Abstract
Background
Systemic sclerosis (SSc), an autoimmune disease with unknown etiology and pathogenesis, is characterized by abnormal autoimmunity, vascular dysfunction, and progressive fibrosis of skin and organs. Studies have shown that a key factor in the pathogenesis of SSc is aberrant activation of CD4+ T cells. Our previous studies have shown that a global hypomethylation state of CD4+ T cells is closely related to aberrant activation. However, the exact mechanism of hypomethylation in CD4+T cells is not yet clear.
Methods
Illumina HiSeq 2500 Platform was used to screen differentially expressed genes and explore the role of OASL, TET1, and IRF1 in the abnormal activation of CD4+T cells in SSc. Finally, double luciferase reporter gene experiments were used to analyze the interaction between IRF1 and TET1.
Results
OASL overexpression could upregulate TET1 to increase the hydroxymethylation levels of CD4+ T cells and induce high expression of functional proteins (CD40L and CD70), thus promoting CD4+T cell aberrant activation. Moreover, OASL upregulated TET1 via IRF1 signaling activation, and a double luciferase reporter gene experiment revealed that IRF1 can bind to the TET1 promoter region to regulate its expression.
Conclusions
OASL participates in the regulation of abnormal hypomethylation of CD4+ T cells in SSc, which implies a pivotal role for IFN signaling in the pathogenesis of SSc. Regulating DNA methylation and IFN signaling may serve as therapeutic treatments in SSc.
Similar content being viewed by others
Introduction
Systemic sclerosis (SSc) is an autoimmune disease characterized by autoimmune abnormalities, microangiopathy, and progressive fibrosis of the skin and various organs [1,2,3]. However, the pathogenesis of SSc has not been fully clarified. Studies have shown that CD4+ T cell aberrant activation is a key factor in the pathogenesis of SSc [4, 5]. The CD4+ T cells of SSc patients are present in an autoreactive condition, which not only stimulates B cells to produce more autoantibodies, but it also releases various cytokines, resulting in inflammation, microvascular damage, and fibrosis [2, 6, 7].
DNA methylation is a form of DNA chemical modification that can change gene expression without changing the DNA sequence [8, 9]. DNA hydroxymethylation is a new type of DNA methylation that converts methyl- to hydroxymethylcytosines by ten-eleven translocation methylcytosine dioxygenase (TET) proteins, subsequently achieving demethylation [10, 11]. Recently, DNA hydroxymethylation has been found to play a pivotal role in tumorigenesis, inflammation, and autoimmune disease [11,12,13,14,15]. Our previous studies have also found that global hypomethylation of CD4+T cells is related to their abnormal expression of functional proteins such as CD40L and CD70, thus contributing to aberrant activation of CD4+T cells in SSc [16,17,18,19,20]. However, the exact mechanism of DNA hypomethylation of CD4+T cells in SSc patients has not been clarified yet.
Type I interferon (IFN) signaling is a key regulator of the innate immune system that modulates immune cell differentiation, proliferation, and cytokine production [21, 22]. 2′-5′-oligoadenylate synthetase like (OASL) is a member of IFN signaling, often as a key antiviral factor induced by IFNs [23]. Recent studies have found that OASL may participate in the pathogenesis of the autoimmune disease [24]. SSc is also defined as an IFN signature disease, which refers to increased expression and activation of IFN-regulated genes, such as MCP1/CCL2, OAS2, IFI30, and STAT1 [25]. It is well known that CD4+ T cells from SSc patients share a substantial number of hyper-expressed genes compared to healthy controls, most of which are induced by IFN signaling [26] and are involved in the pathogenesis of SSc. It remains to be answered whether IFN signaling regulates DNA hypomethylation and thus contributes to the overexpression of immune-related genes such as CD70, CD11a, and CD40L in CD4+T cells of SSc.
In this study, we examined the different gene expression profiles of CD4+ T cells in SSc patients and healthy controls and found that the expression level of OASL was abnormally increased in CD4+ T cells of SSc patients. Furthermore, we found that the overexpression of OASL upregulated TET1, leading to pathological hydroxymethylation of CD4+ T in SSc, increasing the expression of functional proteins CD40L and CD70. In addition, OASL induced IRF1 binding to the promoter region of TET1 to upregulate its expression. These findings revealed a pivotal role for IFN signaling in regulating DNA methylation, which helps clarify the pathogenesis of SSc, and identified OASL as a potential therapeutic target in SSc.
Materials and methods
Samples and patients
SSc patients from the Department of Dermatology, Second Xiangya Hospital were diagnosed as having SSc for one year according to the 2013 ACR/EULAR classification criteria [27]. These patients had never received immunosuppressive treatment which was summarized in Table 1. The healthy controls were obtained from healthy volunteers without any autoimmune diseases. The study was approved by the Institutional Review Board of Central South University and all of the subjects provided informed consent.
