moreover, a more unified definition for cardiac activity is required to facilitate better interpretation of future outcomes. In addition, the level of POCUS training has no influence on the clinical outcomes. However, more high-quality randomized trials are required to support this finding.
Availability of data and materials
Not applicable.
References
Yan S, Gan Y, Jiang N et al (2020) The global survival rate among adult out-of-hospital cardiac arrest patients who received cardiopulmonary resuscitation: a systematic review and meta-analysis. Crit Care 24:61. https://doi.org/10.1186/s13054-020-2773-2
Tsao CW, Aday AW, Almarzooq ZI et al (2022) Heart disease and stroke statistics—2022 update: a report from the american heart association. Circulation 145:e153-639. https://doi.org/10.1161/CIR.0000000000001052
Liberman M, Lavoie A, Mulder D, Sampalis J (1999) Cardiopulmonary resuscitation: errors made by pre-hospital emergency medical personnel. Resuscitation 42:47–55. https://doi.org/10.1016/s0300-9572(99)00082-9
Eberle B, Dick WF, Schneider T, Wisser G, Doetsch S, Tzanova I (1996) Checking the carotid pulse check: diagnostic accuracy of first responders in patients with and without a pulse. Resuscitation 33:107–116. https://doi.org/10.1016/s0300-9572(96)01016-7
Niendorff DF, Rassias AJ, Palac R, Beach ML, Costa S, Greenberg M (2005) Rapid cardiac ultrasound of inpatients suffering PEA arrest performed by nonexpert sonographers. Resuscitation 67:81–87. https://doi.org/10.1016/j.resuscitation.2005.04.007
Breitkreutz R, Walcher F, Seeger FH (2007) Focused echocardiographic evaluation in resuscitation management: concept of an advanced life support-conformed algorithm. Crit Care Med 35:S150-161. https://doi.org/10.1097/01.CCM.0000260626.23848.FC
Long B, Alerhand S, Maliel K, Koyfman A (2018) Echocardiography in cardiac arrest: an emergency medicine review. Am J Emerg Med 36:488–493. https://doi.org/10.1016/j.ajem.2017.12.031
Huisin ‘t Veld MA, Allison MG, Bostick DS, Fisher KR, Goloubeva OG, Witting MD, Winters ME (2017) Ultrasound use during cardiopulmonary resuscitation is associated with delays in chest compressions. Resuscitation 119:95–8. https://doi.org/10.1016/j.resuscitation.2017.07.021
Clattenburg EJ, Wroe P, Brown S, Gardner K, Losonczy L, Singh A, Nagdev A (2018) Point-of-care ultrasound use in patients with cardiac arrest is associated prolonged cardiopulmonary resuscitation pauses: a prospective cohort study. Resuscitation 122:65–68. https://doi.org/10.1016/j.resuscitation.2017.11.056
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535. https://doi.org/10.1136/bmj.b2535
Aichinger G, Zechner PM, Prause G et al (2012) Cardiac movement identified on prehospital echocardiography predicts outcome in cardiac arrest patients. Prehosp Emerg Care 16:251–255. https://doi.org/10.3109/10903127.2011.640414
Atkinson PR, Beckett N, French J, Banerjee A, Fraser J, Lewis D (2019) Does point-of-care ultrasound use impact resuscitation length, rates of intervention, and clinical outcomes during cardiac arrest? A study from the sonography in hypotension and cardiac arrest in the emergency department (SHoC-ED) investigators. Cureus 11:e4456. https://doi.org/10.7759/cureus.4456
Blaivas M, Fox JC (2001) Outcome in cardiac arrest patients found to have cardiac standstill on the bedside emergency department echocardiogram. Acad Emerg Med 8:616–621. https://doi.org/10.1111/j.1553-2712.2001.tb00174.x
Bolvardi E, Pouryaghobi SM, Farzane R, Chokan NMJ, Ahmadi K, Reihani H (2016) The prognostic value of using ultrasonography in cardiac resuscitation of patients with cardiac arrest. Int J Biomed Sci 12:110–114
Breitkreutz R, Price S, Steiger HV et al (2010) Focused echocardiographic evaluation in life support and peri-resuscitation of emergency patients: a prospective trial. Resuscitation 81:1527–1533. https://doi.org/10.1016/j.resuscitation.2010.07.013
