Abstract
Background
SARS-CoV-2 infection, manifesting as COVID-19 pneumonia, constitutes a global pandemic that is disrupting health-care systems. Most patients who are infected are asymptomatic/pauci-symptomatic can safely self-isolate at home. However, even previously healthy individuals can deteriorate rapidly with life-threatening respiratory failure characterized by disproportionate hypoxemic failure compared to symptoms. Ultrasound findings have been proposed as an early indicator of progression to severe disease. Furthermore, ultrasound is a safe imaging modality that can be performed by novice users remotely guided by experts. We thus examined the feasibility of utilizing common household informatic-technologies to facilitate self-performed lung ultrasound.
Methods
A lung ultrasound expert remotely mentored and guided participants to image their own chests with a hand-held ultrasound transducer. The results were evaluated in real time by the mentor, and independently scored by three independent experts [planned a priori]. The primary outcomes were feasibility in obtaining good-quality interpretable images from each anatomic location recommended for COVID-19 diagnosis.
Results
Twenty-seven adults volunteered. All could be guided to obtain images of the pleura of the 8 anterior and lateral lung zones (216/216 attempts). These images were rated as interpretable by the 3 experts in 99.8% (647/648) of reviews. Fully imaging one’s posterior region was harder; only 108/162 (66%) of image acquisitions was possible. Of these, 99.3% of images were interpretable in blinded evaluations. However, 52/54 (96%) of participants could image their lower posterior lung bases, where COVID-19 is most common, with 99.3% rated as interpretable.
Conclusions
Ultrasound-novice adults at risk for COVID-19 deterioration can be successfully mentored using freely available software and low-cost ultrasound devices to provide meaningful lung ultrasound surveillance of themselves that could potentially stratify asymptomatic/paucisymptomatic patients with early risk factors for serious disease. Further studies examining practical logistics should be conducted.
Trial Registration: ID ISRCTN/77929274 on 07/03/2015.
Similar content being viewed by others

Introduction
COVID-19 pneumonia is disrupting life on the planet earth in an unprecedented fashion. While many if not most people have mild or asymptomatic disease, others, even young previously healthy people, may become rapidly sick with severe hypoxia despite exhibiting minimal symptoms, including dyspnea [1,2,3]. The burden on health care systems may be extraordinary, with even well-developed nations’ health care systems being overwhelmed. Health care providers may be particularly susceptible if appropriate infection prevention and control measures are not in place [4]. Thus, solutions need to be sought to provide excellent patient care, but also to protect provider health. COVID-19 is a paradox, as despite the risk to providers, the majority contracting the virus will not develop COVID-19 pneumonia. Most will have none or minor symptoms and can safely self-isolate at home. However, those who develop severe disease, need to be identified early [4].
Compared to chest radiographs and computed tomography (CT), lung ultrasound (LUS) is a simpler, more portable, economical, and potentially home-based technology that might be used for at-risk patients to self-monitor their lungs of for early signs of COVID-19 pneumonia [5,6,7]. Findings from COVID-19 pneumonia are typically present in the lung periphery [8,9,10], an anatomic fact that permits LUS to be used to diagnose and manage all phases of care in COVID-19 [5, 9, 11]. LUS may detect early disease progression as the lungs deteriorate from normal to an alveolar-interstitial pattern of lung disease with single discrete vertical artifacts (B-lines) or confluent B-lines [9, 11]. Through work onboard the International Space Station examining self-performed telementored lung ultrasonography (SPTMLUS) performed by inexperienced point-of-care users guided by remote experts [12], we have long known that accurate ultrasound images of the lungs can be self-obtained [13,14,15,16,17]. What has never been examined, is whether willing but ultrasound-naïve adults can be remotely mentored to obtain meaningful lung ultrasound images upon themselves to triage alveolar-interstitial pneumonic diseases, such as COVID-19. The purpose of this study was thus to examine the feasibility and quality of SPTMLUS of novices when expertly guided. Furthermore, this paradigm may be considered a specific example of a broader concept that may contribute to many facets of patient focused and individualized healthcare.
