Abstract
Autoimmune congenital heart block (ACHB) is a passively acquired immune-mediated disease characterized by the presence of maternal antibodies against components of the Ro/SSA and La/SSB ribonucleoprotein complex that mainly affects the cardiac conducting system. ACHB occurs in 2% of women with positive anti-Ro/SSA and anti-La/SSB antibodies and causes a high risk of intrauterine fetal death, neonatal mortality, and long-term sequelae. In this review, we first describe a case of ACHB to provide preliminary knowledge. Then, we discuss the possible pathogenic mechanisms of ACHB; summarize the pregnancy management of patients with positive anti-Ro/SSA and anti-La/SSB antibodies and/or rheumatic diseases, the prevention of ACHB, and the treatment of ACHB fetuses; and propose routine screening of these antibodies for the general population. Careful follow-up, which consists of monitoring the fetal heart rate, is feasible and reassuring for pregnant women with positive anti-Ro/SSA and/or anti-La/SSB antibodies to lower the risk of ACHB in fetuses. Moreover, maternal administration of hydroxychloroquine may be useful in preventing ACHB in pregnant women with anti-Ro/SSA and/or anti-La/SSB antibodies.
Similar content being viewed by others
Introduction
Autoimmune congenital heart block (ACHB) is an acquired autoimmune disease characterized by dysfunction of the cardiac conducting system, resulting in partial or complete atrioventricular block. It develops in fetuses of women with anti-Ro/SSA and anti-La/SSB autoantibodies who may have autoimmune diseases, such as Sjögren’s syndrome (SS) and systemic lupus erythematosus (SLE); however, it may not be associated with other diseases. In addition, ACHB is detected most often between 18 and 24 weeks of gestation [1]. It has been reported that the morbidity of ACHB fetuses born to women with positive anti-Ro/SSA and/or anti-La/SSB autoantibodies is only 2% [2]. The recurrence rate is 12–25% in women who had a previous child with ACHB [3]. In addition, autoimmune congenital atrioventricular block occurs in approximately 1/20,000 live births, most of which may develop into 3° atrioventricular block from 1° or 2° atrioventricular block. The former is relatively rare but causes significant mortality [4]. In a large US-based registry of ACHB fetuses, the probability of death was 17.5%, and one third of these fetuses died in utero [5].
Herein, we report a case of ACHB and present a systematic review of the literature. This review is an attempt to emphasize the practical data and opinions on the pathogenesis of ACHB, particularly with respect to pregnancy management, prevention of ACHB, and treatment of ACHB fetuses.
Method
To conduct this review, we searched the Web of Science and PubMed through 14 May 2023. Key search words were used that were relevant to autoimmune congenital heart block (“autoantibody-associated congenital heart block” OR “congenital heart block” OR “complete congenital heart block” OR “congenital complete atrioventricular block” OR “congenital heart disease”) and {mechanism (mechanism* OR effect) or pregnancy (pregnant OR pregnant* OR “gestation period”) or management/treatment (treatment* OR management* OR therapy)}.
Studies were included if they met the following eligibility criteria:
-
1)
A quantitative empirical study published in a peer-reviewed journal in English
-
2)
Investigated the potential underlying mechanisms of ACHB
-
3)
Included management of pregnant women with positive anti-Ro/SSA and/or anti-La/SSB antibodies or rheumatic diseases during pregnancy
-
4)
Included prevention and treatment of ACHB (standardized and validated therapies or individual items)
Studies were excluded if the full paper was not available upon request.
Case report
A 39-year-old pregnant woman was admitted to the hospital for “amenorrhea for 37 weeks plus 5 days and fetal bradycardia for more than 8 weeks.” This was the patient’s third pregnancy, which was a natural pregnancy. The pregnant woman had delivered a live baby by cesarean section in November 2008 and terminated her second pregnancy at 40 days of gestation in 2009. She had no history of autoimmune diseases or heart diseases. At 13 weeks of pregnancy, the woman underwent prenatal routine examinations, including routine blood screening, liver function tests, renal function tests, syphilis test, and Down’s screening, and all the examination results were normal. On May 7, 2018, the amniocentesis results revealed alpha thalassemia without significant chromosomal abnormalities. On June 11, 2018, at 29 weeks of gestation, a fetal echocardiogram showed bradycardia of the fetus with a fetal heart rate of approximately 90 beats per minute, as shown in Table 1, and small accumulations of pericardial effusion. At 31 weeks of gestation, a repeat fetal echocardiogram revealed a fetal heart rate of 120 beats per minute. At 33 weeks of gestation, a new fetal echocardiogram was performed, which revealed a fetal heart rate of approximately 66 beats per minute, enlargement of the fetal cardiothoracic ratio, and small accumulations of pericardial effusion. At 37 weeks of gestation, the fetal echocardiogram showed fetal bradycardia with a heart rate between 35 and 43 beats per minute. Obstetric B-mode ultrasound showed an abnormal fetal heart rhythm and suggested possible abnormal heart development. The pregnant woman repeatedly refused further examinations and related treatments. On August 10, 2018, the woman delivered a baby girl via cesarean section. At this time, the baby’s heart rate was 42 beats per minute. The newborn had Apgar scores of 8 and 9 at 1 and 5 min, respectively. The echocardiogram suggested normal left ventricular function, moderate mitral regurgitation, and mild tricuspid and pulmonary regurgitation. High levels of anti-SSA/Ro antibodies (≥ 400.00 RU/mL, normal reference value: 0–20 RU/mL) and anti-Ro52 antibodies (301.77 RU/mL, normal reference value: 0–20 RU/mL) were detected in the serum of the mother, and increased levels of anti-SSA/Ro antibodies (403.00 RU/mL) and anti-Ro52 antibodies (200.72 RU/mL) were also detected in the neonate’s serum. The electrocardiogram revealed that the neonate had third-degree atrioventricular block. Doppler echocardiography indicated patent foramen ovale, moderate mitral regurgitation, and mild tricuspid and pulmonary regurgitation. The neonate was administered isoproterenol and epinephrine to improve heart rates, but it did not have a substantial effect. The parents refused the cardiologist’s recommendation of temporary pacemaker implantation for the neonate, and the neonate died on the second day of life. According to the clinical history, electrocardiogram and cardiac ultrasound results, and positive anti-Ro/SSA and anti-Ro52 antibodies, the neonate was diagnosed with autoimmune congenital complete atrioventricular block (CCAVB).
