Abstract
Background
Low urine pH, which may be mediated by metabolic syndrome (MetS), is common in gout. Tart cherries are shown to improve MetS symptoms and possess anti-inflammatory properties. However, the efficacy of tart cherry supplements on urine pH has yet to be studied.
Objectives
This study aimed to investigate the efficacy and safety of tart cherry supplementary citrate (TaCCi) mixture on urine pH, serum urate (sUA), C-reactive protein (CRP), and gout flares in gout patients initiating urate-lowering therapy (ULT), in comparison to citrate mixture and sodium bicarbonate.
Methods
A prospective, randomized (1:1:1), open-label, parallel-controlled trial was conducted among 282 men with gout and fasting urine pH ≤ 6, who were initiating ULT with febuxostat (initially 20 mg daily, escalating to 40 mg daily if serum urate ≥ 360 μmol/L). Participants were randomized to groups taking either sodium bicarbonate, citrate mixture, or TaCCi mixture. All participants were followed every 4 weeks until week 12. Urine pH and sUA were co-primary outcomes, with various biochemical and clinical secondary endpoints.
Results
Urine pH increased to a similar extent in all three groups. SUA levels declined in all three groups as well, with no significant differences observed between the groups. At week 12, the TaCCi mixture group exhibited a greater reduction in the urine albumin/creatinine ratio (UACR) compared to the other two groups (p < 0.05). Participants taking TaCCi mixture or citrate mixture experienced fewer gout flares than those in the sodium bicarbonate group over the study period (p < 0.05). Additionally, the TaCCi mixture group had a lower CRP level at week 12 relative to the other two groups (p < 0.01). Adverse events were similar across all three groups.
Conclusion
The TaCCi mixture had similar efficacy and safety on urine alkalization and sUA-lowering as the citrate mixture and sodium bicarbonate in patients with gout. However, the TaCCi mixture resulted in greater improvements in UACR and CRP, which suggests that tart cherry supplements may provide additional benefits for renal protection and reduce inflammation in gout, particularly when starting ULT.
Trial registration
This project was registered in ChiCTR (www.chictr.org.cn), with the registration number: ChiCTR2100050749.
Similar content being viewed by others
Background
Gout is the most common inflammatory arthritis worldwide, with a high prevalence of comorbidities including metabolic syndrome (MetS) [1,2,3]. In addition to elevated serum and urinary urate levels, low urine pH is very common among patients with gout and primary hyperuricemia [4, 5]. A previous cross-sectional study among 3565 primary gout patients in our institution found that 46.5% had aciduria (pH < 5.5), and low urine pH was independently linked to diverse kidney injuries, including chronic kidney disease (CKD), nephrolithiasis, renal cyst, traces of hematuria and proteinuria [6]. The low urine pH in gout may be mediated by MetS, which is associated with impaired ammoniagenesis and citrate excretion of the renal proximal tubules [7, 8].
There is growing interest in the role of tart cherries as complementary supplements for gout management [9]. Tart cherries contain high levels of anthocyanins, which have potent anti-inflammatory and anti-oxidative properties [10]. Previous studies suggest tart cherry supplements may provide benefits for MetS. In one animal study, a tart cherry-enriched diet decreased total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C) levels, while increasing the high-density lipoprotein cholesterol (HDL-C) concentration [11]. In humans, cherry juice supplementation was shown to attenuate systolic blood pressure [12] and improve 24-h blood pressure, fasting blood glucose (FBG) levels, TC, LDL-C, and TC/HDL-C ratio [13]. Furthermore, some studies found that cherries and cherry products lowered serum urate (sUA) levels in healthy or obese individuals [14, 15], though this effect has not been consistently observed[16]. Additionally, cherries and cherry products may benefit gout patients, with research finding a 35% lower risk of gout flares [17].
Urine alkalinization may help limit urolithiasis and enhance the effectiveness of urate-lowering therapy (ULT) [18]. However, recommendations regarding urine alkalization in gout management guidelines have been inconsistent due to insufficient evidence. Some guidelines have suggested considering urine alkalization in limited clinical settings [19, 20], while the 2020 American College of Rheumatology (ACR) gout management guideline recommended against urine alkalization [21]. Maintaining the sUA levels below target through ULT is the key strategy for gout management. Xanthine oxidase inhibitors (XOI), such as allopurinol and febuxostat, are strongly recommended as the first-line ULT drugs [21, 22]. XOI treatment decreases the urinary urate concentration and may reduce the formation of uric acid crystals and stones, but this may not necessarily apply to patients with aciduria.