Methods
This study was registered and ethically approved and structured to comply with the SQUIRE reporting guidelines [18]. A healthy cohort of self-isolating participants in Edmonton, Alberta conducted SPTMLUS examinations mentored by a remote expert. The participants were self-isolating among their family units in response to applicable Public Health orders in effect. After informed consent, participants completed an electronic demographic survey (Additional file 1) and received a package containing a disinfected hand-held high-frequency linear ultrasound probe (Philips Lumify, Philips, Amsterdam, NL), and a package of sterile ultrasound-gel. They also watched a brief instructional video on how to hold the probe and where anatomically they would be guided to scan (Additional file 2).
Thereafter, a lung ultrasound expert in Calgary (AWK) guided the subjects to measure their blood pressure (details reported elsewhere) and conduct a standardized lung examination, using Zoom Teleconferencing (Zoom, Hillsboro, OH). The desired examination was based on the 14-zone method proposed by Soldati for International Standardization of the Use of Lung Ultrasound for Patients with COVID-19 [19] (Fig. 1). The study goal was to generate an adequate “Batwing” depiction of the pleura interface between two rib shadows at each location on the thorax [20] (Fig. 2). Outcome measures were, therefore, (1) whether the subject was physically able to reach all 14 desired anatomic locations (Figs. 3 and 4) and (2) whether the quality of images was considered “adequate” for image interpretation and diagnosis. Images were optimized through remote control of ultrasound “knobology” by the mentor using remote access software (Teamviwer, Göppingen, Germany). The pleural interface was interrogated with 2D, M-mode, and color-Power Doppler (CPD) modes[21], and all image acquisition attempts were videorecorded. The mentor scored each of the pleural images real time using the proposed Soldati method from 0 (normal) to 3 (very abnormal) (Additional file 4) [19], and counted the number of B-lines present at each anatomic location. Each participant completed an online post-test evaluation that included their perceptions of the difficulty in performing their self-examination including a 5-point Likert scale rating the examination at each location as being one of; 1—Very Hard, 2—Hard, 3—Neutral, 4—Easy, 5—Very Easy (Additional file 3).

modified from proposal for international standardization of the use of lung ultrasound for patients with COVID‐19 by Soldati et al., J Ultrasound Med 2020, published in Open Access Format
Anatomic locations targeted for the self-performed lung examination. Figure [19]
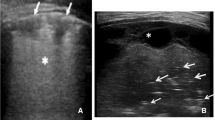
Representative still image of mentee self-performing lung ultrasound to demonstrate the pleural interface. Ultrasound Naïve participant being instructed to demonstrate the pleural interface of the visceral and parietal pleural illustrating the “Batwing” sign of Lichtenstein [20]. It should be noted that all lung ultrasound is a dynamic examination better viewed real time and in video recordings (Additional file 4) than still images
Representative still image of mentee self-performing lung ultrasound to demonstrate the pleural interface of her back. Ultrasound Naïve participant being instructed to demonstrate the pleural interface of the visceral and parietal pleural illustrating the “Seahorse” sign of Lichtenstein [20]. It should be noted that all lung ultrasound is a dynamic examination better viewed real time and in video recordings (Additional file 3) than still images
Representative still image of mentee self-performing lung ultrasound to demonstrate the pleural interface of her back. It should be noted that all lung ultrasound is a dynamic examination better viewed real time and in video recordings (Additional file 3) than still images
Webpage of selected still images and full video recordings of each examination for blinded review. Some individual Participants have consented to the public disclosure of their personal images, while others have not. Thus, despite the large amount of documentation available in the public domain, all the data available to the original reviewers is not available here due to confidentiality considerations. Videorecording of the complete examinations for those subjects who agree to disclose their personal images are available in Additional file 3
Subsequently the mentor retrospectively reviewed all examinations and prepared representative still images and videos at each anatomic location, which were uploaded to a protected website for an a-priori planned independent review by three outside lung ultrasound experts, who rated both the image quality (as adequate vs. inadequate) and the degree of abnormality using the same Soldati scoring protocol [22]. If they had any concerns regarding image quality, they were encouraged to review the entire source examination. Standard descriptive statistics were used. Patients reporting and not reporting any upper body musculoskeletal concerns or shoulder injuries were analyzed separately and the comparisons between groups were made using Fisher’s exact tests. The theoretical ability to complete commonly used and recommended lung ultrasound imaging protocols was calculated by analyzing the ability for SPTMLUS to be completed at each recommended location of the Extended Focused Assessment with Sonography for Trauma [23], the Bedside Lung Ultrasound Assessment [24], and the International evidence-based recommendation for point-of-care lung ultrasound [25], and a theoretical examination just looking at the anterior, lateral, and posterior lung bases (Fig. 5).