Pathogenesis of autoimmune congenital heart block
Apoptosis, inflammation, and extensive fibrosis
A histological study of fetuses that died from ACHB revealed that apoptotic cardiomyocytes were extensively distributed in the fetal heart, especially in the sinoatrial node and atrioventricular node. Furthermore, calcification deposition, scar tissue, and macrophages coclustered with maternal antibodies in extensive fibrosis regions were observed. Macrophages were mostly located in the ventricular septum and thickened fibrous subendocardium region [6]. Human fetal cardiomyocytes are prone to apoptosis, promoting the translocation of SSA and SSB antigens to the surface [7]. During fetal physiologic development, apoptotic cardiomyocytes are cleared by resident cardiomyocytes. However, for patients with anti-Ro/SSA and/or anti-La/SSB, these antibodies may bind to SSA and SSB antigens, respectively, on the surface of apoptotic cardiomyocytes to form immune complexes. The formation of these immune complexes inhibits apoptotic cardiomyocyte physiological clearance. Afterwards, macrophages phagocytose apoptotic cardiomyocytes by binding Fcγ receptors to the immune complexes [8]. Then, the macrophages are activated to release proinflammatory factors such as TGF-β and TNF-α, which stimulate fibroblasts to differentiate into myofibroblasts, eventually leading to scarring [9, 10]. Taken together, the accumulation of apoptotic cardiomyocytes, inflammation, extensive fibrosis, calcification, and scarring play an important role in the induction of ACHB.
Calcium channels’ dysregulation
L-type calcium channels, one of the three main classes of voltage-gated calcium channels, are targeted by calcium channel blockers. Four of the 10 α1 subunits, which include α1S, α1C, α1D, and α1F, form the pores of L-type calcium channels (LTCCs). LTCCs in human hearts include α1C calcium channels and α1D calcium channels [11, 12]. The α1C calcium channels are involved in the electrophysiological activity of the sinoatrial node in the human fetal heart, and α1D calcium channels are involved in the electrophysiological activity of the atrioventricular node [13]. T-type calcium channels in human hearts include α1G and α1H calcium channels, and the Ca current through α1G channels is involved in regulating cardiac impulse conduction through the atrioventricular nodes [14]. Several studies have indicated that anti-Ro/SSA antibodies bind to the α1G or α1D epitopes of cardiomyocytes and inhibit L-type and T-type calcium channels, suppressing the electrophysiological activity of cardiomyocytes in fetuses with ACHB [13, 15]. A recent paper has also addressed the interference role of anti-Ro/SSA antibodies on LTCCs in adults [12]. In particular, the presence of maternal p200-specific anti-Ro52 antibodies has been indicated to increase the risk of ACHB [16]. Utilizing a passive transfer model of ACHB, Ambrosi A and her colleagues [17] injected monoclonal antibodies into pregnant rats and discovered that only antibodies specific for the p200 domain of Ro52 induced ACHB, while antibodies targeting other domains of Ro52 did not. Additionally, p200-specific anti-Ro52 antibodies have been reported to promote intracellular calcium accumulation by recognizing calcium channels and reducing the contractility of cardiomyocytes, even inducing apoptosis [17, 18]. In addition, the activation of 5-HT4 receptors in human atrial cells induces cAMP-mediated activation of L-type calcium channels. Eftekhari et al. found that antibodies against residues 365–382 of the Ro52 peptide recognize and cross-react with residues 165 to 185 of the cardiac 5-HT4 receptor [19]. Then, anti-Ro52 antibodies inhibited the activation of serotonin-induced L-type calcium channels by blocking the activation of 5-HT4 receptors. Moreover, auxilin-deficient fetal mice have been reported to develop arrhythmia-like ACHB symptoms. Interestingly, compared with cardiomyocytes of wild-type mice, auxilin-deficient cardiomyocytes exhibit fewer α1D calcium channels on the cell surface [20]. Overall, anti-Ro/SSA antibodies may disturb the electrical activity of cardiomyocytes in the conduction system by dysregulating intracellular calcium homeostasis.
Interferon
Numerous studies have suggested that type I IFN may contribute to the pathogenesis of ACHB. A clinical study of 9 women with ACHB pregnancies and 14 pregnant women with antibodies against Ro/SSA but without an ACHB complication found high expression of SIGLEC1 (a surrogate marker for the IFN signature that indicates cellular activation) and IFN-α in mothers of affected children [21]. Increased expression of IFN-regulated genes and plasma IFNα levels were detected in neonates exposed to anti-Ro/SSA and/or anti-La/SSB antibodies [22, 23]. In a recent study, researchers simulated cardiac injury conditions in vitro by incubating human fetal cardiac fibroblasts with supernatant from macrophages transfected with SSA/Ro60-associated ssRNA [24]. A transcriptome analysis of stimulated fibroblasts and healthy controls has provided clinical evidence for the upregulation of IFN-regulated genes in stimulated fetal fibroblasts and suggested that maternal autoantibody-induced cardiac damage may be secondary to the effect of type I IFN on fetal fibroblasts. Type I IFN is known to expand and activate NK cells, and a higher proportion of CD56dimCD16hi NK cells has been found in cord blood from anti-Ro/La antibody-exposed neonates than in nonexposed controls [22, 25, 26]. NK cells may activate tissue-resident and infiltrating macrophages via IFN-γ and exaggerate apoptosis, promoting a local inflammatory reaction in the fetal heart. In addition, a single-cell transcriptome study from the heart of one fetus with ACHB has revealed a potential link between type I IFN and fibrosis [27]. Another concern is that IFN-α has been reported to increase the expression of Ro52 and induce apoptosis [28]. Several studies have indicated a potential direct arrhythmogenic effect of type I IFN [29,30,31,32].