We have previously reported that a citrate mixture had comparable efficacy to sodium bicarbonate on urine alkalization in patients with gout and was superior in reducing hematuria as well as gout flares [23]. However, the 24-h urinary albumin/creatinine ratio (UACR) was not measured in that study, nor were any improvements found in MetS components [23]. Here we report the results of a clinical trial testing the efficacy and safety of tart cherry supplementary citrate (TaCCi) mixture in gout patients with XOI febuxostat initiation and acidic urine, compared to a citrate mixture and sodium bicarbonate. The study hypothesized that the tart cherry supplement would have an additional beneficial effect on urine pH and sUA through improvements in MetS.
Methods
Study design and participants
This was an open-labeled, prospective, randomized, parallel controlled trial, conducted between September 2021 and June 2022 at the Gout Clinic of the Affiliated Hospital of Qingdao University. A totally of 354 participants were recruited. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice, and the protocol was reviewed and approved by the Ethics Committee of the Affiliated Hospital of Qingdao University.
The trial was registered in ChiCTR (www.chictr.org.cn), with the registration number ChiCTR2100050749. Eligible participants were male, aged between 18 and 70 years, met the 2015 ACR/EULAR classification criteria for gout, and were about to initiate ULT [24], with fasting urine pH ≤ 6. Due to the low prevalence of gout in women [25] and to minimize the sex confounding influences, only male participants were included in this study. Exclusion criteria included: on ULT or experienced gout flare within 14 days before recruitment; estimated glomerular filtration rate (eGFR) < 60 ml/min/1.73 m2; sUA < 420 μmol/L or > 600 μmol/L; transaminase > two folds of the upper limit of normal (ULN); taking other drugs affecting sUA and/or urine pH; allergic to any drugs or ingredients involved in this trial; secondary gout and secondary hypertension. All participants gave their written informed consents before screening for eligibility. Eligible participants were 1:1:1 randomly assigned to the sodium bicarbonate, citrate mixture, or TaCCi mixture group.
Procedures
All participants were required to undergo a 14-day washout period to withdraw any drug with urate-lowering potential and follow a low-purine diet before baseline data was collected. The random number creator was used to generate a randomization list for those eligible participants, who were 1:1:1 randomly assigned to the sodium bicarbonate group (sodium bicarbonate 1 g, three times a day), the citrate mixture group (citrate mixture: citric acid 50%, sodium citrate 10%, potassium citrate 10%, sodium carbonate 20% and excipient 10%, 3.5 g, twice a day) or the TaCCi mixture group (TaCCi mixture: tart cherry powder 25%, citric acid 30%, sodium citrate 2%, potassium citrate 2%, sodium carbonate 30% and excipient 11%, 3.5 g, twice a day). All participants started ULT with febuxostat (initially 20 mg daily, escalating to 40 mg daily if sUA ≥ 360 μmol/L at first follow-up). Colchicine or other gout flare prophylaxis drugs were not eligible in the trial protocol, while etoricoxib 120 mg once a day for 3–5 days would be prescribed for patients who experienced gout flare during the study. Participants were followed every 4 weeks at a face-to-face visit until week 12.
Demographic and medical history data were acquired at the baseline visit, including age, nephrolithiasis, tophi, smoking, and drinking history. Blood and urine parameters including sUA, triglycerides (TG), TC, FBG, homeostasis model assessment of insulin resistance (HOMA-IR) [26], transaminase, blood urea nitrogen (BUN), serum creatinine (CREA), eGFR, urine pH, urine protein and hemoglobinuria as well as blood pressure (BP) and body mass index (BMI) were obtained at every visit. Other parameters were tested at baseline and week 12 including dual-energy CT (DECT) of affected joints, kidney ultrasonography, 24-h UACR, C-reactive protein (CRP), serum potassium (K+), serum sodium (Na+) and serum chloride (Cl−). Proteinuria and hemoglobinuria were reported as positive when the urinalysis urine protein or hemoglobin reading of ≥ + / − in the urine sample. The pH of the fresh fasting urine samples was determined using a pH electrode (FE28-STANDARD, METTLER Toledo Company, Zurich, Switzerland); Kidney ultrasonography examination was performed using an ALOKA 70 machine (HITACHI, Tokyo, Japan); All symptomatic joints were scanned on a dual x-ray tube 128-detector-row scanner (Somatom Definition Flash, Siemens Healthcare, Forchheim, Germany), with tube A 140kVp/55mAs and tube B 80kVp/255mAs, acquisition at 128 × 0.6 mm, reconstruction at 0.6 mm. Urate volumes were automatically calculated by DECT Syngo via the Gout program (Siemens Healthcare, Germany).
