Abstract
Background
Osteoarthritis (OA) is one of the costliest and most disabling forms of arthritis, and it poses a major public health burden; however, its detailed etiology, pathophysiology, and metabolism remain unclear. Therefore, the purpose of this study was to investigate the key plasma metabolites and metabolic pathways, especially focusing on radiographic OA severity and synovitis, from a large sample cohort study.
Methods
We recruited 596 female volunteers who participated in the Iwaki Health Promotion Project in 2017. Standing anterior-posterior radiographs of the knee were classified by the Kellgren-Lawrence (KL) grade. Radiographic OA was defined as a KL grade of ≥ 2. Individual effusion-synovitis was scored according to the Whole-Organ Magnetic Resonance Imaging Scoring System. Blood samples were collected, and metabolites were extracted from the plasma. Metabolome analysis was performed using capillary electrophoresis time-of-flight mass spectrometry. To investigate the relationships among metabolites, the KL grade, and effusion-synovitis scores, partial least squares with rank order of groups (PLS-ROG) analyses were performed.
Results
Among the 82 metabolites examined in this assay, PLS-ROG analysis identified 42 metabolites that correlated with OA severity. A subsequent metabolite set enrichment analysis using the significant metabolites showed the urea cycle and tricarboxylic acid cycle as key metabolic pathways. Moreover, further PLS-ROG analysis identified cystine (p = 0.009), uric acid (p = 0.024), and tyrosine (p = 0.048) as common metabolites associated with both OA severity and effusion-synovitis. Receiver operating characteristic analyses showed that cystine levels were moderately associated with radiographic OA (p < 0.001, area under the curve 0.714, odds ratio 3.7).
Conclusion
Large sample metabolome analyses revealed that cystine, an amino acid associated with antioxidant activity and glutamate homeostasis, might be a potential metabolic biomarker for radiographic osteoarthritis and early phase synovitis.
Similar content being viewed by others
Introduction
Osteoarthritis (OA), an age-related low-grade inflammatory disease of the synovial joints [1,2,3], is one of the costliest and most disabling forms of arthritis, being highly prevalent and posing a major public health burden [4, 5]. Although prevention and an early diagnosis are prerequisites for a favorable outcome, the detailed etiology, pathophysiology, and metabolism of OA remain unclear [6].
Recently, there has been increased interest in the early stages of knee OA [7, 8]. In this condition, the synovial joints present with various minute pathological changes in the cartilage, ligaments, menisci, subchondral bone, and synovium of the knee, even without radiographic abnormalities [9]. A longitudinal large sample cohort study revealed that bone marrow lesions, effusion-synovitis, and meniscal lesions are key pathologies of OA progression [10]. Additional epidemiological studies using magnetic resonance imaging (MRI) have reported that mild-to-moderate inflammation is detectable in people with early-stage knee OA [3, 11]. Pathophysiologically, synovitis has a strong impact on joint degeneration because the inflamed synovium secretes a variety of proteases and cytokines [12]. However, the precise biological and biomechanical mechanisms of OA are not fully understood yet.
Recent omics profiling approaches combined with clinical measures can comprehensively assess the health status and identify deviations from healthy baselines; these approaches may improve disease risk prediction and early detection [13]. Metabolomics detects many small-molecule metabolites in body fluids or tissues and quantifies these molecules in a single step. This approach promises an immense potential for early diagnosis, therapy monitoring, and understanding of the pathogenesis of many diseases [14]. However, previous omics analyses were affected by limitations in the sample size owing to the high running costs. Large sample cohort studies are necessary to analyze the severity-related changes and assess the influence of confounders like anthropometric factors, bone strength, hormonal condition, or synovitis. After recent improvements in analytical techniques had made it possible to conduct large sample metabolome analyses [15], several large-scale cohort studies successfully identified the etiologies and biomarkers in various fields of health maintenance [16, 17].
We employed targeted plasma metabolome analyses in this large sample cohort study to identify the metabolites and metabolic pathways associated with radiographic severity in OA of the knee. Additionally, we investigated the metabolic pathways related to synovitis and effusion to better understand the etiology of early-stage knee OA and identify the risk factors for the progression of knee OA. We expected that a novel inflammation-related biomarker associated with both synovitis and radiographic severity of knee OA would be identified and that the underlying metabolic pathways related to the etiology of early-stage knee OA would be revealed.
Materials and methods
This was a cross-sectional study with volunteer participants from the Iwaki Health Promotion Project. The Iwaki Health Promotion Project, an annual program for the general population living in the Iwaki area of Hirosaki City located in the western Aomori prefecture, is a preventive medicine program that aims at improving average life expectancy by performing general health checkups [3, 8, 18]. The study of knee OA began in 2008, with annual checkups that followed the natural progression of the condition. At each checkup, physical examination, functional tests, laboratory findings, basic lifestyle information, and imaging data were registered. Furthermore, to discover the disease in its early phase, we performed MRIs on middle-aged female patients, who are known to have the highest risk for knee OA and its progression. The participants were community-dwelling residents who were recruited using mass media advertisements and with the help of public health nurses. All participants provided written informed consent. The study was conducted in agreement with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards and was approved by the ethics committee of Hirosaki University Graduate School of Medicine, Japan (2017-026).
