Abstract
Background
The role of lung ultrasound (LUS) in evaluating the mid- and long-term prognoses of patients with COVID-19 pneumonia is not yet known. The objectives of this study were to evaluate associations between LUS signs at the time of screening and clinical outcomes 1 month after LUS and to assess LUS signs at the time of presentation with known risk factors for COVID-19 pneumonia.
Methods
This was a retrospective study of data prospectively collected 1 month after LUS screening of 447 adult patients diagnosed with COVID-19 pneumonia. Sonographic examination was performed in screening tents with the participants seated. The LUS signs (B-lines > 2, coalescent B-lines, and subpleural consolidations) were captured in six areas of each hemithorax and a LUS aeration score was calculated; in addition, the categories of disease probability based on patterns of LUS findings (high-probability, intermediate-probability, alternate, and low-probability patterns) were evaluated. The LUS signs at patients’ initial evaluation were related to the following outcomes: symptomatology, the need for hospitalization or invasive mechanical ventilation (IMV), and COVID-19-related death.
Results
According to the evaluations performed 1 month after LUS screening, 36 patients were hospitalised, eight of whom required intensive care unit (ICU) admission and three of whom died. The presence of coalescent B-lines was associated with the need for hospitalization (p = 0.008). The presence of subpleural consolidations was associated with dyspnoea (p < 0.0001), cough (p = 0.003), the need for hospitalization (p < 0.0001), the need for ICU admission (p < 0.0001), and death (p = 0.002). A higher aeration score was associated with dyspnoea (p < 0.0001), the need for hospitalization (p < 0.0001), the need for ICU admission (p < 0.0001), and death (p = 0.003). In addition, patients with a high-probability LUS pattern had a higher aeration score (p < 0.0001) and more dyspnoea (p = 0.024) and more often required hospitalization (p < 0.0001) and ICU admission (p = 0.031).
Conclusions
In patients with COVID-19 pneumonia, LUS signs were related to respiratory symptoms 1 month after LUS screening. Strong relationships were identified between LUS signs and the need for hospitalization and death.
Similar content being viewed by others
Background
In the lungs, infection by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is characterized by severe pneumonia and/or acute respiratory distress syndrome (ARDS) in approximately 20% of patients, and mid- and long-term respiratory complications are beginning to be described [1]. However, data from previous coronavirus outbreaks, such as SARS and Middle East respiratory syndrome, suggest that some patients will have these complications after the acute phase of the disease [2]. The possible pulmonary sequelae include pulmonary vascular disease and interstitial lung disease; however, many other respiratory manifestations and changes in pulmonary function can be observed [3, 4].
Amid the coronavirus disease 2019 (COVID-19) pandemic and given the frequent lung involvement observed, new tools are needed to evaluate affected patients and to identify data with potential prognostic implications. Because lung ultrasound (LUS) is a fast test, it has been increasingly used as an alternative imaging method, and evidence supports its ability to identify lung lesions in COVID-19 [5,6,7]. In fact, LUS can be useful at various times, from screening for early diagnosis of lung involvement to decision-making about intensive care unit (ICU) treatment and respiratory support (e.g., mechanical ventilation, prone positioning, positive end-expiratory pressure, recruitment manoeuvres) [8,9,10,11,12]. Because of the strong association between LUS and computed tomography (CT) findings, LUS may be effective for diagnosing peripherally distributed lesions, which are characteristic of SARS-CoV-2 infection [13, 14]. In addition, LUS can identify the persistence of subclinical residual lung damage in COVID-19, even if patients meet the discharge criteria [15].
With the increasing number of patients who have survived COVID-19 worldwide, the most effective follow-up strategies for these patients must be determined. However, few studies have explored the follow-up of such patients after the acute phase of the disease. Severe disease is known to be associated with a higher probability of disability, and patients requiring ICU admission have been observed to have worse mid- and long-term outcomes, with physiological impairment and persistent radiological changes, than those not requiring ICU admission [3]. Because LUS has been increasingly used in the diagnosis of COVID-19 pneumonia, evidence of the prognostic role of LUS should be extended to the management of COVID-19 pneumonia. Thus, the objectives of this study were to evaluate associations between LUS signs at patients’ initial evaluation as a screening tool for pulmonary involvement due to COVID-19 and clinical outcomes 1 month after LUS and to assess LUS signs at the time of presentation with known risk factors for COVID-19 pneumonia.