Adverse events were monitored and managed during the study period. Gout flare was defined as a patient-reported flare with pain VAS score > 3 of 0–10 scale [27]. Newly-onset hypertension, which was defined as a systolic blood pressure ≥ 140 mmHg and/or a diastolic blood pressure ≥ 90 mmHg or under antihypertensive treatment or diagnosed by a physician during the follow-up [28]. Polyene phosphatidylcholine would be administered if the transaminase increased to ≥ 2-folds of the ULN, and etoricoxib 120 mg once a day for 3–5 days would be administrated for gout flares.
Outcomes
The primary outcomes were changes in urine pH and sUA level over 12 weeks. Gout-related outcomes included changes of CRP level, gout flares and changes in DECT-detected monosodium urate (MSU) volume. The renal and metabolic outcomes included changes in eGFR, UACR, and MetS components (including BP, BMI, FBG, HOMA-IR, and blood lipids). Adverse events included changes in serum electrolyte level, newly-onset hemoglobinuria, newly-onset nephrolithiasis/renal cyst examined by kidney ultrasonography, newly-onset hypertension, skin allergy, transaminase elevation, and other adverse events that might lead to treatment interruption or hospitalization.
Sample size
Sampling was based on the joint primary outcomes, urine pH, and sUA. In a separate pilot study conducted in our center, 60 participants were included under the same inclusion/exclusion criteria, the mean change of pH mean ± standard derivation (S.D) was 0.40 ± 0.517, 0.38 ± 0.371, and 0.64 ± 0.478 in the sodium bicarbonate group, citrate mixture group and TaCCi mixture group, respectively. To detect a 0.232 difference in urine pH between sodium bicarbonate and TaCCi mixture, 75 patients in each group provided > 80% power at a significance level of 0.05. In the pilot study, the mean change of sUA (mean ± S.D) was 134 ± 93.5 μmol/L, 137 ± 80.6 μmol/L, and 175 ± 76.1 μmol/L in the sodium bicarbonate group, citrate mixture group, and TaCCi mixture group, respectively. To detect the 38.4 μmol/L difference in sUA between the citrate mixture group and TaCCi mixture group, 69 patients in each group were required. Based on the urine pH and sUA data, 75 participants in each group would provide > 80% power at a significance level of 0.05. Considering a drop-out rate of 20%, at least 281 participants were needed.
Statistical analysis
Demographic and clinical features were displayed using standard descriptive statistics including mean ± S.D, median (interquartile range), or number (percentage) where appropriate. Repeated measures mixed models were used for the urine pH and sUA analysis in the per-protocol (PP) population. A Bonferroni correction was used among 3 treatment comparisons at an alpha level of 0.016. The comparison of response rates based on the distribution of urine pH and sUA levels was calculated by chi-square test among the three groups. The changes in UACR were analyzed by a generalized estimation equation model with baseline values as covariates. Comparisons before and after treatment within the group were made using paired T or rank sum tests. Gout flares were compared by negative binomial regression following the intent-to-treat (ITT: All randomized patients who received ≥ 1 dose of randomized study medication were included in the ITT population) principle, the same population also for adverse events assessments. The baseline and 12 week’s follow-up analyses were performed by the PP principle. SPSS 25.0 (IBM SPSS, Chicago, IL, USA) and R version 4.2.1 (https://www.r-project.org) were used for statistical analysis. All tests were two-sided, and P < 0.05 was regarded as statistically significant.
Results
Patients and baseline characteristics
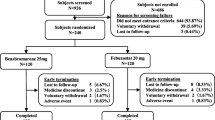
As shown in Fig. 1, a total of 354 patients were screened; 72 patients were excluded for the following reasons: gout flare (n = 18), fasting urine pH > 6 (n = 42), sUA < 420 μmol/L (n = 8), transaminase > two folds of ULN (n = 4); 282 participants were randomized into the sodium bicarbonate, citrate mixture, or TaCCi mixture group at 1:1:1 ratio; and finally, 254 participants completed the study (86 in sodium bicarbonate group, 86 in citrate mixture group, and 82 in TaCCi mixture group). The first study visit was September 2021 and the final study visit was June 2022. Of the 282 participants, 9 withdrew before receiving at least 1 dose of treatment medication, the remaining 273 participants were included in the ITT analyses, and 254 participants who completed the study were included in the PP analyses (Fig. 1).