Participants
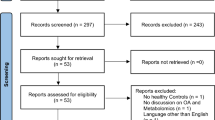
Out of 10,000 community-dwelling residents, 1073 volunteers who participated in the Iwaki Health Promotion Project 2017 were enrolled in this study. First, participants who had not undergone radiographic and blood examinations were excluded. This study aimed to identify the biomarkers useful for the detection of early and advanced stages of OA. Two phases were conducted. Phase 1 analysis aimed to discover the metabolites related to increased radiographic severity in high-risk female participants, who are the definitive high-risk group for knee OA. Therefore, 441 men were excluded, and a total of 597 women were assessed (Fig. 1). In phase 2, we focused on the synovitis without radiographic abnormality, in order to assess the discovered metabolites in the early phase of knee OA. MRIs in phase 2 were performed only on middle-aged women who are at a high risk of early knee OA [8], resulting in a total of 298 participants.
Blood sampling and metabolite extraction
Blood samples were collected from all participants before breakfast after a period of 10 or more hours of fasting. After centrifugation, collected plasma were separately stored at – 80 °C. Metabolite extraction and metabolome analysis were conducted at Human Metabolome Technologies (HMT, Tsuruoka, Yamagata, Japan). Briefly, 50 μL of plasma was added to 450 μL of methanol containing internal standards (Solution ID: H3304-1002, HMT, Inc., Tsuruoka, Japan) at 0 °C to inactivate enzymes. The extract solution was thoroughly mixed with 500 μL of chloroform and 200 μL of Milli-Q water and centrifuged at 2300×g and 4 °C for 5 min; 350 μL of the upper aqueous layer was centrifugally filtered through a Millipore 5-kDa cutoff filter to remove the proteins. The filtrate was centrifugated, the pellet resuspended in 50 μL of Milli-Q water, and then used for metabolome analysis at the HMT.
Metabolome analysis
Metabolome analysis was conducted with the Basic Scan package of the HMT using capillary electrophoresis time-of-flight mass spectrometry (CE-TOFMS) based on the methods described previously [19, 20]. Briefly, CE-TOFMS analysis was carried out using an Agilent CE capillary electrophoresis system equipped with an Agilent 6210 time-of-flight mass spectrometer, Agilent 1100 isocratic HPLC pump for sheath liquid, Agilent G1603A CE-MS adapter kit, and Agilent G1607A CE-ESI-MS sprayer kit (Agilent Technologies, Waldbronn, Germany). The systems were controlled by the Agilent G2201AA ChemStation software version B.03.01 for CE (Agilent Technologies) and connected by a fused silica capillary (50 μm inner diameter × 80 cm total length) with a commercial electrophoresis buffer (H3301-1001 and H3302-1021 for cation and anion analyses, respectively; HMT) as the electrolyte. The spectrometer was scanned from m/z 50 to 1000 [19]. Peaks were extracted using MasterHands, an automatic integration software (Keio University, Tsuruoka, Yamagata, Japan), to obtain peak information including m/z, peak area, and migration time [21]. Signal peaks corresponding to isotopomers, adduct ions, and other product ions of known metabolites were excluded, and the remaining peaks were annotated according to the HMT metabolite database based on their m/z values with the migration times. Areas of the annotated peaks were then normalized based on internal standard levels and sample volumes to obtain relative levels of each metabolite. The relative levels were further normalized using control plasma samples (Sigma-Aldrich Japan, Tokyo, Japan) when measurement conditions were changed by different analysis equipment or capillary replacement in CE-MS measurement. The coefficients of variation (CVs) reported in our previous study were calculated using a portion of the same sample as in this study [22]. The minimum and maximum values of CVs were 3.3% and 81.6%, respectively. For the detected metabolites, 61.6% of the number of metabolites had CVs less than 10%. Moreover, on the basis of the metabolomic results analysis, plasma cystine concentrations (nmol/mL, HPLC) were quantified by the LSI Medience Corporation (LSI Medience Corp., Tokyo, Japan).
Radiographic evaluation
Radiographic examinations of the knee were performed using a digital radiographic device (CXDI-40EG, Canon Inc. Tokyo, Japan). Experienced radiologists and orthopedic surgeons obtained weight-bearing, full-extension, and anterior-posterior radiographs of both the knees with foot map positioning on the day of the checkup. The beam was positioned parallel to the floor and aimed at the joint space, and the sequencing was set at 60 kV, 50 mAs, and 80 ms for all participants. The images were converted into Joint Photographic Experts Group format files. OA severity in each knee was classified by two trained orthopedic surgeons (D.C. and S.O.) as KL grades 0–4 using the KL radiographic atlas [23]. The interclass correlation coefficient of the assessment performed by the two surgeons was 0.815, and the intraclass correlation coefficient was 0.923. These surgeons were unaware of the sequence in which the radiographs were acquired and the clinical status of the participants.
Synovitis evaluated using MRI
Synovitis on MRI was assessed in the right knee. Participants were positioned in supine with their knees in full extension and scanned with a rapid extremity coil and mobile magnetic resonance unit (1.5 T; ECHELON RX, Hitachi, Tokyo, Japan) within 1 week of the other examinations. The scanning sequences were set including the sagittal and coronal planes of T2-weighted fat-suppressed fast-spin echo sequences (repetition time 5000 ms; echo time 80 ms; field of view 16 cm; 288 × 288 matrix; slice thickness of 3 mm with a gap of 1.0 mm between slices). The image database was transferred to an independent computer workstation using the OsiriX software (Newton Graphic, Inc., Hokkaido, Japan). According to the Whole-Organ MRI Scoring (WORMS) method [24], synovitis was semi-quantitatively graded as an effusion-synovitis score from 0 to 3 in terms of the estimated maximal distention of the synovial cavity: 0 = normal, 1 = < 33% of maximum potential distention, 2 = 33–66% of maximum potential distention, and 3 = > 66% of maximum potential distention. Based on the effusion-synovitis scores, participants with KL grades 0 and 1 were divided into low (effusion-synovitis score of 0; n = 205) and high (effusion-synovitis score of 1, 2, and 3; n = 93) effusion-synovitis groups to determine the absence or presence of synovitis, respectively. Furthermore, the effusion-synovitis volume (cm3) was quantified at the suprapatellar fluid equivalent signal on MRI using the OsiriX software by an expert orthopedic surgeon (S.O.). To validate the measurements of effusion synovitis volume, the intra-class correlation coefficient (ICC) was estimated from two separate measurements. The ICC (1,1) of the effusion-synovitis volume was 0.984.