CD4+T cell isolation
We collect 60ml/people peripheral blood for CD4+T cells isolation. CD4+ T cells were isolated by positive selection using CD4 immunomagnetic beads described in our previous study.
RT-qPCR
The methods of Total RNA extraction, cDNA synthesis, and RT-qPCR were described in our previous study. The sequences of primers are presented in Table 2. β-actin was used as the endogenous control, and the relative expression level of each mRNA was calculated using the 2-ΔCT method.
Western blot
A total of 2 × 106 cells were used to extract protein samples with a mixture of 100μlRIPA buffer (Beyotime, # P0013B) and 2 μl PMSF (Sigma, #329-98-6). The concentrations of protein were measured using a BCA Protein Assay Kit (Thermo Fisher Scientific, #NCI3225CH). The total protein (50 μg/sample) added in the loading buffer was boiled at 100 °C for 5 min, separated in a 10% gel at 80 V for 90 min, transferred to a membrane at 4°C using 300 mA for 85 min, and then incubated with QuickBlock™ Western (Beyotime, #P0252) for 10min. The membranes were probed with antihuman TET1 antibody (Abcam #ab191698), anti-OASL (Abcam #ab229136), anti-IRF1 (Abcam #ab191032), and anti-β-actin (Abcam #ab8227)) overnight at 4 °C. Horseradish peroxidase (HRP)-conjugated anti-rabbit IgG antibody (Abcam#) served as a secondary antibody
The level of hydroxymethylation of CD4+ T cell detection
MethylFlashTM Hydroxymethylated DNA Qμantification Kit (Colorimetric) Whole Genomic DNA Hydroxymethylation Kit was used. The protocol is performed by manuscripts. The results were read at 425 nm.
Flow cytometric analysis
Isolated CD4+ T cell suspensions (3 × 106 cells) were stained with antihuman monoclonal antibodies CD4-FITC and CD25-APC (BD, USA), a tube of CD4+T cell (1 × 106 cells) were incubated FITC-CD40L and FITC-CD70 (20μl) as described previously.
siRNA treatment
OASL, TET1, PAX5, SATA4, IRF1, IRF2, and FOXP3 siRNA is provided by Guangzhou RiboBio. The siRNA (30nM) is added in CD4+T cell for 48h.
Plasmid transfection
6 × 106 cells/well planted in the 6-well, the OASL, TET1 Flag plasmid (4μg/well), and IRF1 (5μg/well) plasmid were transfected for 48h.
Dual-luciferase reporter gene analysis
HEK293T cells were plated in a 12-well plate (6 × 105 cells/well) and cultured in DMEM medium overnight, then co-transfected with either plz1 (0 to +600 bp deletion), plz2 (0 to+100 bp deletion), plz3 (− 500 to − 300 deletion), plz4 (0 to +100 and − 500 to − 300 deletion), or plz5 (− 300 to 0bp deletion) mutIRF1 according to the manufacturer’s instructions. Dual-luciferase reporter assays were performed 48 h post-transfection using the dual-luciferase reporter assay system (Promega, #E1910, USA) according to the manufacturer’s instructions.
RNA seq
RNA were extracted from CD4+ T cells of samples using the TRIzol reagent. RNA-Seq was carried out on the Illumina HiSeq 2500 according to the manufacturer’s protocol.
DATA analysis
DESseq2 was used to perform data processing and differential gene selection on the compared count expression profiles. The BH method was used to adjust p-values, and adjusted p values of ≤ 0.05 and absolute values of log2 FoldChange ≥ 1 were used as thresholds to select differentially expressed genes. DESeq2 for GSEA analysis was used, and the selected annotation datasets were KEGG, REACTOME, and GO.BP with p values ≤ 0.05 as thresholds to select up- and downregulated pathways.
Statistical analysis
The data are presented as the mean ± SD and performed in Graphpad6.0. The comparison between each groups were made by using Student’s t test, or the nonparametric test. P < 0.05 is defined as significance (*P < 0.05, **P < 0.01,***P < 0.001,****P < 0.0001).
Results
Differentially expressed genes in peripheral blood CD4+ T cells between SSc patients and healthy controls
To elucidate the function and molecular changes of CD4+ T cells in the pathogenesis of SSc, we first isolated CD4+ T cells from five SSc patients and five age- and sex-matched healthy donors (HDs), and used the Illumina HiSeq 2500 Platform to screen differentially expressed genes (DEGs). After screening and a comparison based on the fold change and false discovery rate (FDR), we use volcano graphs to visualize the expression levels of differential genes between SSc patients and HDs (Fig. 1a). There were a total of 260 DEGs between SSc patients and healthy controls. Of these, 175 genes were upregulated and 85 downregulated (Fig. 1b). To identify the specific pathways that were altered, we performed GO analysis and observed that these genes were mainly enriched in the type I interferon signaling pathway and in response to viruses, as well as in the regulation of lymphocyte activation. KEGG pathway analysis highlighted measles, cytokine-cytokine receptor interaction, RIG-I-like receptor signaling, and pyrimidine metabolism (Fig. 1c, d). Among these genes, we identified two IFN-related genes (OASL and IRF1) that were upregulated in SSc patients and may be involved in the pathogenesis of SSc. We expanded the sample size to verify the expression levels of OASL and IRF1 in CD4+ T cells. The results showed that the mRNA expression levels of OASL and IRF1 were indeed increased in CD4+T cells of SSc patients (Fig. 1e, f), as were the protein levels (Fig. 1g–i).