Cebicci H, Salt O, Gurbuz S, Koyuncu S, Bol O (2014) Benefit of cardiac sonography for estimating the early term survival of the cardiopulmonary arrest patients. Hippokratia 18:125–129
Mojtaba C, Farhad H, Helaleh R, Mahdi S-A, Hamid S, Vafa R-M (2012) Echocardiography integrated ACLS protocol versus conventional cardiopulmonary resuscitation in patients with pulseless electrical activity cardiac arrest. Chin J Traumatol 15:284–287. https://doi.org/10.3760/cma.j.issn.1008-1275.2012.05.005
Chua MT, Chan GW, Kuan WS (2017) Reversible causes in cardiovascular collapse at the emergency department using ultrasonography (REVIVE-US). Ann Acad Med Singap 46:310–316
Cureton EL, Yeung LY, Kwan RO, Miraflor EJ, Sadjadi J, Price DD, Victorino GP (2012) The heart of the matter: utility of ultrasound of cardiac activity during traumatic arrest. J Trauma Acute Care Surg 73:102. https://doi.org/10.1097/TA.0b013e3182569ebc
Flato UAP, Paiva EF, Carballo MT, Buehler AM, Marco R, Timerman A (2015) Echocardiography for prognostication during the resuscitation of intensive care unit patients with non-shockable rhythm cardiac arrest. Resuscitation 92:1–6. https://doi.org/10.1016/j.resuscitation.2015.03.024
Gaspari R, Weekes A, Adhikari S et al (2016) Emergency department point-of-care ultrasound in out-of-hospital and in-ED cardiac arrest. Resuscitation 109:33–39. https://doi.org/10.1016/j.resuscitation.2016.09.018
Hayhurst C, Lebus C, Atkinson PR et al (2011) An evaluation of echo in life support (ELS): is it feasible? What does it add? Emerg Med J 28:119–121. https://doi.org/10.1136/emj.2009.084202
Kim HB, Suh JY, Choi JH, Cho YS (2016) Can serial focussed echocardiographic evaluation in life support (FEEL) predict resuscitation outcome or termination of resuscitation (TOR)? A pilot study. Resuscitation 101:21–26. https://doi.org/10.1016/j.resuscitation.2016.01.013
Lien W-C, Hsu S-H, Chong K-M et al (2018) US-CAB protocol for ultrasonographic evaluation during cardiopulmonary resuscitation: validation and potential impact. Resuscitation 127:125–131. https://doi.org/10.1016/j.resuscitation.2018.01.051
Masoumi B, Azizkhani R, Heydari F, Zamani M, Nasr Isfahani M (2021) The role of cardiac arrest sonographic exam (CASE) in predicting the outcome of cardiopulmonary resuscitation; a cross-sectional study. Arch Acad Emerg Med 9:e48. https://doi.org/10.2203/aaem.v9i1.1272
Ozen C, Salcin E, Akoglu H, Onur O, Denizbasi A (2016) Assessment of ventricular wall motion with focused echocardiography during cardiac arrest to predict survival. Turk J Emerg Med 16:12–16. https://doi.org/10.1016/j.tjem.2015.08.001
Salen P, O’Connor R, Sierzenski P et al (2001) Can cardiac sonography and capnography be used independently and in combination to predict resuscitation outcomes? Acad Emerg Med 8:610–615. https://doi.org/10.1111/j.1553-2712.2001.tb00172.x
Salen P, Melniker L, Chooljian C, Rose JS, Alteveer J, Reed J, Heller M (2005) Does the presence or absence of sonographically identified cardiac activity predict resuscitation outcomes of cardiac arrest patients? Am J Emerg Med 23:459–462. https://doi.org/10.1016/j.ajem.2004.11.007
Tayal VS, Kline JA (2003) Emergency echocardiography to detect pericardial effusion in patients in PEA and near-PEA states. Resuscitation 59:315–318. https://doi.org/10.1016/s0300-9572(03)00245-4
Thandar S, Sahu AK, Sinha TP, Bhoi S (2023) Role of initial cardiac activity assessed by point-of-care ultrasonography in predicting cardiac arrest outcomes: a prospective cohort study. Turk J Emerg Med 23:024–029. https://doi.org/10.4103/2452-2473.366482
Tomruk O, Erdur B, Cetin G, Ergin A, Avcil M, Kapci M (2012) Assessment of cardiac ultrasonography in predicting outcome in adult cardiac arrest. J Int Med Res 40:804–809. https://doi.org/10.1177/147323001204000247