Materials and methods
Participants
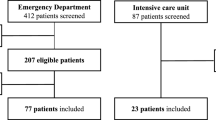
This was a retrospective study of data prospectively collected from LUS examinations performed on patients aged ≥ 18 years with fever and/or acute respiratory symptoms (dyspnoea, cough, and/or coryza) and diagnosed with COVID-19 confirmed by reverse-transcription polymerase chain reaction (RT-PCR). The patients were screened in tents installed in the courtyard of Piquet Carneiro Policlinic, State University of Rio de Janeiro, Rio de Janeiro, Brazil. Patients with LUS exams showing no pathological signs (absence of significant multiple and coalescent B-lines, absence of peripheral and large consolidations) were excluded. The results were analysed at the time of the initial LUS examination, and a clinical follow-up was conducted by phone 1 month after the initial LUS test. The outcomes studied included symptomatology, the need for hospitalization, the need for invasive mechanical ventilation (IMV), and COVID-19-related death. The study was approved by the National Research Ethics Committee of Brazil under number CAAE-30135320.0.0000.5259. All participants read the protocol and agreed to participate in the study.
Lung ultrasound
We used an Aplio XG device (Toshiba Medical Systems, Tokyo, Japan) for the LUS examinations; the device was coupled to a 7.5–10-MHz multifrequency linear transducer or a 3.5- to 5-MHz convex transducer in B mode. The convex transducer was routinely used for analysis, while the linear transducer was used only when doubts remained regarding the analysis of the pleural surface. All LUS evaluations were carried out by a team of 6 pulmonologists employed at the policlinic with experience in the method (three with 13 years of experience, two with 11 years of experience, and one with 8 years of experience in ultrasound at the screening site). The LUS exams were performed with the participants in a sitting position since they were all outpatients and had no ventilatory or haemodynamic instability (individuals with respiratory symptoms were examined in screening tents). All LUS evaluations were performed by two examiners using a standard data collection form, and when disagreements occurred between the examiners, an agreement was reached through a collective discussion. Although no standard has been established with respect to specific scanning protocols, LUS signs were captured in six areas of each hemithorax following a 12-zone protocol [16, 17] as follows: two anterior, two lateral, and two posterior areas (Fig. 1). Each intercostal space of the upper and lower parts of the anterior, lateral, and posterior regions of the left and right chest walls were carefully examined. When evaluating pathological LUS signs, we searched for the following findings: B-lines > 2 (hyperechoic vertical artefacts arising from the pleural line, extending to the bottom of the screen without fading, and vacillating with lung movement, which occurs when the lung loses normal aeration but is not completely consolidated), coalescent B-lines (coalescence of many vertical artefacts to form more extended echogenic patterns corresponding to severe lung aeration loss), and subpleural consolidations (hypoechoic areas that appear as the subpleural density approaches the density of solid tissue, suggesting subpleural fluid-filled alveoli) [14, 18]. These abnormalities were evaluated separately and in combination to obtain an aeration score. To obtain the aeration score, we used a score for each area ranging from 0 to 3 according to the LUS sign as follows: B-lines > 2, 1 point; coalescent B-lines, 2 points; and consolidations, 3 points; the aeration score was calculated as the sum of these points and ranged between 0 and 36 [19]. In addition, we evaluated categories of disease probability based on patterns of LUS findings (high-probability, intermediate-probability, alternate, and low-probability patterns) [20,21,22].