Covariates
The patients’ anthropometric parameters including height, weight, and body mass index (BMI) values were recorded. The difference in muscle volume (kg) of participants was estimated by measuring impedance with the Tanita MC-190 body composition analyzer (Tanita Co., Ltd., Tokyo, Japan), since muscular volume is correlated to the concentration of some metabolites. In this study, the muscle volume was evaluated as that of the right lower extremity, including the thigh, in order to correspond to the side of the obtained MRI.
Furthermore, the serum inflammatory biomarkers were measured from the collected blood samples. After centrifugation, the collected sera were separately stored at – 80 °C. Serum samples were analyzed by the LSI Medience Corporation (LSI Medience Corp., Tokyo, Japan). The company has an ISO-15189-accredited laboratory; therefore, serum assays were performed under strict conditions. As biomarkers for inflammation, highly sensitive C-reactive protein (mg/dL, Nephelometry), matrix metalloproteinase 3 (mg/mL, LA), interleukin-6 (pg/mL, CLEIA), and hyaluronic acid (ng/mL, LA) levels were assessed. The reliabilities of these biomarkers, as shown in the coefficient of variation, were 1.0–1.5%, 2.0–4.8%, 1.5–7.4%, and 2.3–5.0%, respectively.
Statistical analysis
Demographic data and serum inflammation markers are presented as mean ± standard deviation. Age, BMI, muscular volume, and serum inflammation biomarkers among KL grades were compared by the analysis of variance. In the phase 1 analysis, partial least squares (PLS) and PLS with rank order of groups (PLS-ROG) were used. While PLS considers only group differences, PLS-ROG considers the group differences as well as information about the order of the groups. Therefore, PLS-ROG is useful when, in addition to information on the groups, information on the order of groups, such as the KL grade, is available. We first performed PLS and confirmed the association between PLS scores and KL grades. If there was no association between the PLS scores and KL grades, we performed PLS-ROG analyses. Statistically significant metabolites related to the KL grade were identified using PLS or PLS-ROG loading [25] based on Pearson’s correlation coefficient (r) between the PLS-ROG score for the response variable and each metabolite level. We set the smoothing parameter κ as 0.999 in the PLS-ROG analyses. A previous study that explained the theoretical aspects of PLS-ROG [25] reported how the score changes when the smoothing parameter is varied. For practical purposes, it is sufficient to set the smoothing parameter close to 1 (0.999 in this study) and to confirm whether the scores are associated with the order of the groups by visual inspection. Using the identified metabolites, metabolite set enrichment analyses were performed to detect the related pathways by over-representation analysis [26] using an HMT proprietary program written in R [27] and the same metabolite setlist used in our previous study [27]. A heatmap and dendrograms of metabolites were created based on the metabolites’ intensities. In this analysis, Z-scores of the detected metabolites are shown in each row, and samples that were sorted with the Kellgren-Lawrence grade are shown in each column.
In the phase 2 analysis, levels of metabolites between low and high effusion-synovitis score groups were compared using the Mann-Whitney U test. Furthermore, PLS-ROG analysis of the effusion-synovitis score and metabolites was performed, and related metabolites were identified using PLS-ROG loading. Then, Spearman’s correlation coefficients (ρ) were estimated to investigate the relationship between quantified plasma cystine consentrations and inflammation markers.
Finally, to estimate the cutoff value for detecting knee OA with KL grade 2 and higher, a receiver operating characteristic (ROC) curve analysis was performed using these commonly correlated metabolites. Based on the calculated areas under the curve (AUC), the strength with the presence of OA was compared among the metabolites, and their cutoff values were defined as the nearest point to the true positive, that is, the left upper corner of the plot box to maximize the true positive fraction and minimize the false-positive fraction.
Calculations for the statistical analysis were performed using the HMT proprietary R program, SPSS version 25.0J (SPSS Inc., Chicago, IL, USA), and the statistical software BellCurve for Excel (version 3.21; Social Survey Research Information Co., Ltd., Tokyo, Japan). A p-value and q-value, for multiple comparison testing based on the Benjamini-Hochberg [28] approach, < 0.05 were considered statistically significant.
Results
Among the 597 women, a participant who was an outlier, exceeding the 99% confidence interval in a principal component analysis score plot, was excluded (Fig. S1) [27]. Finally, the initial phase 1 analysis included 596 female participants (Fig. 1); their demographic and clinical data are shown in Table 1. In total, 82 metabolites were examined and shown in a heatmap (Fig. S2 and Additional file 1: Table S1). We performed PLS analyses (Fig. 2a, b) of the metabolome data, which failed to confirm an association of PLS scores with OA severity based on the KL grade. Therefore, PLS-ROG analysis for the association between the KL grade and metabolites was performed (Fig. 2c, d). This analysis confirmed an association of the first PLS-ROG score with the KL grade. Of the 82 metabolites, 37 were positively and five were negatively correlated with radiographic severity (q < 0.05). The correlated metabolites (from the highest correlation downwards) were cystine (r = 0.397, p < 0.001, q < 0.001), urea (r = 0.392, p < 0.001, q < 0.001), cis-aconitic acid (r = 0.290, p < 0.001, q < 0.001), ornithine (r = 0.254, p < 0.001, q < 0.001), and tyrosine (r = 0.214, p < 0.001, q < 0.001). A subsequent metabolite set enrichment analysis using the significant metabolites (p < 0.05) found no significantly associated metabolic pathway (Additional file 1: Table S2), whereas five out of six metabolites in the urea cycle (p = 0.059, q =1.000) and all the three metabolites in the tricarboxylic acid (TCA) cycle (p = 0.085, q =1.000) were identified as key metabolites.