Other potential pathogeneses
ACHB may be related to viral infection. It has been reported that Ro/SSA antigens in ACHB fetal cardiomyocytes could translocate to the cell surface following cytomegalovirus infection [33]. The question of whether other viruses contribute to ACHB pathogenesis needs to be further illustrated.
Management of pregnant women with positive anti-Ro/SSA and/or anti-La/SSB antibodies during pregnancy
Hydroxychloroquine
Approximately 2% of fetuses from anti-Ro/SSA-positive mothers develop ACHB, which is extremely serious for the fetuses. Therefore, preventive treatments should be presented to these pregnant women. The 2020 American College of Rheumatology Reproductive Health Management Guidelines for Rheumatoid and Musculoskeletal Diseases conditionally recommend hydroxychloroquine (HCQ) treatment for all pregnant women with positive anti-Ro/SSA and/or anti-La/SSB antibodies to minimize the risk of ACHB [34]. In addition, it is recommended that pregnant women with a previous child with cardiac neonatal lupus syndrome (NLS) receive HCQ treatment [35]. Taking HCQ contributes to a lower risk of the current fetus developing ACHB. This may be because HCQ can inhibit the activation of Toll-like receptor (TLR) signaling and type I IFN [9, 21]. In a retrospective cohort study, 14 pregnant women took HCQ during pregnancy, while 48 pregnant women did not take HCQ [36]. Of the mothers taking HCQ throughout pregnancy, five approached 200 mg/day of HCQ for oral administration, while nine consumed 400 mg/day. One newborn in the HCQ group (7.1%) developed ACHB, while 7 newborns in the non-HCQ group developed ACHB (14.6%). The mother of the ACHB child in the HCQ group took HCQ again during her second pregnancy and gave birth to a healthy infant. A cohort study also observed that the incidence of ACHB fetuses in pregnant women taking HCQ (1/18, 5.5%) was much lower than that in the group not taking HCQ (6/21, 28.6%) [37]. These studies have shown that HCQ treatment possibly plays a vital role in reducing the prevalence of ACHB in fetuses.
Intravenous immunoglobulin
Intravenous immunoglobulin (IVIG) can reduce transplacental autoantibody passage and increase the release of anti-inflammatory factors. However, whether IVIG treatment administered to the mother influences any outcome in ACHB is a current matter of debate. The risk of the fetus developing ACHB is approximately 2% in pregnant women with anti-Ro/SSA and/or anti-La/SSB autoantibodies, and the recurrence rate is 12–25% in women who had a previous child with ACHB [3]. In 2003, maternal administration of IVIG was initially proposed after a case series of 8 patients reported only 1 case of recurrent ACHB in mothers with a previously affected child [38]. However, in a small nonrandomized study, 20 pregnant women with positive anti-Ro/SSA antibodies who had a previous child with ACHB or neonatal lupus rash were administered 400 mg/kg IVIG every 3 weeks from 12 to 24 weeks of gestation, and 3 fetuses (3/20, 15%) were diagnosed with ACHB at the 19th, 20th, and 24th weeks of gestation [39]. The results suggest that low-dose IVIG does not decrease the recurrence of ACHB in high-risk pregnancies. Whether IVIG at higher doses would be more effective needs further study [40, 41].
Daily ambulatory fetal heart rate monitoring and fetal ultrasound echocardiography
For pregnant women who have previously delivered infants with ACHB or NLS, the 2020 American College of Rheumatology Reproductive Health Management Guidelines for Rheumatology and Musculoskeletal Diseases recommend weekly fetal echocardiography, beginning at weeks 16–18 and continuing through week 26.
The recommendations conditionally recommend serial (less frequent than weekly; interval not determined) fetal echocardiography, beginning at weeks 16–18 and continuing through week 26 for pregnant women with anti-Ro/SSA and/or anti-La/SSB antibodies but no infant history of ACHB or NLS [34]. In fact, the transition from a normal rhythm to a third-degree atrioventricular block (AVB) occurs within 24 h, which highlights the importance of a closer surveillance of rhythm, eventually performed at home directly by the patients. It can prompt quicker access to dedicated care and improve outcomes at birth. In contrast, it is difficult for weekly monitoring to detect an early stage of ACHB before it progresses to third degree, and all data on therapies seem to emphasize that the earlier the stage at detection, the better the results of various therapies are. Daily ambulatory fetal heart rate monitoring (FHRM) allows for early detection of rhythm alterations and the administration of timely targeted treatments [42, 43].
Pregnancy management in patients with rheumatic diseases
Pregnancy management in patients with systemic lupus erythematosus
The 2020 American College of Rheumatology Guideline for the Management of Reproductive Health in Rheumatic and Musculoskeletal Diseases recommends that lupus patients should be screened for anti-Ro/SSA and/or anti-La/SSB antibodies before or during pregnancy. Moreover, given the titer and persistence of these antibodies, it is recommended that no repeated detection is needed during pregnancy [34]. According to the Canadian Rheumatology Association Recommendations for the Assessment and Monitoring of Systemic Lupus Erythematosus, anti-Ro/SSA and/or anti-La/SSB should be detected before pregnancy and during the first 3 months of pregnancy for women with SLE to monitor and implement prophylaxis treatment in a timely manner, mitigating the risk of gestating a fetus with ACHB and improving prognosis [44].