Flow of study participants. Totally 354 participants were screened, and then 282 eligible patients were enrolled and randomized into sodium bicarbonate, citrate mixture, and TaCCi mixture groups. Nine participants withdrew before receiving at least 1 dose of treatment medication and were excluded from ITT analysis; furthermore, 19 participants were excluded from PP analysis for loss to follow-up. Sb, sodium bicarbonate; TaCCi mixture, tart cherry supplementary citrate mixture, ITT, intention to treat; PP, per protocol
At baseline, the demographic and clinical features were comparable across these three groups. As shown in Table 1, the median (interquartile range) baseline urine pH levels were 5.56 (5.32–5.77) in the sodium bicarbonate group, 5.42 (5.25–5.88) in the citrate mixture group and 5.51 (5.34–5.72) in the TaCCi mixture group. The mean ± S.D of baseline sUA levels in these 3 groups were 538 ± 68.3 μmol/L, 533 ± 59.3 μmol/L, and 524 ± 68.1 μmol/L respectively (Table 1).
Primary outcomes
The urine pH increased in all groups at week 4 and remained stable until week 12, median (interquartile range) 5.56 (5.32–5.77) to 5.82 (5.47–6.40) in the sodium bicarbonate group, 5.42 (5.25–5.88) to 6.02 (5.67–6.65) in the citrate mixture group, and 5.51 (5.34–5.72) to 5.88 (5.55–6.28) in the TaCCi mixture group, respectively, with no significant difference observed between groups in pH level, nor in the proportion when sub-grouped with pH (< 5.5, 5.5–6.2, and > 6.2) (Table 2, Fig. 2A, B), and age did not change the treatment response of the 3 groups (Table S1).
Urine pH categories and UACR changes. A Proportion of patients with pH < 5.5 or ≥ 5.5 at baseline. B Proportion of patients with pH < 5.5, 5.5–6.2, or > 6.2 at week 12. C UACR at baseline and week 12. TaCCi mixture, tart cherry supplementary citrate mixture group; UACR, 24-h urinary albumin-creatinine ratio
The sUA level decreased significantly in all groups (mean ± S.D) 538 ± 68.3 to 370 ± 82.3 μmol/L in the sodium bicarbonate group, 533 ± 59.3 to 360 ± 64.6 μmol/L in the citrate mixture group, and 524 ± 68.1 to 360 ± 59.0 μmol/L in the TaCCi mixture group. There were no significant differences between groups in sUA level, nor in the proportion achieving sUA targets (sUA < 360 or < 300 μmol/L) at each visit (Table 2), and age didn’t change the treatment response of the 3 groups (Table S1).
Gout-related outcomes
The incidence of gout flare in the intent-to-treat population is shown in Table S2; the proportion of patients with gout flares at weeks 4, 8, and 12 was 25 (27.2%), 28 (30.4%), and 19 (20.7%) in the sodium bicarbonate group; 16 (17.4%), 22 (23.9%), and 15 (16.3%) in the citrate mixture group; and 18 (20.2%), 14 (15.7%), and 14 (15.7%) in the TaCCi mixture group. Participants in the citrate mixture group and the TaCCi mixture group experienced fewer gout flares than participants in the sodium bicarbonate group during the study (0.58 ± 0.87 and 0.56 ± 0.88 per participant versus 0.94 ± 1.45 per participant, p = 0.033 and 0.024, respectively), with no difference between the citrate mixture group and the TaCCi mixture group (Table S2). For CRP there was no significant difference between groups at baseline, while it was lower in TaCCi mixture at week 12 when compared to the other two groups (p < 0.01) (Table 2).
The DECT detected urate volume (Ln transformed) decreased significantly from baseline in all groups, whereas no significant between-group difference was observed at baseline or at week 12 (Fig. S1).
Renal and metabolic outcomes
No significant changes in eGFR were observed in the groups over time, except for an increase at the week 8 time point in the TaCCi mixture group (Table 2). The UACR improved from 3.01 (0.56–11.2) mg/g to 1.72 (0.73–7.08) mg/g in the sodium bicarbonate group (p = 0.253), from 3.29 (0.44–10.3) mg/g to 1.17 (0.71–4.14) mg/g in the citrate mixture group (p = 0.007) and from 7.42 (0.72–11.9) mg/g at baseline to 0.89 (0.40–1.82) mg/g at week 12 in the TaCCi mixture group (p < 0.001) (Table 2). Notably, significant differences in UACR were observed between groups at week 12, of which the mean difference between the sodium bicarbonate group and the TaCCi mixture group was − 4.40 mg/g (95% CI, − 6.87 to − 1.94, p < 0.001)), between the citrate mixture group and the TaCCi mixture group was − 1.51 mg/g (95% CI, − 2.94 to − 0.085, p = 0.034) and between the sodium bicarbonate group and the citrate mixture group was − 2.89 mg/g (95%CI, − 5.46 to –0.81, p = 0.021) (Fig. 2C).