PLS and PLS-ROG analyses of the Kellgren-Lawrence (KL) grade and metabolites. Results of the partial least squares (PLS) and PLS with rank order of groups (PLS-ROG) analyses of the metabolome data. The KL grade (0–4) was set as the response variable. A scatter plot of the first and second PLS scores for the explanatory variable (a). A bar plot (b) of the first PLS score for the response variable and a scatter plot (c) of the first and second PLS-ROG score for the explanatory variable, are shown. A bar plot (d) of the first PLS-ROG score for the response variable is shown
The phase 2 analysis included 298 women who had undergone MRI examinations. In this population, the mean effusion-synovitis volume was 2.6 ± 2.7 (range 0.1–24.6) cm3, which increased with the effusion-synovitis score (p < 0.001; Additional file 1: Table S3). There were no significant differences in age, BMI, and muscle volume in the effusion-synovitis score in the variance test analysis (Additional file 1: Table S3). In the comparison between the low and high effusion-synovitis score groups, alanine (p = 0.016, q = 0.587), cystine (p = 0.039, q = 0.587), methionine (p = 0.043, q = 0.587), propionylcarnitine (p = 0.046, q = 0.587), and uric acid (p = 0.048, q = 0.587) were identified as metabolites with significantly increased levels in patients with high synovitis scores (p < 0.05; Fig. 3a–e).
Effusion-synovitis-related metabolites. Levels of metabolites between the low (effusion-synovitis score 0) and high (effusion-synovitis scores 1, 2, and 3) groups are compared by the Mann-Whitney U test. Levels of alanine (a), cystine (b), methionine (c), propionylcarnitine (d), and uric acid (e) in the high group are significantly higher than those of the lower group. The box and center line indicate the first quartile, median, and third quartile. The dots indicate the outliers. Partial least squares with rank order of group (PLS-ROG) results for metabolome data (e–g). The effusion-synovitis score (from 0 to 3) was set as the response variable. A scatter plot of the first and second PLS-ROG scores for the explanatory variable (f). A bar plot of the first PLS-ROG score for the response variable (g). Significant metabolites in the PLS-ROG analysis with effusion-synovitis scores (h). Metabolites that correlate with both the Kellgren-Lawrence grade and effusion-synovitis score are indicated with an asterisk (*)
Regarding the metabolomics results related to effusion-synovitis scores in patients with KL grades 0 and 1, the PLS-ROG analysis detected eight significantly correlated metabolites, which were alanine, cystine, 2-hydroxyvaleric acid, uric acid, methionine, tyrosine, 3-hydroxybutyric acid, and S-methylcysteine (p < 0.05; Fig. 3f–h). Among these metabolites, cystine (r = 0.151, p = 0.009, q = 0.375), uric acid (r = 0.131, p = 0.024, q = 0.403), and tyrosine (r = 0.115, p = 0.048, q = 0.437) were identified as common key metabolites associated with both the KL grade and effusion-synovitis score (Fig. 3h). Further ROC analyses showed that the cystine level was moderately associated with the presence of radiographic OA (p < 0.001; AUC 0.714; 95% CI 0.667, 0.761; cutoff value 2.23; odds ratio 3.7; Fig. 4). The quantified plasma cystine consentration had a mean value of 28.4 ± 9.1 nmol/mL and was significantly correlated with the levels of serum inflammatory markers such as high-sensitive C-reactive protein (ρ = 0.252, p < 0.001), matrix metalloproteinase 3 (ρ = 0.137, p < 0.001), interleukin-6 (ρ = 0.224, p < 0.001), and hyaluronic acid (ρ = 0.467, p < 0.001).
Receiver operating characteristic (ROC) analysis of metabolites for the presence of radiographic knee osteoarthritis. ROC analysis was performed to estimate the predictivity of cystine (a), uric acid (b), and tyrosine (c) for the presence of radiographic knee osteoarthritis. Cystine has the highest area under the curve value with a cutoff value of 2.23 and an odds ratio of 3.7. TPF, true-positive fraction; FPF, false-positive fraction
Discussion
This cross-sectional study accomplished a large sample metabolome analysis focusing on both radiographic knee OA and synovitis on MRI. Our results revealed that cystine, uric acid, and tyrosine were common metabolites related to both radiographic severity and degree of synovitis effusion. According to the ROC analysis, cystine highly reflected the presence of OA. High cystine levels may be a key biomarker in identifying patients at high risk of knee OA progression caused by synovitis. In addition, we demonstrated that a metabolomic approach could identify the key metabolites and metabolic pathways related to radiographic severity and effusion-synovitis, although the correlation was extremely weak.
Metabolomics has become an advanced method for the identification of OA biomarkers and can be used to systematically study small-molecule metabolites across biological systems. The metabolome constitutes the final downstream product of distinct regulatory complexes such as the genome, transcriptome, and proteome that are proximal to the metabolome constituting the disease phenotype [29]. Synovial fluid is an ideal specimen for OA studies because metabolite concentrations in the synovial fluid directly reflect the biological processes of the synovium or cartilage. However, obtaining synovial fluid samples is invasive and not practical for patients without joint effusion. Thus, one of the most frequently used biological samples in OA research is blood. Zhang et al. reported that correlations between blood plasma and synovial fluid metabolite concentrations are modest. Metabolite ratios, which are considered proxies for enzymatic reaction rates owing to their high correlation, should be considered when using blood plasma as a surrogate for synovial fluid in OA biomarker identification, while there are possibilities of including different metabolites or concentrations [30].