Results
Demographics
The study population consisted of a convenience sample of 27 self-isolating inhabitants in Edmonton, Alberta, Canada. All participants stated they were comfortable with typical informatic tools of modern society, such as smart-phones and tablet computers, but none were informatic technology experts. Three [11.1%] had prior ultrasound exposure but none had ever performed LUS (Table 1). Fifteen [14.8%) of participants had a Masters or higher Education degree, 7 [26%] reported an upper body musculoskeletal problem and 8 [29.6%] reported a previous shoulder injury (Table 1).
Ability to complete a comprehensive pleural ultrasound examination
Overall, all 27 participants were able to obtain images from all (100%) of the eight anatomic locations on the anterolateral chest. The back was relatively harder, although possible on the lower back in 52/54 (96.3%) of attempts; 38/54 (70.4%) midback, and only18/54 (33%) on the upper back (Table 2). When asked the subjective difficulty in performing the examination, participants rated imaging their own anterior and lateral chest as between “easy” and “very easy” in all locations, while the back was rated between “neutral” to “easy” in all locations (Table 3). There was no statistical relation between the ability/inability to obtain images and a history of shoulder injury at any of the anatomic location.
Real-time subjective mentored evaluation and a-priori independent review
Although not all desired locations in all participants could be imaged, of the sites that could, the image quality of 322/324 (99.7%) of real-time determinations was felt to be adequate in real time assessment and adequate in 1124/1128 (99.6%) of independent reviews (Table 2). An examination of suspected reasons for inadequate images is presented in Table 4. Results regarding evaluated lung score and B-line counts are available (Additional file 5), recognizing that no patient had known COVID-19 during examination.
Selected standardized lung ultrasound performance difficulty
The theoretical feasibility and participant-rated ease of performance of commonly used LUS protocols is presented in Table 5. The Extended Focused Assessment with Sonography for Trauma (EFAST) examination [23] was 100% feasible and would be “very easy” (mean Likert score 4.67 out of 5) as assessed by the participants, as was the Bedside Lung Ultrasound in Emergency (BLUE), (feasibility 100%; ease of performance score 4.56) [20]. Similarly, the International evidence-based recommendations for point-of care lung ultrasound (ICC-LUS) was 100% feasible and easy (mean score 4.56) [26]. Even the overall Soldati COVID examination was feasible in 85.7% of locations and overall rated easy (mean score 4.07). A new theoretical examination involving the lowermost lung fields of the anterior, lateral, and posterior lung bases would be 98.8% feasible and easy to very easy (mean score 4.37) (Table 5).
Discussion
Our results demonstrate that adults without prior ultrasound experience were remarkably adept at accurately imaging their own chests to generate clinically meaningful images under the guidance of a remote mentor. Every participant was able to satisfactorily image their anterior, lateral, and lower posterior thoracic areas. With the exception of one participant who later contracted COVID-19 [27], all participants were at risk, but remained clinically well. In our opinion, the most important interpretation of these results, beyond the implications to assist with the surveillance of COVID-19, is the potential to explore the paradigm of mentored self-care to other conditions involving remote, self-isolated, or vulnerable populations to better enable and empower their own health maintenance. Furthermore, although the technique required an economical hand-held ultrasound device, all other communication technologies are widely available in many if not most homes currently, thus minimizing costs and maximizing potential opportunities.