Zengin S, Yavuz E, Al B, Cindoruk Ş, Altunbaş G, Gümüşboğa H, Yıldırım C (2016) Benefits of cardiac sonography performed by a non-expert sonographer in patients with non-traumatic cardiopulmonary arrest. Resuscitation 102:105–109. https://doi.org/10.1016/j.resuscitation.2016.02.025
Link MS, Berkow LC, Kudenchuk PJ et al (2015) Part 7: adult advanced cardiovascular life support. Circulation 132:S444–S464. https://doi.org/10.1161/CIR.0000000000000261
Morrison LJ, Deakin CD, Morley PT et al (2010) Part 8: advanced life support: 2010 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation 122:S345-421. https://doi.org/10.1161/CIRCULATIONAHA.110.971051
Reynolds JC, Issa MS, Nicholson CT et al (2020) Prognostication with point-of-care echocardiography during cardiac arrest: a systematic review. Resuscitation 152:56–68. https://doi.org/10.1016/j.resuscitation.2020.05.004
Hansen C, Lauridsen KG, Schmidt AS, Løfgren B (2018) Decision-making in cardiac arrest: physicians’ and nurses’ knowledge and views on terminating resuscitation. Open Access Emerg Med 11:1–8. https://doi.org/10.2147/OAEM.S183248
Aagaard R, Caap P, Hansson NC, Bøtker MT, Granfeldt A, Løfgren B (2017) Detection of pulmonary embolism during cardiac arrest-ultrasonographic findings should be interpreted with caution. Crit Care Med 45:e695-702. https://doi.org/10.1097/CCM.0000000000002334
Aagaard R, Granfeldt A, Bøtker MT, Mygind-Klausen T, Kirkegaard H, Løfgren B (2017) The right ventricle is dilated during resuscitation from cardiac arrest caused by hypovolemia: a porcine ultrasound study. Crit Care Med 45:e963–e970. https://doi.org/10.1097/CCM.0000000000002464
Labovitz AJ, Noble VE, Bierig M et al (2010) Focused cardiac ultrasound in the emergent setting: a consensus statement of the American society of echocardiography and American college of emergency physicians. J Am Soc Echocardiogr 23:1225–1230. https://doi.org/10.1016/j.echo.2010.10.005
Amaya SC, Langsam A (1999) Ultrasound detection of ventricular fibrillation disguised as asystole. Ann Emerg Med 33:344–346. https://doi.org/10.1016/s0196-0644(99)70372-0
Limb C, Siddiqui MA (2015) Apparent asystole: are we missing a lifesaving opportunity? BMJ Case Rep 2015:2014208364. https://doi.org/10.1136/bcr-2014-208364
Ávila-Reyes D, Acevedo-Cardona AO, Gómez-González JF, Echeverry-Piedrahita DR, Aguirre-Flórez M, Giraldo-Diaconeasa A (2021) Point-of-care ultrasound in cardiorespiratory arrest (POCUS-CA): narrative review article. Ultrasound J 13:46. https://doi.org/10.1186/s13089-021-00248-0
Gaspari R, Weekes A, Adhikari S et al (2017) A retrospective study of pulseless electrical activity, bedside ultrasound identifies interventions during resuscitation associated with improved survival to hospital admission a REASON study. Resuscitation 120:103–107. https://doi.org/10.1016/j.resuscitation.2017.09.008
Acknowledgements
The publication of this article is funded by the Qatar National Library.
Funding
Open Access funding provided by the Qatar National Library.
Author information
Authors and Affiliations
Contributions
HAZ, EES: conceptualization; data curation; formal analysis; writing—original draft preparation. HI, MN, KB: conceptualization; project administration; writing- reviewing and editing. AMA, BHA, NS, WE: supervision; validation; writing—reviewing and editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
See Table 2
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zaki, H.A., Iftikhar, H., Shaban, E.E. et al. The role of point-of-care ultrasound (POCUS) imaging in clinical outcomes during cardiac arrest: a systematic review. Ultrasound J 16, 4 (2024). https://doi.org/10.1186/s13089-023-00346-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13089-023-00346-1