Representation of the 12 zones on the chest. a The anterior and axillary zones; b the posterior zones. The zones 1 and 2 are limited by the parasternal and anterior axillary lines. The zones 3 and 4 are limited by the anterior axillary and posterior axillary lines. Finally, the zones 5 and 6 are limited by the paravertebral and posterior axillary lines and by the contour of the scapula
Statistical analysis
A nonparametric method was applied because the variables did not have a Gaussian distribution as indicated by rejection of the null hypothesis of a normal distribution by the Shapiro–Wilk test and graphical analysis of histograms. Thus, the most appropriate measures for summarizing the results were quartiles (the medians and interquartile ranges) for numerical data and the frequency (percentage) for categorical data. For numerical data, associations between pathological LUS signs, clinical findings detected 1 month after LUS, and poor outcomes were evaluated by the Mann–Whitney test for comparisons between two subgroups or Kruskal–Wallis ANOVA for comparisons between three subgroups (Dunn’s multiple comparison test was applied to identify which subgroups differed significantly from each other). For categorical data, associations were evaluated using the Chi-square or Fisher’s exact test. The significance level adopted was 5%. Statistical analysis was performed using the statistical software SAS 6.11 (SAS Institute, Inc., Cary, NC, USA).
Results
Among the 460 patients eligible for inclusion in the study, 13 were lost to follow-up because they could not be reached by telephone. Among the 447 participants with COVID-19 pneumonia diagnosed by LUS included in the study, 305 (68.2%) were women, and the median age was 40 (34–50) years. The median time since symptom onset was 5 (2–7) days. The most prevalent comorbidities in this sample were hypertension (98, 21.9%) and diabetes (52, 11.6%). On LUS, the most frequent pathological sign was B-lines > 2, and the median aeration score at the time of COVID-19 pneumonia diagnosis was 4 (2–7). The demographic and clinical characteristics of the sample and the LUS data at the time of diagnosing pulmonary involvement are provided in Table 1.
According to the evaluations performed 1 month after LUS screening for COVID-19 pneumonia, 36 (8.1%) patients required hospitalization due to pulmonary complications, with a median length of stay of 7 (4–10) days; among those who required hospitalization, 8 (1.8%) required admission to the ICU, and 3 (0.7%) died. In the sample studied 1 month after the initial evaluation, 121 (27.1%) reported general fatigue, while 62 (13.9%) complained of dyspnoea. Cough and fever were reported by 39 (8.7%) and 3 (0.7%) patients, respectively.
We evaluated associations between LUS findings at the time of screening, clinical outcomes, and the presence of symptoms 1 month after LUS. In this analysis, the presence of B-lines > 2 was not associated with any clinical finding or outcome. However, the presence of coalescent B-lines was associated with older age (p = 0.035), a higher body mass index (p = 0.027), diabetes (p = 0.027), dyspnoea (p = 0.037), and the need for hospitalization (p = 0.008). The presence of subpleural consolidations on LUS was associated with diabetes (p = 0.002), chronic heart failure (CHF, p = 0.021), general fatigue (p = 0.013), dyspnoea (p < 0.0001), cough (p = 0.003), fever (p = 0.043), the need for hospitalization (p < 0.0001), the need for ICU admission (p < 0.0001), and death (p = 0.002) (Fig. 2).
We evaluated the relationships of the LUS aeration score with clinical outcomes and symptoms 1 month after ultrasound examination. In this analysis, a higher aeration score was associated with hypertension (p = 0.032), diabetes (p = 0.0001), dyspnoea (p < 0.0001), cough (p = 0.041), the need for hospitalization (p < 0.0001), the need for ICU admission (p < 0.0001), and death (p = 0.003) (Fig. 3).
Additionally, we evaluated categories of the probability of COVID-19 based on patterns of LUS signals. In this analysis, 234 cases (52.3%) had high-probability patterns, 180 cases (40.3%) had intermediate-probability patterns, and 33 cases (7.4%) had alternate LUS patterns; no participant had low-probability patterns. Although all participants were positive for COVID-19 by RT-PCR, the alternate LUS patterns were as follows: chronic heart failure (n = 18), bacterial pneumonia (n = 6), tuberculosis sequelae (n = 4), systemic sclerosis (n = 3), and rheumatoid arthritis (n = 2). Interestingly, we observed that patients with a high-probability LUS pattern had a higher aeration score (p < 0.0001) and more dyspnoea (p = 0.024) and more often required hospitalization (p < 0.0001) and ICU admission (p = 0.031) (Table 2).