As for MetS components, in the TaCCi mixture group, the SBP, DBP, and TC levels decreased significantly from baseline to week 12, and the FBG level as well as HOMA-IR decreased significantly from baseline at all visits (p < 0.05). In the sodium bicarbonate group, SBP and BMI increased from baseline to week 12 (p < 0.05) (Table 2). However, no significant between-group differences were observed at any visit (Table 2).
Adverse events
The serum electrolytes, Na+, K+, and Cl−, were similar between groups and over time (Fig. S2A–C). There was no significant difference regarding adverse events across the three groups: the incidence of newly-onset hemoglobinuria was 4 (4.35%), 2 (2.17%), and 3 (3.37%); newly-onset nephrolithiasis was 1 (1.09%), 2 (2.17%) and 1 (1.12%); newly-onset renal cyst was 6 (6.98%), 4 (4.65%), and 2 (2.44%); newly-onset hypertension was 3 (3.26%), 3 (3.26%), and 1 (1.12%) in sodium bicarbonate, citrate mixture, and TaCCi mixture group, respectively (Table 3). All three treatments were well tolerated, and no other adverse events that might lead to treatment interruption or hospitalization occurred during this study.
Discussion
The aim of this study was to investigate the efficacy and safety of a tart cherry supplementary citrate (TaCCi) mixture on urine pH, sUA, and gout flares in gout patients initiating ULT with XOI febuxostat. The hypothesis was that tart cherry supplement could ameliorate acidic urine by improving MetS components. Our findings indicate that the TaCCi mixture had similar efficacy and safety to sodium bicarbonate or citrate mixture for urine alkalization. However, the TaCCi mixture group demonstrated greater improvement in UACR and CRP compared to the other two groups, and fewer gout flares than the sodium bicarbonate group. No between-group differences were observed for MetS components over the 12-week study period.
Low urine pH is a major risk factor for renal system uric acid crystallization formation and stone development [29]. However, a prior study found that urine alkalization with sodium bicarbonate for three months did not retard the progress of kidney damage as assessed by eGFR and hematuria in patients with gout, though citrate mixture lowered hematuria at comparable urine pH levels [23]. In our study, both the TaCCi mixture and citrate mixture significantly reduced the UACR after 12 weeks, with UACR being lower in the TaCCi mixture group than in the citrate mixture or the sodium bicarbonate groups at week 12. UACR is a sensitive and reliable index for glomeruli injury and a key criterion in CKD classification as recommended by the KDIGO guidelines [30]. The improvement of UACR may partly reflect citrate-induced resolution of uric acid crystal in renal tubules relieving pressure in the Bowman's capsule of glomerulus, as suggested by our prior study [23]. The greater reductions of UACR in the TaCCi mixture group align with anthocyanins from tart cherry lowering UACR in high-fat diet mice [31]. Anthocyanins and metabolites possess potent anti-oxidative and anti-inflammatory properties, and may attenuate renal injury in mice through reduced oxidative stress and protected mitochondrial function [32]. Uric acid crystals can induce renal inflammatory, proliferative and maladaptive changes, manifesting as renal cysts, nephrolithiasis, and eventual CKD, while soluble urate may similarly impact glomeruli in a manner unaffected by urine pH [33]. Oxidative stress and activation of the NLRP3 inflammasome in kidney cells correlate with these changes [33]. Taken together, the TaCCi mixture may provide extra renal protection, though the mechanism requires further study.
Observational studies have suggested that tart cherry intake may reduce gout flare frequency [17, 34]. Additionally, in vitro studies showed tart cherry inhibits IL-1β secretion, closely linked to gout flare initiation [34]. The urate-lowering effects of tart cherry are controversial, with variable results reported [16, 17, 34, 35]. The current data did not find greater serum urate-lowering among study participants all initiating febuxostat during the study period. In addition, the incidence of gout flare was lower in both the TaCCi mixture and the citrate mixture groups versus the sodium bicarbonate group. Decreased gout flares with the citrate mixture agree with prior work [23]. Citrate participates in the tricarboxylic acid cycle and other multiple metabolic pathways [36]. During inflammation, citrate withdraws from the Krebs cycle and exports to the cytosol, where it is cleaved and exerts anti-inflammatory function [36]. Lower gout flare incidence with citrate and TaCCi mixture may relate to their citrate component. CRP levels were similar at baseline, but decreased more in the TaCCi mixture group compared with the other two groups at week 12. CRP elevation reflects inflammation from potentially diverse sources of gout. These data suggested that tart cherry supplement lowered systemic inflammation in gout patients at 12 weeks, although gout flares were similar between the TaCCi and the citrate mixture groups.