HCQ treatment should be recommended in pregnant SLE patients with anti-Ro/SSA antibodies to reduce the risk of fetuses with ACHB. A case–control study involving anti-Ro/SSA antibody-positive patients with SLE in pregnancy was published [45]. A pregnancy was considered exposed to HCQ if the patient took ≥ 200 mg/day during pregnancy, while a pregnancy was considered unexposed if HCQ was never taken or was discontinued after confirming pregnancy. Seven (14%) of the heart injury-related NLS children were exposed to HCQ compared with 56 (37%) of the controls (noncardiac neonatal lupus), indicating that exposure to HCQ reduces the risk of cardiac NLS.
Pregnancy management in patients with Sjogren’s syndrome
Similarly, the British Society for Rheumatology guidelines for the management of adults with primary Sjögren’s syndrome suggest serial Doppler echocardiography to monitor atrioventricular time intervals during pregnancy in patients with Sjogren’s syndrome [46]. For all pregnant women with rheumatic diseases and positive anti-Ro/SSA and/or anti-La/SSB antibodies, HCQ treatment is conditionally recommended when they have low disease activity (Table 2) [34].
Treatment for ACHB fetuses
Fluorinated steroids
Fluorinated steroids may prevent the fetus from progressing from incomplete atrioventricular block to complete atrioventricular block [49]. Notably, for pregnant women with anti-Ro/SSA and/or anti-La/SSB antibodies and echocardiography showing fetal incomplete heart block, the 2020 American College of Rheumatology Guideline for the Management of Reproductive Health in Rheumatic and Musculoskeletal Diseases recommends oral dexamethasone of 4 mg per day [34]. However, the adverse effects of fluorinated steroids should not be ignored. Mothers are prone to suffer from hypertension, hyperglycemia, and excessive weight gain, while fetuses have a high risk for growth restriction, adrenal insufficiency, and oligohydramnios [50]. Whether dexamethasone prevents disease progression, reduces mortality, and avoids pacemaker implantation and cardiomyopathy in cases of second-degree and third-degree AVB is controversial [51, 52], but recent studies do not support its use [53].
Plasmapheresis
Plasmapheresis may be a potential effective therapeutic strategy for ACHB fetuses. The efficacy of plasmapheresis in removing anti-Ro/SSA and anti-La/SSB antibodies was evaluated in 10 consecutive pregnant women with ACHB fetuses, and the degree of ACHB at detection and at delivery was recorded. In fact, 8 of the women showed a steady and significant decrease in anti-Ro/SSA and anti-La/SSB antibodies as the pregnancy progressed. Among them, the block reverted from a second to first degree in two fetuses and from second degree to sinus rhythm in the third fetus, indicating that plasmapheresis has beneficial effects on the reversal of incomplete atrioventricular block [54]. Regrettably, even receiving plasmapheresis, third-degree AVB in these fetuses seems to be permanent.
Intravenous immunoglobulin
Regarding the effect of IVIG treatment on the improvement of ACHB, some case reports are promising. In a case report, the fetus of a mother with positive anti-Ro/La antibodies was diagnosed with 2:1 AV block and intermittent complete heart block at 28 weeks’ gestation [40]. The mother promptly received therapy with IVIG (400 mg/kg per day) for 5 days. Improvement in sinus rhythm with intermittent AV block was recorded throughout the remainder of the pregnancy. In a 10-year retrospective study of NLS in China, five babies who had a prolonged PR interval on ECG at birth were treated with intravenous immunoglobulin (IVIG) at a dose of 1 g/kg for 2 days, all of whom reverted to a normal sinus rhythm, providing evidence for the effectiveness of IVIG [55]. In a case series, 6 pregnant women with the fetuses diagnosed with second- and third-degree blocks were treated with a combination of IVIG 1 g/kg every 2 weeks, dexamethasone, and weekly plasmapheresis. Three of 3 cases of second-degree AVB reverted to normal sinus rhythm prior to delivery. All 3 cases with third-degree heart block remained stable prenatally, and only 1 needed pacemaker implantation at age 10 months [41]. This provided further support for the possible benefit of IVIG treatment in ACHB. However, further research is needed before definitive conclusions can be drawn regarding IVIG in ACHB treatment.
Pacemaker implantation
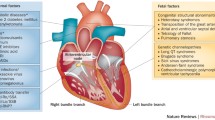
In a study of 16 ACHB fetuses diagnosed with third-degree block (12 diagnosed in utero and 4 at birth), 15 patients underwent pacemaker implantation during the first 2 weeks, and one patient underwent pacemaker implantation at 7 months [56]. During follow-up, all 15 patients had normal left ventricle (LV) function in the early postnatal period, and LV function significantly decreased in one patient. In a nationwide study of patients with complete atrioventricular block, all 127 patients underwent pacemaker implantation (median age at pacemaker implantation, 3.2 years) [57]. The survival rate was 96% at follow-up after approximately 9 years of pacing, indicating that pacemaker implantation may be a potential effective therapy for improving the survival rate of ACHB patients with complete atrioventricular block. However, pacemaker implantation has an adverse side effect that induces abnormal electrical activation patterns [58]. According to the American guidelines (2018) [59] and European guidelines (2013) [60], pacemaker implantation is recommended for ACHB fetus with third-degree block. In the European guidelines (2013), permanent pacing is indicated in symptomatic ACHB patients with third-degree block and is reasonable in asymptomatic patients. Figure 1 shows the potential pathogenesis of autoimmune congenital heart block and its prevention and treatment.