Discussion
LUS has played a critical role in the pandemic caused by SARS-CoV-2, with growing evidence of its usefulness both to screen for lung involvement and to evaluate disease severity. Sequential LUS has also been used as an efficient tool to monitor the progression of lung lesions in individuals with more severe lung disease. Despite all these indications, the prognostic power of LUS in COVID-19 is also important to evaluate. Here, we searched for correlations between pathological LUS signs and patient outcomes. The main results of the present study were that 1 month after LUS screening, LUS signs (especially subpleural consolidations) were associated with the presence of persistent respiratory symptoms and general fatigue. The LUS findings strongly predicted the need for hospitalization (including in the ICU) and death. In addition, the LUS aeration score was correlated with the persistence of respiratory manifestations at 1 month after ultrasound examination. Finally, we also observed a strong relationship between aeration scores and poor outcomes, such as the need for hospitalization (including in the ICU) and death. To our knowledge, this study is the first to evaluate the relationship between pathological LUS signs and 1-month clinical outcomes.
The mid- and long-term complications of COVID-19, including those related to the respiratory system, should be identified, and affected patients require follow-ups by appropriate services. In the present study, we observed that respiratory manifestations (such as dyspnoea and cough) and systemic manifestations (such as fever and general fatigue) were associated with LUS signs diagnosed 1 month before the prospective evaluation. Since ultrasound equipment is increasingly available and LUS can be performed at the bedside within a few minutes and in patients with mild disease or even in unstable patients [23], we believe that these interrelationships may be of great clinical interest in the context of the COVID-19 pandemic. Although LUS signs may not be specific for COVID-19 compared to some other lung diseases, the identification of certain patterns in the epidemiological context of the pandemic can certainly help clinicians to identify individuals likely to exhibit worsening clinical conditions and to develop sequelae after resolution of the acute phase of the disease [24, 25].
The clinical significance of B-lines depends mainly on their quantity (the number of B-lines per area examined and the presence or absence of confluent B-lines) and is usually associated with interstitial syndromes [2]. As the disease progresses, the air content decreases and lung density and the number of B-lines increase, leading to confluent areas equivalent to ground-glass opacities (GGOs) on CT [2, 24]. In the present study, we observed that coalescent B-lines were associated with older age, obesity, and diabetes, which are considered risk factors for COVID-19 exacerbation [4, 26, 27]. However, the strongest associations with persistent respiratory symptoms 1 month after LUS were observed for previously diagnosed subpleural consolidations. In fact, as the pneumonic process progresses in COVID-19, lung density increases even more because of alveolar infiltration with inflammatory cells, causing loss of aeration and consolidation areas [2, 24]. Thus, the finding of subpleural consolidations on LUS may not only predict worsening of symptoms in the course of the disease [23] but may also indicate a more severe course in terms of pulmonary sequelae in SARS-CoV-2 infection.
The use of a scoring system to assess pathological LUS signs in patients infected with SARS-CoV-2 has been increasingly widespread during the pandemic [11, 28]. In our study, we observed that the LUS aeration score was strongly related to dyspnoea and cough 1 month after ultrasound screening. Interestingly, Lichter et al. [29] evaluated 120 consecutive patients with COVID-19 who underwent LUS within 24 h after admission. These authors observed that clinical deterioration was associated with increased follow-up LUS scores, mainly due to loss of aeration in anterior lung segments. Using an LUS scoring system and the need for supplemental oxygen at the time of examination, Manivel et al. [30] proposed a protocol to assist clinicians with decision-making in patients with COVID-19 and to facilitate provision planning within emergency departments. Because our study examined 1-month outcomes, we think that our results provide additional evidence that the LUS score can be used as a prediction tool for clinical outcomes of the disease after the acute phase and can be used as an additional tool for clinical reasoning.