The study had several limitations. Firstly, it was conducted at a single clinical center and was open label. Secondly, only male patients with eGFR > 60 ml/min/1.73m2 were enrolled. Thirdly, as a short-term study, long-term efficacy, safety, and impacts on other conditions of the mixtures remain unclear. Thus, a double-blind, placebo-controlled study of varied patients over longer follow-up is needed to better evaluate the role of urine alkalinization in gout management and confirm these results.
Conclusions
This study demonstrated that tart cherry supplementary citrate mixture has similar efficacy on urine pH in gout patients initiating XOI therapy when compared to a citrate mixture and sodium bicarbonate. The tart cherry mixture also showed similar safety. However, the tart cherry mixture resulted in greater improvements in UACR and CRP levels, which suggests that tart cherry supplements might offer extra therapeutic value beyond just urine alkalization.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- MetS:
-
Metabolic syndrome
- CKD:
-
Chronic kidney disease
- TC:
-
Total cholesterol
- LDL-C:
-
Low-density lipoprotein cholesterol
- HDL-C:
-
High-density lipoprotein cholesterol
- FBG:
-
Fasting blood glucose
- sUA:
-
Serum urate
- ACR:
-
American College of Rheumatology
- ULT:
-
Urate-lowing therapy
- XOI:
-
Xanthine oxidase inhibitors
- UACR:
-
Twenty-four-hour urinary albumin/creatinine ratio
- TaCCi:
-
Tart cherry supplementary citrate
- eGFR:
-
Estimated glomerular filtration rate
- ULN:
-
Upper limit of normal
- CRP:
-
C-reactive protein
- TG:
-
Triglycerides
- HOMA-IR:
-
Homeostasis model assessment of insulin resistance
- BUN:
-
Blood urea nitrogen
- CREA:
-
Serum creatinine
- BP:
-
Blood pressure
- BMI:
-
Body mass index
- DECT:
-
Dual-energy CT
- MSU:
-
Monosodium urate
- S.D:
-
Standard derivation
- PP:
-
Per protocol
- ITT:
-
Intent-to-treat
- KDIGO:
-
Kidney Disease: Improving Global Outcomes
- NLRP3:
-
NOD-like receptor thermal protein domain associated protein 3
References
Richette P, Clerson P, Perissin L, Flipo RM, Bardin T. Revisiting comorbidities in gout: a cluster analysis. Ann Rheum Dis. 2015;74(1):142–7.
Bevis M, Blagojevic-Bucknall M, Mallen C, Hider S, Roddy E. Comorbidity clusters in people with gout: an observational cohort study with linked medical record review. Rheumatology (Oxford). 2018;57(8):1358–63.
Dao HH, Harun-Or-Rashid M, Sakamoto J. Body composition and metabolic syndrome in patients with primary gout in Vietnam. Rheumatology (Oxford). 2010;49(12):2400–7.
Maalouf NM, Cameron MA, Moe OW, Sakhaee K. Metabolic basis for low urine pH in type 2 diabetes. Clin J Am Soc Nephrol. 2010;5(7):1277–81.
Daudon M, Traxer O, Conort P, Lacour B, Jungers P. Type 2 diabetes increases the risk for uric acid stones. J Am Soc Nephrol. 2006;17(7):2026–33.
He Y, Xue X, Terkeltaub R, Dalbeth N, Merriman TR, Mount DB, et al. Association of acidic urine pH with impaired renal function in primary gout patients: a Chinese population-based cross-sectional study. Arthritis Res Ther. 2022;24(1):32.
Maalouf NM, Cameron MA, Moe OW, Adams-Huet B, Sakhaee K. Low urine pH: a novel feature of the metabolic syndrome. Clin J Am Soc Nephrol. 2007;2(5):883–8.
Maalouf NM, Sakhaee K, Parks JH, Coe FL, Adams-Huet B, Pak CY. Association of urinary pH with body weight in nephrolithiasis. Kidney Int. 2004;65(4):1422–5.