The potential pathogenesis of autoimmune congenital heart block and its prevention and treatment. A Accumulation of apoptotic cardiomyocytes, inflammation, extensive fibrosis, calcification, and scarring play an important role in the induction of ACHB. B Antibodies may disturb the electrical activity of cardiomyocytes in the conduction system through dysregulating intracellular calcium homeostasis. C Management in pregnant women with positive anti-Ro/SSA and/or anti-La/SSB antibodies or rheumatic diseases during pregnancy. D Treatment for ACHB fetuses
Routine screening of anti-Ro/SSA and anti-La/SSB antibodies among women of childbearing age
Although ACHB in neonates born to mothers with positive anti-Ro/SSA and/or anti-La/SSB antibodies is uncommon, it can burden their lives and their families. A recent observational cohort study tested 7339 veterans for anti-Ro/SSA, 612 of whom were anti-Ro/SSA-positive (8.3%) [61]. Other studies have also reported the detection of anti-Ro/SSA (0.5–2.7%) in the general population [62,63,64]. Furthermore, a large observational study screened 2181 serum samples from the general population for disease-specific antinuclear antibodies (ANAs) and showed that the prevalence of anti-Ro/SSA in the general population was 2.7% (3.5% in females and 1.0% in males), and that of anti-Ro/SSB was 0.2% [64]. Therefore, it is important to screen for the antibodies described above in women of childbearing age. In addition, we can use predictive therapeutic strategies for these women to prevent the birth of ACHB fetuses.
Conclusion
Autoimmune congenital heart block is rare, but it is associated with an increased risk of intrauterine fetal death, neonatal mortality, and long-term sequelae. To date, an increasing number of studies on the pathogenesis, prevention, and treatment of ACHB have been conducted. A deep understanding of its pathogenesis and knowledge of reasonable pregnancy management strategies and the latest therapeutic options are important to greatly reduce morbidity and mortality. The presence of anti-Ro/SSA and/or anti-La/SSB antibodies during pregnancy causes a significant risk for fetuses to develop ACHB. New evidence indicates the important role of type I interferon in the occurrence and development of ACHB. In addition, routine screening of anti-Ro/SSA and anti-La/SSB antibodies among women of childbearing age, daily ambulatory fetal heart rate monitoring, and monitoring of fetal echocardiography for pregnant women with positive anti-Ro/SSA and/or anti-La/SSB antibodies are feasible and reassuring methods to lower the risk of ACHB fetuses. Finally, prophylactic use of HCQ is important for pregnant women and is recommended in all women at risk for recurrence of CHB. Knowledge in this field of ACHB is continuously developing, and continuous updates are needed.
Availability of data and materials
Not applicable.
Abbreviations
- ACHB:
-
Autoimmune congenital heart block
- SS:
-
Sjögren’s syndrome
- SLE:
-
Systemic lupus erythematosus
- CCAVB:
-
Congenital complete atrioventricular block
- HCQ:
-
Hydroxychloroquine
- NLS:
-
Neonatal lupus syndrome
- TLR:
-
Toll-like receptor
- IVIG:
-
Intravenous immunoglobulin
- LV:
-
Left ventricle
- ANAs:
-
Antinuclear antibodies
- AVB:
-
Atrioventricular block
- FHRM:
-
Fetal heart rate monitoring
References
Tsuboi H, Sumida T, Noma H, Yamagishi K, Anami A, Fukushima K, Horigome H, Maeno Y, Kishimoto M, Takasaki Y, et al. Maternal predictive factors for fetal congenital heart block in pregnant mothers positive for anti-SS-A antibodies. Mod Rheumatol. 2016;26(4):569–75.
Ambrosi A, Sonesson SE, Wahren-Herlenius M. Molecular mechanisms of congenital heart block. EXP CELL RES. 2014;325(1):2–9.
Hansahiranwadee W. Diagnosis and management of fetal autoimmune atrioventricular block. Int J Womens Health. 2020;12:633–9.
Di Mauro A, Caroli CV, Favia GG, Calderoni G, Cicinelli E, Laforgia N. Antenatal and postnatal combined therapy for autoantibody-related congenital atrioventricular block. BMC Pregnancy Childbirth. 2013;13:220.
Izmirly PM, Saxena A, Kim MY, Wang D, Sahl SK, Llanos C, Friedman D, Buyon JP. Maternal and fetal factors associated with mortality and morbidity in a multi-racial/ethnic registry of anti-SSA/Ro-associated cardiac neonatal lupus. Circulation. 2011;124(18):1927–35.
Clancy RM, Kapur RP, Molad Y, Askanase AD, Buyon JP. Immunohistologic evidence supports apoptosis, IgG deposition, and novel macrophage/fibroblast crosstalk in the pathologic cascade leading to congenital heart block. Arthritis Rheum. 2004;50(1):173–82.
Miranda-Carus ME, Askanase AD, Clancy RM, Di Donato F, Chou TM, Libera MR, Chan EK, Buyon JP. Anti-SSA/Ro and anti-SSB/La autoantibodies bind the surface of apoptotic fetal cardiocytes and promote secretion of TNF-alpha by macrophages. J Immunol. 2000;165(9):5345–51.
Clancy RM, Neufing PJ, Zheng P, O’Mahony M, Nimmerjahn F, Gordon TP, Buyon JP. Impaired clearance of apoptotic cardiocytes is linked to anti-SSA/Ro and -SSB/La antibodies in the pathogenesis of congenital heart block. J Clin Invest. 2006;116(9):2413–22.
Clancy RM, Alvarez D, Komissarova E, Barrat FJ, Swartz J, Buyon JP. Ro60-associated single-stranded RNA links inflammation with fetal cardiac fibrosis via ligation of TLRs: a novel pathway to autoimmune-associated heart block. J Immunol. 2010;184(4):2148–55.