Challenges related to COVID-19 include the high infectivity of the virus, rapid mutations of more aggressive variants, the frequency of asymptomatic carriers, and the fact that pauci-symptomatic patients may shed the most virus immediately before exhibiting any symptoms. This makes in-person medical assessments a potentially dangerous undertaking, and one that contributed to the near collapse of many healthcare systems [28, 29]. Furthermore, COVID-19 is predicted to be just one of many future zoonotic-based pandemics that will afflict humans in the future [30]. Thus, early experiences with nearly overwhelmed health systems, prompted recommendations to employ telemedical capabilities to provide advanced outreach capabilities, for the “entire population not only for hospitals” [31]. Such an approach would hospitalize only those with severe disease, with asymptomatic or paucisymptomatic observed at home, thereby decreasing contagion and preserving personal protective equipment [8, 31]. Such an approach necessitates the ability to quickly recognize those who exhibiting signs of deterioration, and “rescuing” them quickly [1, 8]. Previous work has demonstrated that with remote guidance, non-expert point of care providers, who may be as inexperienced as children, can be guided to place an inexpensive ultrasound probe onto the chest to assess the visceral–parietal pleural surface [17].
LUS, a relatively new discipline based on the science of artifact analysis, is now established in emergency and resuscitative medicine [26]. We and others recognize its near unlimited value to manage the COVID-19 crisis [11, 19, 32]. The gold standard diagnostic test for COVID-19 diagnosis is the identification of viral nucleic acids (PCR). However, compared to PCR, CT scan may show disease at an earlier time frame [33], as may LUS [34], even in cases, where the PCR was initially negative [35]. As lung ultrasound is a technology that may diagnosis COVID-19 earlier than PCR testing, is portable and able to go to the patient, relatively economical, can discern the presence of progression of COVID-19 pneumonia, and is easily repeatable over the projected time course of disease, lung ultrasound may be beneficial to follow pleural health over the complete evolution of disease [11]. Through marrying the ability to perform ultrasound upon oneself using remote guidance, all these potential uses might be provided without ever requiring a physical encounter with a health-care provider to completely reduce health care exposure to infection during the initial assessment and risk stratification of healthy but at-risk individuals.
In health, the pleural interface of the normal lung of a healthy human, is typically the only part of the lung that can be viewed with ultrasound [36], as air has the highest acoustic density of any other component of the human body, effectively preventing transmission of ultrasound waves beyond the pleura. Thus, when examining the interface of the parietal pleura of the chest wall and the visceral pleura of the lung, only this interface can be seen. When the lung becomes diseased with COVID pneumonia it has been observed that abnormal lung artifacts arise at the pleural junction [11]. Of note, the patchy bilateral, multifocal ground-glass opacity and abnormalities typically found on CT associated with COVID-19 are predominantly identified in the lower [9, 37, 38] and posterior lung zones [9, 39], the so called COVID hot spots. At the bedside, a critical “tipping-point” for concern stratifying those with home-manageable minor diseases from potentially severe deterioration may be an evolution from the normal A-line pattern of health to a progressively abnormal alveolar-interstitial pattern of lung disease illustrated by initially single or discernable B line artifacts, progressing to confluent vertical artifacts (B-lines) or confluent vertical artifacts, ultimately culminating with white lungs and/or consolidated effusions of severe COVID-19 [11].
Although standard examinations have been proposed [9, 22], there is no single currently accepted “standard” examination. It was not unexpected that participants would be less adept at examining their backs, and a posterior examination does not comprise part of many common lung ultrasound protocols (Table 5). Nonetheless, 96.3% of all participants could image their own lower back, higher than was originally expected, and emphasizing the remarkable abilities of motivated laypersons when mentored.
COVID-19 may be particularly treacherous as there may be profound disassociation between the severity of hypoxemia and preservation of respiratory muscle mechanics, lung compliance, and an absence of dyspnea [40]. Thus, there are patients with COVID-19 who exhibit oxygen levels incompatible with life without dyspnea, sometimes termed “happy hypoxia” but is more precisely termed silent hypoxemia [2]. There are also more than a few limitations of pulse oximetry such as O2 Saturation monitoring may not be accurate at very low PaO2 [2]. This condition may have an alarming frequency. Busana noted that among patients presenting to hospital with hypoxia consistent with acute respiratory failure, one-third were not dyspneic, including 18% with severely abnormal PaO2/FiO2 ratios of between 50 and 150, and overall, these patients had a mortality rate of 17.6% [1]. Thus, lung ultrasound offers a technique to potentially detect these patients at the earliest signs of lung swelling. Opportunities for future research include formal statistical comparisons of test performance characteristics of each of these modalities individually. However, we suspect that in actual clinical practice, these modalities would be complementary when used together. Recent guidelines for the use of Lung Ultrasound in managing COVID-19 did NOT recommend serial LUS re-examinations despite its potential utility in managing the patient’s status due to concerns regarding infection transmission risks [9]. However, with the technique of RTMSPLUS these risks are completely obviated. Furthermore, these same guidelines, recommend that LUS should, however, be the initial lung imaging modality of choice in patients with minimal symptoms as LUS has higher sensitivity and lower radiation risks [9]. We have also demonstrated unmanned arial vehicle (drone) delivery of RTMSPLUS, with unlimited potential to access remote and disadvantaged populations [41].