Sautner J, Eichbauer-Sturm G, Gruber J, Lunzer R, Puchner RJ. 2022 update of the Austrian Society of Rheumatology and Rehabilitation nutrition and lifestyle recommendations for patients with gout and hyperuricemia. Wien Klin Wochenschr. 2022;134(13–14):546–54.
Golovinskaia O, Wang CK. Review of functional and pharmacological activities of berries. Molecules. 2021;26(13):3904.
Papp N, Blazovics A, Febel H, Salido S, Altarejos J, Feher E, et al. Antihyperlipidemic effects of sour cherries characterized by different in vitro antioxidant power and polyphenolic composition. Plant Foods Hum Nutr. 2015;70(4):408–13.
Keane KM, George TW, Constantinou CL, Brown MA, Clifford T, Howatson G. Effects of Montmorency tart cherry (Prunus Cerasus L.) consumption on vascular function in men with early hypertension. Am J Clin Nutr. 2016;103(6):1531–9.
Desai T, Roberts M, Bottoms L. Effects of short-term continuous Montmorency tart cherry juice supplementation in participants with metabolic syndrome. Eur J Nutr. 2021;60(3):1587–603.
Jacob RA, Spinozzi GM, Simon VA, Kelley DS, Prior RL, Hess-Pierce B, et al. Consumption of cherries lowers plasma urate in healthy women. J Nutr. 2003;133(6):1826–9.
Martin KR, Coles KM. Consumption of 100% tart cherry juice reduces serum urate in overweight and obese adults. Curr Dev Nutr. 2019;3(5):nzz011.
Stamp LK, Chapman P, Frampton C, Duffull SB, Drake J, Zhang Y, et al. Lack of effect of tart cherry concentrate dose on serum urate in people with gout. Rheumatology (Oxford). 2020;59(9):2374–80.
Zhang Y, Neogi T, Chen C, Chaisson C, Hunter DJ, Choi HK. Cherry consumption and decreased risk of recurrent gout attacks. Arthritis Rheum. 2012;64(12):4004–11.
Yan F, Xue X, Lu J, Dalbeth N, Qi H, Yu Q, et al. Superiority of low-dose benzbromarone to low-dose febuxostat in a prospective, randomized comparative effectiveness trial in gout patients with renal uric acid underexcretion. Arthritis Rheumatol. 2022;74(12):2015–23.
Hui M, Carr A, Cameron S, Davenport G, Doherty M, Forrester H, et al. The British Society for Rheumatology Guideline for the Management of Gout. Rheumatology (Oxford). 2017;56(7):1246.
Yu KH, Chen DY, Chen JH, Chen SY, Chen SM, Cheng TT, et al. Management of gout and hyperuricemia: multidisciplinary consensus in Taiwan. Int J Rheum Dis. 2018;21(4):772–87.
FitzGerald JD, Dalbeth N, Mikuls T, Brignardello-Petersen R, Guyatt G, Abeles AM, et al. 2020 American College of Rheumatology Guideline for the Management of Gout. Arthritis Care Res (Hoboken). 2020;72(6):744–60.
Richette P, Doherty M, Pascual E, Barskova V, Becce F, Castaneda-Sanabria J, et al. 2016 updated EULAR evidence-based recommendations for the management of gout. Ann Rheum Dis. 2017;76(1):29–42.
Xue X, Liu Z, Li X, Lu J, Wang C, Wang X, et al. The efficacy and safety of citrate mixture vs sodium bicarbonate on urine alkalization in Chinese primary gout patients with benzbromarone: a prospective, randomized controlled study. Rheumatology (Oxford). 2021;60(6):2661–71.
Neogi T, Jansen TL, Dalbeth N, Fransen J, Schumacher HR, Berendsen D, et al. 2015 Gout classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2015;74(10):1789–98.
Singh JA. Racial and gender disparities among patients with gout. Curr Rheumatol Rep. 2013;15(2):307.
Gonzalez-Gonzalez JG, Violante-Cumpa JR, Zambrano-Lucio M, Burciaga-Jimenez E, Castillo-Morales PL, Garcia-Campa M, et al. HOMA-IR as a predictor of health outcomes in patients with metabolic risk factors: a systematic review and meta-analysis. High Blood Press Cardiovasc Prev. 2022;29(6):547–64.
Gaffo AL, Schumacher HR, Saag KG, Taylor WJ, Dinnella J, Outman R, et al. Developing a provisional definition of flare in patients with established gout. Arthritis Rheum. 2012;64(5):1508–17.
Zhang Y, Zhang Y, Yang S, Ye Z, Wu Q, Liu M, et al. U-shaped relation of dietary thiamine intake and new-onset hypertension. Nutrients. 2022;14(16):3251.