Clancy RM, Markham AJ, Reed JH, Blumenberg M, Halushka MK, Buyon JP. Targeting downstream transcription factors and epigenetic modifications following Toll-like receptor 7/8 ligation to forestall tissue injury in anti-Ro60 associated heart block. J Autoimmun. 2016;67:36–45.
Striessnig J, Pinggera A, Kaur G, Bock G, Tuluc P. L-type Ca channels in heart and brain Wiley interdisciplinary reviews. Membrane Transport Signal. 2014;3(2):15–38.
Lazzerini PE, Murthy Ginjupalli VK, Srivastava U, Bertolozzi I, Bacarelli MR, Verrengia D, Salvini V, Accioli R, Carbone SF, Santoro A, et al. Anti-Ro/SSA antibodies blocking calcium channels as a potentially reversible cause of atrioventricular block in adults. JACC Clin Electrophysiol. 2023;9:1631–48.
Qu YS, Lazzerini PE, Capecchi PL, Laghi-Pasini F, El SN, Boutjdir M. Autoimmune calcium channelopathies and cardiac electrical abnormalities. Front Cardiovasc Med. 2019;6:54.
Cribbs L. T-type calcium channel expression and function in the diseased heart. Channels (Austin). 2010;4(6):447–52.
Karnabi E, Qu Y, Mancarella S, Yue Y, Wadgaonkar R, Boutjdir M. Silencing of Cav1.2 gene in neonatal cardiomyocytes by lentiviral delivered shRNA. Biochem Biophys Res Commun. 2009; 384(4):409–414.
De Carolis S, Garufi C, Garufi E, De Carolis MP, Botta A, Tabacco S, Salvi S. Autoimmune congenital heart block: a review of biomarkers and management of pregnancy. Front Pediatr. 2020;8: 607515.
Brucato A, Cimaz R, Caporali R, Ramoni V, Buyon J. Pregnancy outcomes in patients with autoimmune diseases and anti-Ro/SSA antibodies. Clin Rev Allergy Immunol. 2011;40(1):27–41.
Ambrosi A, Dzikaite V, Park J, Strandberg L, Kuchroo VK, Herlenius E, Wahren-Herlenius M. Anti-Ro52 monoclonal antibodies specific for amino acid 200–239, but not other Ro52 epitopes, induce congenital heart block in a rat model. Ann Rheum Dis. 2012;71(3):448–54.
Eftekhari P, Sallé L, Lezoualc’H F, Mialet J, Gastineau M, Briand JP, Isenberg DA, Fournié GJ, Argibay J, Fischmeister R, et al. Anti-SSA/Ro52 autoantibodies blocking the cardiac 5-HT4 serotoninergic receptor could explain neonatal lupus congenital heart block. Eur J Immunol. 2000; 30(10):2782–2790.
Meisgen S, Hedlund M, Ambrosi A, Folkersen L, Ottosson V, Forsberg D, Thorlacius GE, Biavati L, Strandberg L, Mofors J, et al. Auxilin is a novel susceptibility gene for congenital heart block which directly impacts fetal heart function. Ann Rheum Dis. 2022;81(8):1151–61.
Lisney AR, Szelinski F, Reiter K, Burmester GR, Rose T, Dorner T. High maternal expression of SIGLEC1 on monocytes as a surrogate marker of a type I interferon signature is a risk factor for the development of autoimmune congenital heart block. Ann Rheum Dis. 2017;76(8):1476–80.
Ivanchenko M, Thorlacius GE, Hedlund M, Ottosson V, Meneghel L, Bjorkander S, Ossoinak A, Tingstrom J, Bremme K, Sverremark-Ekstrom E, et al. Natural killer cells and type II interferon in Ro/SSA and La/SSB autoantibody-exposed newborns at risk of congenital heart block. Ann Rheum Dis. 2021;80(2):194–202.
Hedlund M, Thorlacius GE, Ivanchenko M, Ottosson V, Kyriakidis N, Lagnefeldt L, Tingstrom J, Sirsjo A, Bengtsson AA, Aronsson E, et al. Type I IFN system activation in newborns exposed to Ro/SSA and La/SSB autoantibodies in utero. RMD Open. 2020;6(1):e000989.
Clancy RM, Markham AJ, Jackson T, Rasmussen SE, Blumenberg M, Buyon JP. Cardiac fibroblast transcriptome analyses support a role for interferogenic, profibrotic, and inflammatory genes in anti-SSA/Ro-associated congenital heart block. American journal of physiology. Heart Circul Physiol. 2017; 313(3):H631-H640.
Müller L, Aigner P, Stoiber D. Type I interferons and natural killer cell regulation in cancer. Front Immunol. 2017;8:304.
Ivarsson MA, Loh L, Marquardt N, Kekäläinen E, Berglin L, Björkström NK, Westgren M, Nixon DF, Michaëlsson J. Differentiation and functional regulation of human fetal NK cells. J Clin Investig. 2013;123(9):3889–901.
Suryawanshi H, Clancy R, Morozov P, Halushka MK, Buyon JP, Tuschl T. Cell atlas of the foetal human heart and implications for autoimmune-mediated congenital heart block. Cardiovasc Res. 2020;116(8):1446–57.
Strandberg L, Ambrosi A, Espinosa A, Ottosson L, Eloranta ML, Zhou W, Elfving A, Greenfield E, Kuchroo VK, Wahren-Herlenius M. Interferon-alpha induces up-regulation and nuclear translocation of the Ro52 autoantigen as detected by a panel of novel Ro52-specific monoclonal antibodies. J Clin Immunol. 2008;28(3):220–31.