In COVID-19, lung lesions play a key role in determining the clinical course and prognosis [31]. In this sense, one of the objectives of the current study was to evaluate the correlations of LUS findings with poor outcomes. We observed that the pathological LUS signs (particularly subpleural consolidations) and a higher aeration score were associated with the need for hospitalization, the need for ICU admission, and death. In line with our findings, some researchers have observed that early LUS signs (including the LUS score) are effective for assessing the need for prolonged hospitalization [15, 32]. Other studies have shown the potential of LUS to stratify early risk in COVID-19 patients who visit emergency departments according to mortality risk and the need for IMV, with this risk being higher in individuals with more pathological lung areas [29, 33, 34]. Given the scarcity of equipment and trained health professionals, the use of a relatively simple diagnostic procedure that does not use ionizing radiation, such as LUS, may have clinical and public health implications [34]. In contrast to studies evaluating short-term consequences, our study assessed 1-month outcomes, which may have important implications from the perspective of utilizing scarce resources in disadvantaged socioeconomic areas.
When using LUS, the criteria for positivity must be defined considering the possibility of alternative diagnoses and levels of probability [20, 21]. Because LUS signs are nonspecific, LUS cannot be used alone to establish a definitive diagnosis of COVID-19 infection [35]. In fact, almost 10% of our cases had LUS patterns more consistent with other diagnoses (including cardiogenic pulmonary oedema and bacterial pneumonia), although the diagnosis of COVID-19 was confirmed in all participants by RT-PCR. Importantly, our study showed that patients with high-probability LUS patterns had higher aeration scores and more dyspnoea 1 month after LUS screening and more often required hospitalization and ICU admission. Although more studies are needed, the use of categories of the probability of COVID-19 pneumonia can be an interesting strategy to predict the evolution of the disease in the short and medium term.
Several limitations in our study should be noted. First, our study was conducted at a single reference centre during the screening of COVID-19 pneumonia; therefore, generalization of our results to other periods of the pandemic should be executed with caution. However, with the emergence of the second wave of the pandemic in many regions of the world, the role of LUS may become even more prominent in the process of implementing local and global measures efficiently. Second, ultrasound evaluates only approximately 1/16 of the total lung and detects only changes closely related to the pleural surface [36]; therefore, other chest imaging methods may have a greater prognostic role than LUS. Third, we performed only a single evaluation by LUS; serial LUS examinations can be useful for tracking the clinical trajectory of an apparently unpredictable disease course, thus improving the prediction of clinical outcomes.
Conclusion
In patients with COVID-19 pneumonia, LUS signs, including the aeration score, were related to respiratory and systemic symptoms 1 month after ultrasound screening examination. In these patients, a strong relationship was also found between LUS findings and poor outcomes, such as the need for hospitalization (including in the ICU) and death. Future studies should evaluate the relationship between LUS signs at the time of diagnosing lung involvement and long-term consequences.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Abbreviations
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- ARDS:
-
Acute respiratory distress syndrome
- COVID-19:
-
Coronavirus disease 2019
- LUS:
-
Lung ultrasound
- ICU:
-
Intensive care unit
- CT:
-
Computed tomography
- RT-PCR:
-
Reverse-transcription polymerase chain reaction
- IMV:
-
Invasive mechanical ventilation
- GGO:
-
Ground-glass opacities
References
Chen N, Zhou M, Dong X et al (2020) Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395:507–513. https://doi.org/10.1016/S0140-6736(20)30211-7