In our analyses, cystine was identified as a potential biomarker for identifying patients with both radiographic abnormality and active synovitis. Patients with active synovitis are considered a high-risk group for OA progression because synovitis leads to the secretion of several proteases and subsequent cartilage degeneration [1, 12]. Cystine is an amino acid formed from two molecules of the amino acid cysteine, which is well known as an anti-oxidative agent in the cystine-cysteine cycle [31]. Under excessive oxidative stress, cysteine is oxidized by hydrogen peroxide to cystine and transported into cells via the cystine-glutamate exchange transporter in the plasma membrane (Fig. 5) [31]. In macrophages, this transporter is also induced and activated by stimulation with reactive oxygen species [32]. The transported cystine is intracellularly reduced to cysteine and used for the synthesis of proteins and glutathione [33]. Glutathione has important roles in reducing reactive oxygen species [34]. Therefore, increased extracellular cystine levels reflect the presence of oxidative stress in or around cells. It is well known that oxidative stress causes inflammation of the synovium, thereby accelerating chondrocyte death, cartilage degeneration, and bone fragility [35]. Based on the described etiology and metabolism, cystine might be a novel biomarker for knee OA with synovitis.
Schema of the cystine-cysteine cycle in knee osteoarthritis. Under excessive oxidative stress, cysteine is oxidized by hydrogen peroxide to cystine and transported into cells via the cystine-glutamate exchange transporter in the plasma membrane, and the transported cystine is intracellularly reduced to cysteine and used for the synthesis of proteins and glutathione. Glutathione has important roles in reducing reactive oxygen species. Increased extracellular cystine levels reflect the presence of oxidative stress in or around cells. Glutamate released via the cystine-glutamate transporter damages the chondrocytes, and high glutamate levels in the synovial fluid are accompanied by severe joint destruction in animal models of osteoarthritis. Although excessive oxidative stress can be controlled via the cystine-cysteine cycle, higher glutamate would also result in worse metabolic conditions for joint homeostasis
Furthermore, glutamate released via the cystine-glutamate transporter is known to damage chondrocytes, and high glutamate levels in the synovial fluid were accompanied by severe joint destruction in an OA animal model [36]. In this model, inhibition of the cystine-glutamate transporter could prevent cartilage destruction [37]. Although excessive oxidative stress can be controlled via the cystine-cysteine cycle, higher glutamate would also result in worse metabolic conditions for joint homeostasis. From this perspective, an effective activation of the cystine-cysteine cycle without causing glutamate accumulation in the synovial fluid might be the key to reduce inflammation and the damage of chondrocytes and thereby and to alleviate the progression of the symptoms in knee OA.
Our metabolome analysis identified urea and TCA cycles as pathways related to knee OA severity; however, their correlation was very weak, and further studies on this would be needed. A previous targeted metabolome analysis showed that both cycles were key metabolic pathways for blood and synovial fluid metabolites [30]. The urea cycle, transferring nitrogen of an amino acid to the end metabolite urea in the mitochondria, is necessary to reduce ammonia toxicity. In progressed stages of OA, higher levels of synovitis are present [38], requiring more energy. To maintain the energy balance, for example, collagen and amino acid breakdown and TCA cycle might be upregulated, leading to the release of excessive amounts of amino groups. These amino groups are usually converted to ammonia, which also exerts toxicity. To reduce the excess amino group, the urea cycle might be activated. In our study, arginine levels in the KL grade 4 group were lower than those in the KL grade 0–3 group. Thus, our results showed that arginine was depleted in patients with progressed OA, similar to the findings in a previous report [39]. In a targeted metabolomics analysis. Zhang et al. found that depletion of plasma arginine is highly correlated with knee OA progression and might be decreased owing to its metabolic consumption [39]. Arginine is a semi-essential amino acid, a collagen precursor, and a natural inhibitor of cathepsins, which is considered as an important factor of OA pathogenesis [40].
Previous reports have suggested that the TCA cycle is one of the major pathways in immunometabolism [41]. The mitochondria are powerhouses of the cell, providing energy in the form of adenosine triphosphate for a range of activities including movement, cellular differentiation, cell death, regulation of signaling, and cell cycle control [42]. However, mitochondrial dysfunction and oxidative stress are hallmarks of OA [43], and increased mitochondrial DNA damage can be detected in chondrocytes from patients with OA compared with chondrocytes from healthy individuals [44]. Degeneration and inflammation sometimes limit the pyruvate metabolism in the mitochondrial TCA cycle during oxidative phosphorylation in the articular cartilage, subchondral bone, and synovium in patients with OA owing to the activity of synovial macrophages, even under aerobic conditions [45], and this may cause a stagnation of the TCA cycle, leading to a gradual accumulation of TCA intermediates in the blood in patients with high KL grades.