Pneumothoraces, posterior lung fields, and the lung bases
Although the additional modalities of M-Mode and CPD are not necessarily critical to the inference of COVID-19, they contribute to the diagnosis of pneumothorax which recent guidelines recommend assessing for during a COVID-19 lung ultrasound examination [9]. Recent guidelines also strongly recommend that in addition to the usual anterolateral lungs, that posterior lung zones should be scanned whenever possible [9]. This is an aspect, wherein the healthier asymptomatic/Paucisymptomatic patients are advantaged in not being bedbound and supine and were able to image their lower posterior lung fields 100% of the time.
A potential limitation of our study was that our participants were not sick and were feeling well. Therefore, the majority of the LUS images were normal. However, this is appropriate for a screening test intended for asymptomatic/pauci-symptomatic populations. Our participants were relatively young and nearly all completed high school. Thus, our results may not generalize to an older, or less educated population. Our participants all had access to a computer and internet. Remote guidance may be more challenging in situations, where internet is not available, although we agree that internet availability should be a basic human right [42]. Three participants had previous ultrasound exposure, but none had examined the pleura before. In general males were less able to image their complete backs than females, but this may be less critical for lung surveillance in COVID-19, given the propensity for early COVID to affect the lung bases. Therefore, it may be more important for RTMSPLUS to examine the lung bases (anterior, lateral, and posterior locations) and in our study, the ability to image these locations was 100% (Table 2).
This study constitutes a proof of concept that we hope generates further discussion and analysis of the capabilities, logistical requirements, and human factors challenges in assisting in remotely mentored self-care. Clearly innumerable details require study before such a paradigm is ready for clinical application. Given these practicalities, however, we contend that the concept potentially empowers those remote from fixed hospital care to empower their own healthcare through permitting required imaging of their own anatomy and physiology wherever they are geographically as long as they are connected. Just as necessity drove space medicine to consider innumerable innovative ways to incorporate remotely mentored ultrasound into care paradigms [43], just-in-time mentoring of the isolated may have innumerable applications that deserve examination.
Conclusions
We contend that providing home-SPRTLUS may be a useful method to provide surveillance of at-risk populations. Besides earlier diagnosis and rescue of severe cases, we postulate that such a proactive approach empowering the willing to manage their own health might also potentially reduce anxiety and increase the “connectedness” of patients and health care providers, an intangible commodity that is severely threatened in these times of strict self-isolation.
Availability of data and materials
The data sets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Change history
04 May 2023
A Correction to this paper has been published: https://doi.org/10.1186/s13089-023-00316-7
References
Busana M, Gasperetti A, Giosa L, Forleo GB, Schiavone M, Mitacchione G et al (2021) Prevalence and outcome of silent hypoxemia in COVID-19. Minerva Anestesiol 87(3):325–333
Tobin MJ, Laghi F, Jubran A (2020) Why COVID-19 silent hypoxemia is baffling to physicians. Am J Respir Crit Care Med 202(3):356–360
Nouri-Vaskeh M, Sharifi A, Khalili N, Zand R, Sharifi A (2020) Dyspneic and non-dyspneic (silent) hypoxemia in COVID-19: possible neurological mechanism. Clin Neurol Neurosurg 198:106217
Nacoti M, Ciocca A, Giupponi A, Brambillasca P, Lussana F, Pisano M, et al. At the Epicenter of the Covid-19 Pandemic and Humanitarian Crises in Italy: Changing Perspectives on Preparation and Mitigation. Innov Care Deliv. 2020.