Allinovi M, Parise A, Giacalone M et al (2020) Lung ultrasound may support diagnosis and monitoring of COVID-19 pneumonia. Ultrasound Med Biol 46:2908–2917. https://doi.org/10.1016/j.ultrasmedbio.2020.07.018
George PM, Barratt SL, Condliffe R et al (2020) Respiratory follow-up of patients with COVID-19 pneumonia. Thorax 75:1009–1016. https://doi.org/10.1136/thoraxjnl-2020-215314
Mo X, Jian W, Su Z et al (2020) Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur Respir J 55:2001217. https://doi.org/10.1183/13993003.01217-2020
Smargiassi A, Soldati G, Torri E et al (2021) Lung ultrasound for COVID-19 patchy pneumonia. J Ultrasound Med 40:521–528. https://doi.org/10.1002/jum.15428
Fonsi GB, Sapienza P, Brachini G et al (2020) Is lung ultrasound imaging a worthwhile procedure for severe acute respiratory syndrome coronavirus 2 pneumonia detection? J Ultrasound Med. https://doi.org/10.1002/jum.15487 (Epub ahead of print)
Zhu F, Zhao X, Wang T et al (2020) Ultrasonic characteristics and severity assessment of lung ultrasound in COVID-19 pneumonia in Wuhan, China: a retrospective, observational study. Engineering. https://doi.org/10.1016/j.eng.2020.09.007 (Epub ahead of print)
Lopes AJ, Mafort TT, da Costa CH et al (2020) Comparison between lung ultrasound and computed tomographic findings in patients with COVID-19 pneumonia. J Ultrasound Med. https://doi.org/10.1002/jum.15521 (Epub ahead of print)
Mafort TT, Lopes AJ, da Costa CH et al (2020) Changes in lung ultrasound of symptomatic healthcare professionals with COVID-19 pneumonia and their association with clinical findings. J Clin Ultrasound 48:515–521. https://doi.org/10.1002/jcu.22905
Zieleskiewicz L, Markarian T, Lopez A et al (2020) Comparative study of lung ultrasound and chest computed tomography scan in the assessment of severity of confirmed COVID-19 pneumonia. Intensive Care Med 46:1707–1713. https://doi.org/10.1007/s00134-020-06186-0
Perrone T, Soldati G, Padovini L (2020) A new lung ultrasound protocol able to predict worsening in patients affected by severe acute respiratory syndrome coronavirus 2 pneumonia. J Ultrasound Med. https://doi.org/10.1002/jum.15548 (Epub ahead of print)
Hussain A, Via G, Melniker L et al (2020) Multi-organ point-of-care ultrasound for COVID-19 (PoCUS4COVID): international expert consensus. Crit Care 24:702. https://doi.org/10.1186/s13054-020-03369-5
Bernheim A, Mei X, Huang M et al (2020) Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology 295:200463. https://doi.org/10.1148/radiol.2020200463
Sultan LR, Sehgal CM (2020) A review of early experience in lung ultrasound in the diagnosis and management of COVID-19. Ultrasound Med Biol 46:2530–2545. https://doi.org/10.1016/j.ultrasmedbio.2020.05.012
Gaspardone C, Meloni C, Preda A et al (2021) Lung ultrasound in COVID-19 a role beyond the acute phase? J Ultrasound Med 40:503–511. https://doi.org/10.1002/jum.15425
Soummer A, Perbet S, Brisson H et al (2012) Ultrasound assessment of lung aeration loss during a successful weaning trial predicts postextubation distress. Crit Care Med 40:2064–2072. https://doi.org/10.1097/CCM.0b013e31824e68ae
Tung-Chen Y, Algora-Martín A, Llamas-Fuentes R et al (2021) Point-of-care ultrasonography in the initial characterization of patients with COVID-19. Med Clin. https://doi.org/10.1016/j.medcli.2020.12.007 (Epub ahead of print)
Saraogi A (2015) Lung ultrasound: present and future. Lung India 32:250–257. https://doi.org/10.4103/0970-2113.156245
Tung-Chen Y, de Gracia MM, Díez-Tascón A et al (2020) Correlation between chest computed tomography and lung ultrasonography in patients with coronavirus disease 2019 (COVID-19). Ultrasound Med Biol 46:2918–2926. https://doi.org/10.1016/j.ultrasmedbio.2020.07.003
Volpicelli G, Lamorte A, Villén T (2020) What’s new in lung ultrasound during the COVID-19 pandemic. Intensive Care Med 46:1445–1448. https://doi.org/10.1007/s00134-020-06048-9
Millington SJ, Koenig S, Mayo P et al (2021) Lung ultrasound for patients with coronavirus disease 2019 pulmonary disease. Chest 159:205–211. https://doi.org/10.1016/j.chest.2020.08.2054
Volpicelli G, Gargani L, Perlini S et al (2021) Lung ultrasound for the early diagnosis of COVID-19 pneumonia: an international multicenter study. Intensive Care Med. https://doi.org/10.1007/s00134-021-06373-7 (Epub ahead of print)