This study has several limitations. First, the biomarker candidates are limited to highly polar ionic metabolites because the metabolome analysis was performed using only CE-MS. Second, dietary intake, which can influence the levels of plasma metabolites, was not recorded in detail [46]. To reduce the risk of bias in our study, plasma was sampled after at least 10 h of fasting. Third, all the participants were women, which poses a risk of selection bias. The etiology of OA in men and women seems to be partially different [4]. The reduction of estrogen concentration with menopause is essential, and it influences the chondrocytes and cartilage [47, 48]. The influence of menopause should be assessed in future studies. In this study, we focused on middle-aged and older adult women because the highest prevalence of early-stage knee OA was observed in this age bracket [7]. For this reason, our results may not be completely applicable to men. Furthermore, the relationship with synovitis through all KL grades was not investigated in this cohort. Fourth, knee symptoms and functional tests were not included in this study. Patient-reported outcomes and functional scores reflect synovitis, knee symptoms, and severity of knee OA; the analysis of these data would have supplied important additional information. Fifth, in the ROC analysis, the AUC of cystine was below 0.8, which represents a fair correlation with the presence of radiographic OA. This reflects the difficulty in explaining the multifactorial etiology of this disease with one metabolite. In addition, the use of inflammatory markers and serum or plasma measurements has a risk of selection bias in this analysis. Sixth, while radiographs are very sensitive, they may not be appropriate for defining the stages of diseases because their reproducibility is considered to be low. Therefore, we added MRI evaluations to radiographic evaluations to investigate synovitis. Additionally, due to the high cost and time consumption of MRIs, they were only performed on the right knees. Finally, this was a cross-sectional study that used data collected in a cohort study; further studies with a longitudinal design are needed to reveal the causal relationships between these metabolic markers and OA.
Conclusions
In summary, large sample metabolome analyses revealed that, in women, the plasma cystine level increased with an increase in both radiographic OA severity and MRI-based synovitis stage. Thus, cystine level is a potential biomarker for patients who have knee OA with active synovitis. In contrast, the urea and TCA cycles were considered as possible key metabolic pathways related to the severity of radiographic knee OA, although the association was very weak. Further longitudinal observations might reveal the prognostic power of the identified metabolites.
Availability of data and materials
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Abbreviations
- BMI:
-
Body mass index
- OA:
-
Osteoarthritis
- PLS:
-
Partial least square
- PLS-ROG:
-
PLS with rank order of groups
- TCA:
-
Tricarboxylic acid
- ROC:
-
Receiver operating characteristic
- AUC:
-
Area under the curve
- KL:
-
Kellgren-Lawrence
- WORMS:
-
Whole-Organ MRI Scoring
References
Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthritis Cartilage. 2013;21:16–21. https://doi.org/10.1016/j.joca.2012.11.012.
Loeser RF. Aging and osteoarthritis. Curr Opin Rheumatol. 2011;23:492–6. https://doi.org/10.1097/BOR.0b013e3283494005.
Ishibashi K, Sasaki E, Ota S, Chiba D, Yamamoto Y, Tsuda E, et al. Detection of synovitis in early knee osteoarthritis by MRI and serum biomarkers in Japanese general population [Sci. rep.:12310]. Sci Rep. 2020;10:12310. https://doi.org/10.1038/s41598-020-69328-w.
Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393:1745–59. https://doi.org/10.1016/S0140-6736(19)30417-9.
Hiligsmann M, Cooper C, Arden N, Boers M, Branco JC, Luisa Brandi ML, et al. Health economics in the field of osteoarthritis: an expert’s consensus paper from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Semin Arthritis Rheum. 2013;43:303–13. https://doi.org/10.1016/j.semarthrit.2013.07.003.
Vina ER, Kwoh CK. Epidemiology of osteoarthritis: literature update. Curr Opin Rheumatol. 2018;30:160–7. https://doi.org/10.1097/BOR.0000000000000479.
Luyten FP, Bierma-Zeinstra S, Dell’Accio F, Kraus VB, Nakata K, Sekiya I, et al. Toward classification criteria for early osteoarthritis of the knee. Semin Arthritis Rheum. 2018;47:457–63. https://doi.org/10.1016/j.semarthrit.2017.08.006.
Sasaki E, Ota S, Chiba D, Kimura Y, Sasaki S, Yamamoto Y, et al. Early knee osteoarthritis prevalence is highest among middle-aged adult females with obesity based on new set of diagnostic criteria from a large sample cohort study in the Japanese general population. Knee Surg Sports Traumatol Arthrosc. 2020;28:984–94. https://doi.org/10.1007/s00167-019-05614-z.
Scanzello CR. Role of low-grade inflammation in osteoarthritis. Curr Opin Rheumatol. 2017;29:79–85. https://doi.org/10.1097/BOR.0000000000000353.
van Oudenaarde K, Jobke B, Oostveen AC, Marijnissen AC, Wolterbeek R, Wesseling J, et al. Predictive value of MRI features for development of radiographic osteoarthritis in a cohort of participants with pre-radiographic knee osteoarthritis-the CHECK study. Rheumatology (Oxford). 2017;56:113–20. https://doi.org/10.1093/rheumatology/kew368.
Guermazi A, Niu J, Hayashi D, Roemer FW, Englund M, Neogi T, et al. Prevalence of abnormalities in knees detected by MRI in adults without knee osteoarthritis: population based observational study (Framingham Osteoarthritis Study). BMJ. 2012;345:e5339. https://doi.org/10.1136/bmj.e5339.
Sellam J, Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol. 2010;6:625–35. https://doi.org/10.1038/nrrheum.2010.159.
Schüssler-Fiorenza Rose SM, Contrepois K, Moneghetti KJ, Zhou W, Mishra T, Mataraso S, et al. A longitudinal big data approach for precision health. Nat Med. 2019;25:792–804. https://doi.org/10.1038/s41591-019-0414-6.
Gowda GA, Zhang S, Gu H, Asiago V, Shanaiah N, Raftery D. Metabolomics-based methods for early disease diagnostics. Expert Rev Mol Diagn. 2008;8:617–33. https://doi.org/10.1586/14737159.8.5.617.