Volpicelli G, Gargani L, Perlini S, Spinelli S, Barbieri G, Lanotte A et al (2021) Lung ultrasound for the early diagnosis of COVID-19 pneumonia: an international multicenter study. Intensive Care Med 47(4):444–454
Gargani L, Soliman-Aboumarie H, Volpicelli G, Corradi F, Pastore MC, Cameli M (2020) Why, when, and how to use lung ultrasound during the COVID-19 pandemic: enthusiasm and caution. Eur Heart J Cardiovasc Imaging 21(9):941–948
Volpicelli G, Gargani L (2020) Sonographic signs and patterns of COVID-19 pneumonia. Ultrasound J 12(1):22
Di Gennaro F, Pizzol D, Marotta C, Antunes M, Racalbuto V, Veronese N et al (2020) Coronavirus diseases (COVID-19) current status and future perspectives: a narrative review. Int J Environ Res Public Health 17(8):2690
Ma IWY, Hussain A, Wagner M, Walker B, Chee A, Arishenkoff S et al (2020) Canadian Internal Medicine Ultrasound (CIMUS) expert consensus statement on the use of lung ultrasound for the assessment of medical inpatients with known or suspected coronavirus disease 2019. J Ultrasound Med 40(9):1879–1882
Ng MY, Lee EYP, Yang J, Yang F, Li X, Wang H et al (2020) Imaging profile of the COVID-19 infection: radiologic findings and literature review. Radiol Cardiothorac Imaging. 2(1):e200034
Soldati G, Smargiassi A, Inchingolo R, Buonsenso D, Perrone T, Briganti DF et al (2020) Is there a role for lung ultrasound during the COVID-19 pandemic? J Ultrasound Med 39(7):1459–1462
Sargsyan AE, Hamilton DR, Jones JA, Melton S, Whitson PA, Kirkpatrick AW et al (2005) FAST at MACH 20: clinical ultrasound aboard the International Space Station. J Trauma 58(1):35–39
Biegler N, McBeth PB, Tevez-Molina M, McMillan JR, Crawford I, Hamilton DR et al (2012) Just-in-time cost-effective off-the-shelf remote telementoring of paramedical personnel in bed-side lung sonography—a technical case study. Telemed J E Health 18(10):807–809
Biegler N, McBeth PB, Tiruta C, Hamilton DR, Xiao Z, Crawford I et al (2013) The feasibility of nurse practitioner-performed, telementored lung telesonography with remote physician guidance—’a remote virtual mentor’. Crit Ultrasound J 5(1):5
Crawford I, McBeth PB, Mitchelson M, Tiruta C, Ferguson J, Kirkpatrick AW (2011) Telementorable “just-in-time” lung ultrasound on an iPhone. J Emerg Trauma Shock 4(4):526–527
Crawford I, Tiruta C, Kirkpatrick AW, Mitchelson M, Ferguson J (2011) Big brother could actually help quite easily: telementored “just-in-time” telesonography of the FAST over a smartphone. Ann Emerg Med 58(3):312–314
McBeth PB, Crawford I, Blaivas M, Hamilton T, Musselwhite K, Panebianco N et al (2011) Simple, almost anywhere, with almost anyone: remote low-cost telementored resuscitative lung ultrasound. J Trauma 71(6):1528–1535
Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D (2016) SQUIRE 2.0-standards for quality improvement reporting excellence-revised publication guidelines from a detailed consensus process. J Am Coll Surg 222(3):317–323
Soldati G, Smargiassi A, Inchingolo R, Buonsenso D, Perrone T, Briganti DF et al (2020) Proposal for international standardization of the use of lung ultrasound for COVID-19 patients; a simple, quantitative, reproducible method. J Ultrasound Med 39(7):1413–1419
Lichtenstein DA, Meziere GA (2008) Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol. Chest 134(1):117–125
Cunningham J, Kirkpatrick AW, Nicolaou S, Liu D, Hamilton DR, Lawless B et al (2002) Enhanced recognition of “lung sliding” with power color Doppler imaging in the diagnosis of pneumothorax. J Trauma 52(4):769–771
Soldati G, Smargiassi A, Inchingolo R, Buonsenso D, Perrone T, Briganti DF et al (2020) Proposal for international standardization of the use of lung ultrasound for patients With COVID-19: a simple, quantitative, reproducible method. J Ultrasound Med 39(7):1413–1419
Kirkpatrick AW, Sirois M, Laupland KB, Liu D, Rowan K, Ball CG et al (2004) Hand-held thoracic sonography for detecting post-traumatic pneumothoraces: the Extended Focused Assessment with Sonography for Trauma (EFAST). J Trauma 57(2):288–295
Lichtenstein DA, Meziere GA (2008) Relevance of lung ultrasound in the diagnosis of acute respiratory distress. Chest 134(1):117–125
Lamperti M, Bodenham AR, Pittiruti M, Blaivas M, Augoustides JG, Elbarbary M et al (2012) International evidence-based recommendations on ultrasound-guided vascular access. Intensive Care Med 38(7):1105–1117
Volpicelli G, Elbarbary M, Blaivas M, Lichtenstein DA, Mathis G, Kirkpatrick AW et al (2012) International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med 38(4):577–591
Kirkpatrick AW, McKee JL, Conly JM (2021) Longitudinal remotely mentored self-performed lung ultrasound surveillance of paucisymptomatic Covid-19 patients at risk of disease progression. Ultrasound J 13(1):27
Chirico F, Nucera G, Magnavita N. COVID-19: Protecting Healthcare Workers is a priority. Infect Control Hosp Epidemiol. 2020:1–4.