Tung-Chen Y (2020) Lung ultrasound in the monitoring of COVID-19 infection. Clin Med 20:e62–e65. https://doi.org/10.7861/clinmed.2020-0123
Vetrugno L, Baciarello M, Bignami E et al (2020) The “pandemic” increase in lung ultrasound use in response to Covid-19: can we complement computed tomography findings? A narrative review Ultrasound J 12:39. https://doi.org/10.1186/s13089-020-00185-4
Convissar DL, Gibson LE, Berra L et al (2020) Application of lung ultrasound during the COVID-19 pandemic: a narrative review. Anesth Analg 131:345–350. https://doi.org/10.1213/ANE.0000000000004929
Zheng Z, Peng F, Xu B et al (2020) Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect 81:e16–e25. https://doi.org/10.1016/j.jinf.2020.04.021
Korakas E, Ikonomidis I, Kousathana F et al (2020) Obesity and COVID-19: immune and metabolic derangement as a possible link to adverse clinical outcomes. Am J Physiol Endocrinol Metab 319:E105–E109. https://doi.org/10.1152/ajpendo.00198.2020
Vetrugno L, Bove T, Orso D et al (2020) Our Italian experience using lung ultrasound for identification, grading and serial follow-up of severity of lung involvement for management of patients with COVID-19. Echocardiography 37:625–627. https://doi.org/10.1111/echo.14664
Lichter Y, Topilsky Y, Taieb P et al (2020) Lung ultrasound predicts clinical course and outcomes in COVID-19 patients. Intensive Care Med 46:1873–1883. https://doi.org/10.1007/s00134-020-06212-1
Manivel V, Lesnewski A, Shamim S et al (2020) CLUE: COVID-19 lung ultrasound in emergency department. Emerg Med Australas 32:694–696. https://doi.org/10.1111/1742-6723.13546
Istvan-Adorjan S, Ágoston G, Varga A et al (2020) Pathophysiological background and clinical practice of lung ultrasound in COVID-19 patients: a short review. Anatol J Cardiol 24:76–80. https://doi.org/10.14744/AnatolJCardiol.2020.33645
Jin YH, Cai L, Cheng ZS et al (2020) A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version). Mil Med Res 7:4. https://doi.org/10.1186/s40779-020-0233-6
Brahier T, Meuwly J-Y, Pantet O et al (2020) Lung ultrasonography for risk stratification in patients with COVID-19: a prospective observational cohort study. Clin Infect Dis. https://doi.org/10.1093/cid/ciaa1408 (Epub ahead of print)
Bonadia N, Carnicelli A, Piano A et al (2020) Lung ultrasound findings are associated with mortality and need for intensive care admission in COVID-19 patients evaluated in the emergency department. Ultrasound Med Biol 46:2927–2937. https://doi.org/10.1016/j.ultrasmedbio.2020.07.005
Volpicelli G, Gargani L (2020) Sonographic signs and patterns of COVID-19 pneumonia. Ultrasound J 12:22. https://doi.org/10.1186/s13089-020-00171-w
Serafino MD, Notaro M, Rea G et al (2020) The lung ultrasound: facts or artifacts? In the era of COVID-19 outbreak. Radiol Med 125:738–753. https://doi.org/10.1007/s11547-020-01236-5
Acknowledgements
Not applicable.
Funding
The authors thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq: 302215/2019-0) and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ: #E-26/202.679/2018 and #E-26/010.002124/2019).
Author information
Authors and Affiliations
Contributions
TTM, RR, CHC, and AJL developed the hypothesis. All authors were involved in the literature search and refinement of the hypothesis. TTM, MSC, LBM, PFL, LLZP, and ASESM performed and interpreted imaging exams and collected data. AJL prepared the initial draft of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was conducted in accordance with the Helsinki Declaration of 1964 and its later amendments. The study protocol was approved by the National Research Ethics Committee of Brazil (Number: CAAE-30135320.0.0000.5259). All participants read the protocol and agreed to participate in the study.
Consent for publication
No identifiable data from individual patients are included in this paper.
Competing interests
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mafort, T.T., Rufino, R., da Costa, C.H. et al. One-month outcomes of patients with SARS-CoV-2 infection and their relationships with lung ultrasound signs. Ultrasound J 13, 19 (2021). https://doi.org/10.1186/s13089-021-00223-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13089-021-00223-9