Dunn WB, Broadhurst D, Begley P, Zelena E, Francis-McIntyre S, Anderson N, et al. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat Protoc. 2011;6:1060–83. https://doi.org/10.1038/nprot.2011.335.
Holmes E, Loo RL, Stamler J, Bictash M, Yap IK, Chan Q, et al. Human metabolic phenotype diversity and its association with diet and blood pressure. Nature. 2008;453:396–400. https://doi.org/10.1038/nature06882.
Steinhubl SR. The future of individualized health maintenance. Nat Med. 2019;25:712–4. https://doi.org/10.1038/s41591-019-0443-1.
Ota S, Chiba D, Sasaki E, Kumagai G, Yamamoto Y, Nakaji S, et al. Symptomatic bone marrow lesions induced by reduced bone mineral density in middle-aged women: a cross-sectional Japanese population study. Arthritis Res Ther. 2019;21:113. https://doi.org/10.1186/s13075-019-1900-4.
Ohashi Y, Hirayama A, Ishikawa T, Nakamura S, Shimizu K, Ueno Y, et al. Depiction of metabolome changes in histidine-starved Escherichia coli by CE-TOFMS. Mol BioSyst. 2008;4:135–47. https://doi.org/10.1039/b714176a.
Ooga T, Sato H, Nagashima A, Sasaki K, Tomita M, Soga T, et al. Metabolomic anatomy of an animal model revealing homeostatic imbalances in dyslipidaemia. Mol BioSyst. 2011;7:1217–23. https://doi.org/10.1039/c0mb00141d.
Sugimoto M, Wong DT, Hirayama A, Soga T, Tomita M. Capillary electrophoresis mass spectrometry-based saliva metabolomics identified oral, breast and pancreatic cancer-specific profiles. Metabolomics. 2010;6:78–95. https://doi.org/10.1007/s11306-009-0178-y.
Yamamoto H, Suzuki M, Matsuta R, Sasaki K, Kang MI, Kami K, et al. Capillary electrophoresis mass spectrometry-based metabolomics of plasma samples from healthy subjects in a cross-sectional Japanese population study. Metabolites. 2021;11:314. https://doi.org/10.3390/metabo11050314.
Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. https://doi.org/10.1136/ard.16.4.494.
Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthr Cartil. 2004;12:177–90. https://doi.org/10.1016/j.joca.2003.11.003.
Yamamoto H, PLS. PLS-ROG: partial least squares with rank order of groups. J Chemom. 2017;31:e2883. https://doi.org/10.1002/cem.2883.
Draghici S, Khatri P, Martins RP, Ostermeier GC, Krawetz SA. Global functional profiling of gene expression. Genomics. 2003;81:98–104. https://doi.org/10.1016/s0888-7543(02)00021-6.
Yamamoto H, Fujimori T, Sato H, Ishikawa G, Kami K, Ohashi Y. Statistical hypothesis testing of factor loading in principal component analysis and its application to metabolite set enrichment analysis. BMC Bioinformatics. 2014;15:51. https://doi.org/10.1186/1471-2105-15-51.
Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57:289–300. https://doi.org/10.1111/j.2517-6161.1995.tb02031.x.
Zhai G, Wang-Sattler R, Hart DJ, Arden NK, Hakim AJ, Illig T, et al. Serum branched-chain amino acid to histidine ratio: a novel metabolomic biomarker of knee osteoarthritis. Ann Rheum Dis. 2010;69:1227–31. https://doi.org/10.1136/ard.2009.120857.
Zhang W, Likhodii S, Aref-Eshghi E, Zhang Y, Harper PE, Randell E, et al. Relationship between blood plasma and synovial fluid metabolite concentrations in patients with osteoarthritis. J Rheumatol. 2015;42:859–65. https://doi.org/10.3899/jrheum.141252.
Banjac A, Perisic T, Sato H, Seiler A, Bannai S, Weiss N, et al. The cystine/cysteine cycle: a redox cycle regulating susceptibility versus resistance to cell death. Oncogene. 2008;27:1618–28. https://doi.org/10.1038/sj.onc.1210796.
Sato H, Tamba M, Ishii T, Bannai S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. J Biol Chem. 1999;274:11455–8. https://doi.org/10.1074/jbc.274.17.11455.
Meister A. Glutathione metabolism and its selective modification. J Biol Chem. 1988;263:17205–8. https://doi.org/10.1016/S0021-9258(19)77815-6.
Iwata S, Hori T, Sato N, Hirota K, Sasada T, Mitsui A, et al. Adult T cell leukemia (ATL)-derived factor/human thioredoxin prevents apoptosis of lymphoid cells induced by L-cystine and glutathione depletion: possible involvement of thiol-mediated redox regulation in apoptosis caused by pro-oxidant state. J Immunol. 1997;158:3108–17.
Carlo MD, Loeser RF. Increased oxidative stress with aging reduces chondrocyte survival: correlation with intracellular glutathione levels. Arthritis Rheum. 2003;48:3419–30. https://doi.org/10.1002/art.11338.
Jean YH, Wen ZH, Chang YC, Lee HS, Hsieh SP, Wu CT, et al. Hyaluronic acid attenuates osteoarthritis development in the anterior cruciate ligament-transected knee: association with excitatory amino acid release in the joint dialysate. J Orthop Res. 2006;24:1052–61. https://doi.org/10.1002/jor.20123.
Tsai WY, Tsai RY, Liu CC, Wu JL, Wong CS. Sulfasalazine attenuates ACL transection and medial menisectomy-induced cartilage destruction by inhibition of cystine/glutamate antiporter. J Orthop Res. 2016;34:650–7. https://doi.org/10.1002/jor.23069.