R M, E P. Virus Knocks Thousands of Health Workers Out of Action in Europe New York: New York Times; 2020 [cited 2020 April 12 2020]. https://www.nytimes.com/2020/03/24/world/europe/coronavirus-europe-covid-19.html.
Daszak P, das Neves C, Amuasi J, Hayman D, Kuiken T, Roche B, Zambrana-Torrelio C, Buss P, Dundarova H, Feferholtz Y, Foldvari G, Igbinosa E, Junglen S, Liu Q, Suzan G, Uhart M, Wannous C, Woolaston K, Mosig Reidl P, O'Brien K, Pascual U, Stoett P, Li H, Ngo HT, IPBES secretariat. Workshop report on biodiversity and pandemics of the intergovernmental platform on biodiversity and ecosystem services. Bonn, Germany; 2020. Contract No.: 10.5281/zenodo.4147317.
Nacoti N, Ciocca A, Giupponi G, Bonacina D, Fazzi F, Naspro R, et al. At the epicentre of the Covid-19 pademic and humanitarian crises in Italy: chaging perspectives on preparation and mitigation. NEJM Catalyst. 2020;1(2).
Kirkpatrick AW, McKee JL. Lung ultrasound findings in a 64-year-old woman with Covid-19: This examination could be remote! CMAJ [Internet]. 2020. https://www.cmaj.ca/content/192/15/E399/tab-e-letters#re-lung-ultrasound-findings-in-a-64-year-old-woman-with-covid-19-this-examination-could-be-remote.
Yang W, Sirajuddin A, Zhang X, Liu G, Teng Z, Zhao S et al (2020) The role of imaging in 2019 novel coronavirus pneumonia (COVID-19). Eur Radiol 30(9):4874–4882
Poggiali E, Dacrema A, Bastoni D, Tinelli V, Demichele E, Mateo Ramos P et al (2020) Can lung US Help critical care clinicians in the early diagnosis of novel coronavirus (COVID-19) pneumonia? Radiology 295(3):200847
Kalafat E, Yaprak E, Cinar G, Varli B, Ozisik S, Uzun C et al (2020) Lung ultrasound and computed tomographic findings in pregnant woman with COVID-19. Ultrasound Obstet Gynecol 55(6):835–837
Kirkpatrick AW (2007) Clinician-performed focused sonography for the resuscitation of trauma. Crit Care Med 35:S162–S172
Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A (2020) Coronavirus disease 2019 (COVID-19) imaging reporting and data system (COVID-RADS) and common lexicon: a proposal based on the imaging data of 37 studies. Eur Radiol 30(9):4930–4942
Caruso D, Zerunian M, Polici M, Pucciarelli F, Polidori T, Rucci C et al (2020) Chest CT features of COVID-19 in Rome. Italy Radiology 296(2):E79–E85
Song F, Shi N, Shan F, Zhang Z, Shen J, Lu H et al (2020) Emerging 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology 295(1):210–217
Gattinoni L, Chiumello D, Rossi S (2020) COVID-19 pneumonia: ARDS or not? Crit Care 24(1):154
Kirkpatrick AW, McKee JL, Moeini S, Conly JM, Ma IWY, Baylis B et al (2021) Pioneering remotely piloted aerial systems (Drone) Delivery of a remotely telementored ultrasound capability for self diagnosis and assessment of vulnerable populations-the sky is the limit. J Digit Imaging 34(4):841–845
La Rue F. Promotion and protection of all human rights, civil, political, economic, social and cultural rights, including the right to development 2011 [A/HRC/17/27]. http://www2.ohchr.org/english/bodies/hrcouncil/docs/17session/A.HRC.17.27_en.pdf.