Ramos GK, Goldfarb DS. Update on uric acid and the kidney. Curr Rheumatol Rep. 2022;24(5):132–8.
Kidney Disease: Improving Global Outcomes Glomerular Diseases Work G. KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int . 2021;100(4S):S1–276. https://doi.org/10.1016/j.kint.2021.05.021.
Shan Q, Zheng Y, Lu J, Zhang Z, Wu D, Fan S, et al. Purple sweet potato color ameliorates kidney damage via inhibiting oxidative stress mediated NLRP3 inflammasome activation in high fat diet mice. Food Chem Toxicol. 2014;69:339–46.
Wei J, Wu H, Zhang H, Li F, Chen S, Hou B, et al. Anthocyanins inhibit high glucose-induced renal tubular cell apoptosis caused by oxidative stress in db/db mice. Int J Mol Med. 2018;41(3):1608–18.
Johnson RJ, Bakris GL, Borghi C, Chonchol MB, Feldman D, Lanaspa MA, et al. Hyperuricemia, acute and chronic kidney disease, hypertension, and cardiovascular disease: report of a scientific workshop organized by the National Kidney Foundation. Am J Kidney Dis. 2018;71(6):851–65.
Chen PE, Liu CY, Chien WH, Chien CW, Tung TH. Effectiveness of cherries in reducing uric acid and gout: a systematic review. Evid Based Complement Alternat Med. 2019;2019:9896757.
Hillman AR, Uhranowsky K. Acute ingestion of Montmorency tart cherry reduces serum uric acid but has no impact on high sensitivity C-reactive protein or oxidative capacity. Plant Foods Hum Nutr. 2021;76(1):83–9.
Iacobazzi V, Infantino V. Citrate–new functions for an old metabolite. Biol Chem. 2014;395(4):387–99.
Acknowledgements
We would like to thank all participants enrolled in this study, and the efforts all authors did for this work.
Funding
This study was supported by the National Key Research and Development Program of China (2022YFC2503300, 2022YFE0107600), Projects of International Cooperation and Exchanges NSFC (82220108015), Shandong Provincial Key Research and Development Plan Major Scientific and Technological Innovation Project (2021CXGC011103) and Qingdao Medical and Health Research Program (2021-WJZD168).
Author information
Authors and Affiliations
Contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Changgui Li and Mingshu Sun were responsible for study conception and design; Can Wang and Wenyan Sun were responsible for data analysis and manuscript preparation; Zhongjun Wang, Xuefeng Wang, Xiaopeng Ji, Lin Han, Lingling Cui, Xinde Li, Zhen Liu, Aichang Ji, and Yuwei He all participated in data and sample acquisition; Nicola Dalbeth and Xiaomei Xue participated in data interpretation and manuscript revision.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee from the Affiliated Hospital of Qingdao University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests. Nicola Dalbeth has received consulting fees, speaker fees, or grants from AstraZeneca, Dyve Biosciences, Horizon, Amgen, Selecta, Arthrosi, JW Pharmaceutical Corporation, PK Med, PTC Therapeutics, Protalix, Cello Health, Abbvie, and Janssen, outside the submitted work.
Other authors have nothing to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Age related treatment response of the three groups:Participants in the three treatment arms were grouped into > 40 years old or ≤ 40 years old subgroup. Those main outcomes were compared between participants of each subgroup within and between/among these three treatment groups, including ΔUACR, ΔSU, ΔpH, pH ≥ 6.2 at week12 and SU < 360 μmol/L at week 12 in vs in three treatment groups, and no significant difference was observed between subgroups. Table S2. Gout flares in the intent-to-treat set. Fig. S1. DECT urate volumes. Significant differences (p < 0.05) were observed in Sodium bicarbonate, Citrate mixture and TaCCi mixture group between baseline and week 12, while no significant difference was observed between groups. TaCCi Mixture: Tart Cherry supplementary Citrate Mixture. Fig. S2. Serum electrolytes levels during follow up. No significant difference was observed between baseline and week 12 in Sodium bicarbonate, Citrate mixture and TaCCi mixture group. A: Serum potassium,B: serum sodium and C: serum chlorine levels.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, C., Sun, W., Dalbeth, N. et al. Efficacy and safety of tart cherry supplementary citrate mixture on gout patients: a prospective, randomized, controlled study. Arthritis Res Ther 25, 164 (2023). https://doi.org/10.1186/s13075-023-03152-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13075-023-03152-1