Roemer FW, Kassim Javaid MK, Guermazi A, Thomas M, Kiran A, Keen R, et al. Anatomical distribution of synovitis in knee osteoarthritis and its association with joint effusion assessed on non-enhanced and contrast-enhanced MRI. Osteoarthr Cartil. 2010;18:1269–74. https://doi.org/10.1016/j.joca.2010.07.008.
Zhang W, Sun G, Likhodii S, Liu M, Aref-Eshghi E, Harper PE, et al. Metabolomic analysis of human plasma reveals that arginine is depleted in knee osteoarthritis patients. Osteoarthr Cartil. 2016;24:827–34. https://doi.org/10.1016/j.joca.2015.12.004.
Tong BC, Barbul A. Cellular and physiological effects of arginine. Mini-Rev Med Chem. 2004;4:823–32. https://doi.org/10.2174/1389557043403305.
Mobasheri A, Rayman MP, Gualillo O, Sellam J, van der Kraan P, Fearon U. The role of metabolism in the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2017;13:302–11. https://doi.org/10.1038/nrrheum.2017.50.
Shutt TE, Shadel GS. A compendium of human mitochondrial gene expression machinery with links to disease. Environ Mol Mutagen. 2010;51:360–79. https://doi.org/10.1002/em.20571.
Loeser RF, Collins JA, Diekman BO. Ageing and the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016;12:412–20. https://doi.org/10.1038/nrrheum.2016.65.
Grishko VI, Ho R, Wilson GL, Pearsall AW 4th. Diminished mitochondrial DNA integrity and repair capacity in OA chondrocytes. Osteoarthr Cartil. 2009;17:107–13. https://doi.org/10.1016/j.joca.2008.05.009.
Gomez R, Lago F, Gomez-Reino J, Dieguez C, Gualillo O. Adipokines in the skeleton: influence on cartilage function and joint degenerative diseases. J Mol Endocrinol. 2009;43:11–8. https://doi.org/10.1677/JME-08-0131.
Wu G, Morris SM Jr. Arginine metabolism: nitric oxide and beyond. Biochem J. 1998;336:1–17. https://doi.org/10.1042/bj3360001.
Xu X, Li X, Liang Y, Ou Y, Huang J, Xiong J, et al. Estrogen modulates cartilage and subchondral bone remodeling in an ovariectomized rat model of postmenopausal osteoarthritis. Med Sci Monit. 2019;25:3146–53. https://doi.org/10.12659/MSM.916254.
Richette P, Corvol M, Bardin T. Estrogens, cartilage, and osteoarthritis. Joint Bone Spine. 2003;70:257–62. https://doi.org/10.1016/s1297-319x(03)00067-8.
Acknowledgements
We thank all the members of the staff for helping us conduct the Iwaki Health Promotion Project and Editage (www.editage.jp) for English editing.
Authors’ contributions
Funding
This study was supported in part by JST COI Grant Number JPMJCE1302, Grants-in-Aid from the Japanese Society for the Promotion of Science (nos. 21500676, 18K16606, and 18K09091), Health Labor Sciences Research Grant, JOA-Subsidized Science Project Research from the Japanese Orthopaedic Association, and a grant of the Japan Orthopaedics and Traumatology Research Foundation (No. 421).
Author information
Authors and Affiliations
Contributions
E.S., H.Y., T.A., R.M., and Y.I. contributed to the conception and design of the study. E.S., H.Y., T.A., R.M., S.O., Y.K., and S.S. contributed to the acquisition of the data. E.S., H.Y., T.A., and R.M. contributed to the analysis and/or interpretation of the data. E.S., H.Y., T.A., and R.M. drafted the manuscript. K.K., Y.Y., E.T., and Y.I. revised the manuscript critically for important intellectual content. All authors approved the final version of the manuscript to be published. Y.I. is the guarantor. The corresponding author attests that all listed authors meet the authorship criteria and that no other authors meeting the criteria have been omitted.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All participants provided written informed consent. The study was conducted in agreement with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards and was approved by the ethics committee of our institute (2017-026).
Consent for publication
All authors approved the manuscript and give their consent for submission and publication.
Competing interests
H.Y., R.M., and K.K. are employees of Human Metabolome Technologies, Inc. The Department of Metabolomics Innovation is a joint research department supported by Human Metabolome Technologies, Inc. The other authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Principal component analysis plot of metabolites with Kellgren-Lawrence grade. The 99.999% confidence interval of each component is shown as a circle around the dot plot. An outlier was omitted for the statistical analysis. Figure S2. Heatmap of metabolites in relation with Kellgren-Lawrence grade. Z-scores of detected metabolites are presented in each row, and samples that were sorted with the Kellgren–Lawrence grade are presented in each column. Table S1. Results of the first PLS-ROG loading in correlation with the Kellgren-Lawrence grade. Table S2. Metabolite set enrichment analysis to detect metabolic pathway with severity of knee osteoarthritis. “All” includes the number of metabolites registered in metabolic pathway list. “Detected” includes the number of detected metabolites. “Selected” includes statistically significant metabolites with a p < 0.05. The p-value was computed by Fisher’s exact test and the q-value is its multiple comparison correction by Benjamini-Hochberg method. Table S3. Demographic data of participants according to their effusion-synovitis score. Data are presented as the mean ± standard deviation. Groups are compared by analysis of variance and Tukey test. BMI: body mass index.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sasaki, E., Yamamoto, H., Asari, T. et al. Metabolomics with severity of radiographic knee osteoarthritis and early phase synovitis in middle-aged women from the Iwaki Health Promotion Project: a cross-sectional study. Arthritis Res Ther 24, 145 (2022). https://doi.org/10.1186/s13075-022-02830-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13075-022-02830-w