Kwon D, Bouffard JA, van Holsbeeck M, Sargsyan AE, Hamilton DR, Melton SL et al (2007) Battling fire and ice: remote guidance ultrasound to diagnose injury on the International Space Station and the ice rink. Am J Surg 193(3):417–420
Lichtenstein DA, Menu Y (1995) A bedside ultrasound sign ruling out pneumothorax in the critically ill. Lung sliding Chest 108(5):1345–1348
Acknowledgements
The authors would like to acknowledge Jill and Ryan Buehler, Karra and Chris Egato, Katherine and Mark Farr, Chantelle Fortin, Henry Friese, Terry Han, Jennifer and Andrew Laycock, Wei Li, Jessica and Ian McKee, Jordan Schroeder, William James Slater and Virginia Slater, Shawna and Jason Sopka, and Brenda and Dennis Southwick for their time and efforts to conduct this work.
Funding
This work was partially supported by an unrestricted grant from the Office of Surgical Research of the Department of Surgery, University of Calgary, Calgary Alberta, and personal contributions from the Andrew W Kirkpatrick Professional Corporation.
Author information
Authors and Affiliations
Contributions
AWK made substantial contributions to the conception AND design of the work; AND the acquisition, analysis, AND interpretation of data; AND drafted the work AND substantively revised it AND approved the submitted version AND agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. JLM made substantial contributions to the conception AND design of the work; AND the acquisition, analysis, AND interpretation of data; AND approved the submitted version AND agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. CGB made substantial contributions to the acquisition, analysis, AND interpretation of data; AND substantively revised the work AND approved the submitted version AND agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. IWYM made substantial contributions to the acquisition, analysis, AND interpretation of data; AND substantively revised the work AND approved the submitted version AND agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. LAM made substantial contributions to the acquisition, analysis, AND interpretation of data; AND substantively revised the work AND approved the submitted version AND agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This research was approved by the CHREB of the University of Calgary, REB14-0634_MOD4.Consent for publication. All subjects provided written informed consent after full explanation of the study.
Consent for Publication
The research participants freely consent to publication of the main manuscript and Supplementary material.
Competing interests
Andrew W Kirkpatrick has consulted for the Zoll, Innovative Trauma Care, CSL Behring and SAM Medical Corporations, and is the PI of a Prospective Randomized Controlled Trial partially supported by the Acelity Corporation ( https://clinicaltrials.gov/ct2/show/NCT03163095). He discloses a personal relationship with JL McKee. Jessica L McKee is the Research Director of Innovative Trauma Care and has consulted for Zoll, Aceso, SAM Medical Products and Acelity. She also discloses a personal relationship with AW Kirkpatrick. Chad G Ball MD, reported no disclosures. Irene W. Y. Ma, is a chair holder of the John A. Buchanan Chair in General Internal Medicine at the University of Calgary. She has no other conflicts of interest to disclose. Lawrence A. Melniker, reported no disclosures.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Subject pre-test evaluation form.
Additional file 2
. Pre-examination Introductory Video.
Additional file 3
. Complete video recordings of examinations.
Additional file 4
. Pleural Scoring System for COVID 19.
Additional file 5
. Mentors and Blinded Reviewers Assessment of Pleural Lung Health Scoring.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kirkpatrick, A.W., McKee, J.L., Ball, C.G. et al. Empowering the willing: the feasibility of tele-mentored self-performed pleural ultrasound assessment for the surveillance of lung health. Ultrasound J 14, 2 (2022). https://doi.org/10.1186/s13089-021-00250-6
Accepted:
Published:
DOI: https://doi.org/10.1186/s13089-021-00250-6