Parrens E, Chevalier JM, Rougier M, Douard H, Labbé L, Quiniou G, Broustet A, Broustet JP. Third degree atrio-ventricular block induced by interferon alpha. Report of a case. Arch Mal Coeur Vaiss. 1999; 92(1):53–56.
Rechciński T, Matusik D, Rudziński T, Bednarkiewicz Z, Paprotna K, Deroń Z, Kurpesa M, Krzemińska-Pakuła M. Cardiotoxic properties of interferon: aggravation of atrio-ventricular block during treatment of chronic hepatitis C with peginterferon–a case report. Pol Arch Med Wewn. 2007;117:49–52.
Odashiro K, Hiramatsu S, Yanagi N, Arita T, Maruyama T, Kaji Y, Harada M. Arrhythmogenic and inotropic effects of interferon investigated in perfused and in vivo rat hearts: influences of cardiac hypertrophy and isoproterenol. Circ J. 2002;66(12):1161–7.
Lee KH, GJAM. Cardiac arrhythmia in a CML patient treated with interferons. Tex Med. 1989;85(4):36–8.
Proceedings and abstracts of the International Workshop on Clinical and Molecular Aspects of Congenital Heart Block. Stockholm, Sweden. September 17–19, 2010. Scand J Immunol. 2010; 72(3):17–241, 262–276.
Sammaritano LR, Bermas BL, Chakravarty EE, Chambers C, Clowse M, Lockshin MD, Marder W, Guyatt G, Branch DW, Buyon J, et al. 2020 American College of Rheumatology Guideline for the Management of Reproductive Health in Rheumatic and Musculoskeletal Diseases. Arthritis Rheumatol. 2020;72(4):529–56.
Izmirly P, Kim M, Friedman DM, Costedoat-Chalumeau N, Clancy R, Copel JA, Phoon C, Cuneo BF, Cohen RE, Robins K, et al. Hydroxychloroquine to prevent recurrent congenital heart block in fetuses of anti-SSA/Ro-positive mothers. J Am Coll Cardiol. 2020;76(3):292–302.
Mollerach FB, Scolnik M, Catoggio LJ, Rosa J, Soriano ER. Causes of fetal third-degree atrioventricular block and use of hydroxychloroquine in pregnant women with Ro/La antibodies. Clin Rheumatol. 2019;38(8):2211–7.
Martinez-Sanchez N, Perez-Pinto S, Robles-Marhuenda A, Arnalich-Fernandez F, Martin CM, Hueso ZE, Bartha JL. Obstetric and perinatal outcome in anti-Ro/SSA-positive pregnant women: a prospective cohort study. Immunol Res. 2017;65(2):487–94.
Kaaja R, Julkunen H. Prevention of recurrence of congenital heart block with intravenous immunoglobulin and corticosteroid therapy: comment on the editorial by Buyon et al. Arthritis Rheum. 2003; 48(1):280–281, 281–282.
Friedman DM, Llanos C, Izmirly PM, Brock B, Byron J, Copel J, Cummiskey K, Dooley MA, Foley J, Graves C, et al. Evaluation of fetuses in a study of intravenous immunoglobulin as preventive therapy for congenital heart block: results of a multicenter, prospective, open-label clinical trial. Arthritis Rheum. 2010;62(4):1138–46.
David AL, Ataullah I, Yates R, Sullivan I, Charles P, Williams D. Congenital fetal heart block: a potential therapeutic role for intravenous immunoglobulin. Obstet Gynecol. 2010;116:543–547.
Ruffatti A, Marson P, Svaluto-Moreolo G, Marozio L, Tibaldi M, Favaro M, Calligaro A, Grava C, Hoxha A, Pengo V, et al. A combination therapy protocol of plasmapheresis, intravenous immunoglobulins and betamethasone to treat anti-Ro/La-related congenital atrioventricular block. A case series and review of the literature. Autoimmun Rev. 2013;12(7):768–773.
Milazzo R, Ligato E, Laoreti A, Ferri G, Basili L, Serati L, Brucato A, Cetin I. Home fetal heart rate monitoring in anti Ro/SSA positive pregnancies: literature review and case report. Eur J Obstet Gynecol Reprod Biol. 2021;259:1–6.
Kaplinski M, Cuneo BF. Novel approaches to the surveillance and management of fetuses at risk for anti-Ro/SSA mediated atrioventricular block. Semin Perinatol. 2022;46(4): 151585.
Keeling SO, Alabdurubalnabi Z, Avina-Zubieta A, Barr S, Bergeron L, Bernatsky S, Bourre-Tessier J, Clarke A, Baril-Dionne A, Dutz J, et al. Canadian Rheumatology Association Recommendations for the Assessment and Monitoring of Systemic Lupus Erythematosus. J Rheumatol. 2018;45(10):1426–39.
Izmirly PM, Kim MY, Llanos C, Le PU, Guerra MM, Askanase AD, Salmon JE, Buyon JP. Evaluation of the risk of anti-SSA/Ro-SSB/La antibody-associated cardiac manifestations of neonatal lupus in fetuses of mothers with systemic lupus erythematosus exposed to hydroxychloroquine. Ann Rheum Dis. 2010;69(10):1827–30.
Price EJ, Rauz S, Tappuni AR, Sutcliffe N, Hackett KL, Barone F, Granata G, Ng WF, Fisher BA, Bombardieri M, et al. The British Society for Rheumatology guideline for the management of adults with primary Sjogren’s Syndrome. Rheumatology (Oxford). 2017;56(10):1643–7.
Price EJ, Rauz S, Tappuni AR, Sutcliffe N, Hackett KL, Barone F, Granata G, Ng WF, Fisher BA, Bombardieri M, et al. The British Society for Rheumatology guideline for the management of adults with primary Sjögren’s Syndrome. Rheumatology (Oxford). 2017;56(10):e24–48.
Andreoli L, Bertsias GK, Agmon-Levin N, Brown S, Cervera R, Costedoat-Chalumeau N, Doria A, Fischer-Betz R, Forger F, Moraes-Fontes MF, et al. EULAR recommendations for women’s health and the management of family planning, assisted reproduction, pregnancy and menopause in patients with systemic lupus erythematosus and/or antiphospholipid syndrome. Ann Rheum Dis. 2017;76(3):476–85.
Sonesson SE, Salomonsson S, Jacobsson LA, Bremme K, Wahren-Herlenius M. Signs of first-degree heart block occur in one-third of fetuses of pregnant women with anti-SSA/Ro 52-kd antibodies. Arthritis Rheum. 2004;50(4):1253–61.
Brucato A, Tincani A, Fredi M, Breda S, Ramoni V, Morel N, Costedoat-Chalumeau N. Should we treat congenital heart block with fluorinated corticosteroids? Autoimmun Rev. 2017;16(11):1115–8.
Cuneo BF, Lee M, Roberson D, Niksch A, Ovadia M, Parilla BV, Benson DW. A management strategy for fetal immune-mediated atrioventricular block. J Matern Fetal Neonatal Med. 2010;23(12):1400–5.
Friedman DM, Kim MY, Copel JA, Llanos C, Davis C, Buyon JP. Prospective evaluation of fetuses with autoimmune-associated congenital heart block followed in the PR Interval and Dexamethasone Evaluation (PRIDE) Study. Am J Cardiol. 2009;103(8):1102–6.
Izmirly PM, Saxena A, Sahl SK, Shah U, Friedman DM, Kim MY, Buyon JP. Assessment of fluorinated steroids to avert progression and mortality in anti-SSA/Ro-associated cardiac injury limited to the fetal conduction system. Ann Rheum Dis. 2016;75(6):1161–5.
Tonello M, Ruffatti A, Marson P, Tison T, Marozio L, Hoxha A, De Silvestro G, Punzi L. Plasma exchange effectively removes 52- and 60-kDa anti-Ro/SSA and anti-La/SSB antibodies in pregnant women with congenital heart block. Transfusion. 2015;55(7):1782–6.
Yu Y, Du L, Pan J, Zheng J, Chen A, Chen L. A 10-year retrospective study of neonatal lupus erythematous in China. Asian Pac J Allergy. 2016;34(2):174–8.
Moak JP, Barron KS, Hougen TJ, Wiles HB, Balaji S, Sreeram N, Cohen MH, Nordenberg A, Van Hare GF, Friedman RA, et al. Congenital heart block: development of late-onset cardiomyopathy, a previously underappreciated sequela. J Am Coll Cardiol. 2001;37(1):238–42.
Eliasson H, Sonesson SE, Salomonsson S, Skog A, Wahren-Herlenius M, Gadler F. Outcome in young patients with isolated complete atrioventricular block and permanent pacemaker treatment: A nationwide study of 127 patients. Heart Rhythm. 2015;12(11):2278–84.
Brito-Zerón P, Izmirly PM, Ramos-Casals M, Buyon JP, Khamashta MA. Autoimmune congenital heart block: complex and unusual situations. Lupus. 2016;25(2):116–28.
Kusumoto FM, Schoenfeld MH, Barrett C, Edgerton JR, Ellenbogen KA, Gold MR, Goldschlager NF, Hamilton RM, Joglar JA, Kim RJ, et al. 2018 ACC/AHA/HRS guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2019;140(8):e382–482.
Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, Breithardt OA, Cleland J, Deharo JC, Delgado V, Elliott PM, et al. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur Heart J. 2013; 34(29):2281–2329.
Lazzerini PE, Cevenini G, Qu YS, Fabris F, El-Sherif N, Acampa M, Cartocci A, Laghi-Pasini F, Capecchi PL, Boutjdir M, et al. Risk of QTc interval prolongation associated with circulating anti-Ro/SSA antibodies among US veterans: an observational cohort study. J Am Heart Assoc. 2021;10(4): e18735.
Satoh M, Chan EK, Ho LA, Rose KM, Parks CG, Cohn RD, Jusko TA, Walker NJ, Germolec DR, Whitt IZ, et al. Prevalence and sociodemographic correlates of antinuclear antibodies in the United States. Arthritis Rheum. 2012;64(7):2319–27.
Guo YP, Wang CG, Liu X, Huang YQ, Guo DL, Jing XZ, Yuan CG, Yang S, Liu JM, Han MS, et al. The prevalence of antinuclear antibodies in the general population of China: a cross-sectional study. Curr Ther Res Clin Exp. 2014;76:116–9.
Hayashi N, Koshiba M, Nishimura K, Sugiyama D, Nakamura T, Morinobu S, Kawano S, Kumagai S. Prevalence of disease-specific antinuclear antibodies in general population: estimates from annual physical examinations of residents of a small town over a 5-year period. Mod Rheumatol. 2008;18(2):153–60.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from Guangzhou Science and Technology Program Project (Grant No. 202201010926), Guangdong Basic and Applied Basic Research Foundation (2022A0505050040), and the President Foundation of The Third Affiliated Hospital of Southern Medical University, Guangzhou, China (YM2021007 and YL202205).
Author information
Authors and Affiliations
Contributions
Ying Huang wrote the manuscript. J.D. and J.L. edited the manuscript and designed the tables. F.Y. and Yi He contributed to the manuscript and figure design. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Huang, Y., Deng, J., Liu, J. et al. Autoimmune congenital heart block: a case report and review of the literature related to pathogenesis and pregnancy management. Arthritis Res Ther 26, 8 (2024). https://doi.org/10.1186/s13075-023-03246-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13075-023-03246-w