Identification of differentially expressed genes between systemic sclerosis and normal. a Volcano plot of the differential gene expression analysis. X-axis: fold change difference (log 2 scale); y-axis: BH-adjusted p values for each probe (-log10 scale). b Hot map for differential gene expression analysis. The vertical dotted lines represent an absolute cutoff value of 1.5-fold change. The horizontal dotted line represents the significant cutoff of p < 0.05. c GO enrichment analysis of DEGs; d KEGG pathway analysis of DGEs. The horizontal axes shows -log10 transformed P value and p < 0.05 is considered significant. e, f The mRNA expression level of OASL and IRF1 in CD4+T cells of SSc patients (n=15). g–i The protein expression level of OASL and IRF1 in CD4+T cells Of SSc patients (n=6)
Overexpression of OASL upregulates CD40L and CD70 expression levels via increasing DNA hydroxymethylation in CD4+ T cells of SSc patients
It has recently been found that OASL may act as a biomarker or predictor of therapeutic responses in autoimmune disease [24, 28,29,30,31]. In previous studies, we found that hypomethylation of CD40L and CD70 in their promoter regions contributes to their overexpression in CD4+ T cells of SSc patients and induces an aberrant autoimmune response [16, 17]. We first confirmed that the overall level of hydroxymethylation significantly increased in CD4+T cells of SSc patients (Fig. 2a). We next sought to identify whether overexpression of OASL is involved in this pathological process. We transfected normal CD4+ T cells with OASL-Flag recombinant plasmids to confirm whether OASL overexpression can mimic the abnormal activation state in CD4+ T cells of SSc. The results showed that the overall level of hydroxymethylation significantly increased in normal CD4+ T cells after OASL plasmid transfection (Fig. 2b). Also, the expression levels of CD40L and CD70 significantly increased (Fig. 2c–e).
Overexpression of TET1 upregulated the CD40L and CD70 expression level via increasing of DNA hydroxymethylation in CD4+ T cells of SSc patients. a Global 5-hydroxymethylcytosine level of CD4+T cells in SSc patients (n=10). b The hydroxymethylation level of CD4+ T cells after OASL-Flag recombinant plasmid treatment in normal CD4+T cells. c The mRNA expression level of CD40L and CD70 in CD4+T cells of SSc. d–f The percentage of CD40L and CD70 staining cells (n=6)
TET1 overexpression upregulates CD40L and CD70 expression levels via increased DNA hydroxymethylation in CD4+ T cells of SSc patients
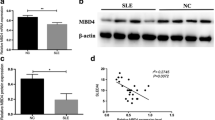
TET1 is a member of the TET family that can oxidize 5-methylcytosine (5mC) to 5-hydroxymethylcytosine (5hmC), thereby driving the completion of DNA demethylation. Recently, Tet protein has been considered to be the main mediator of the increase in global 5hmC levels and the present hypomethylation state in cells, which plays an important role in the process of tumorigenesis, inflammation, and autoimmune diseases [32, 33]. We hypothesized that the DNA demethylase TET1 also participates in hypomethylation in CD4+ T cells of SSc patients. We first identified its expression levels in SSc patients and normal healthy controls (Fig. 3a, b) and found that TET1 is enhanced in CD4+ T cells of SSc. We further explored the exact role of TET1 in the activation of CD4+ T cells. Our findings demonstrated that overexpression of TET1 with a plasmid can upregulate the hydroxymethylation level (Fig. 3c) in addition to the CD40L and CD70 expression levels in normal CD4+ T cells (Fig. 3d–h). These results indicate that TET1 may participate in the pathogenesis of SSc via increasing hydroxymethylation levels in CD4+ T cells.
Overexpression of OASL upregulated the CD40L and CD70 expression level via increasing of DNA hydroxymethylation in CD4+ T cells of SSc patients. a, b The expression level of Tet1 in CD4+T cells of SSc. c The hydroxymethylation level of CD4+ T cells after TET1-Flag recombinant plasmid treatment in normal CD4+T cells. d, e The mRNA expression level of CD40L and CD70 in CD4+T cells after OASL-Flag recombinant plasmid treatment in normal CD4+T cells. f–h The percentage of CD40L and CD70 staining cells after OASL-Flag recombinant plasmid treatment in normal CD4+T cells (n=6)
OASL upregulates the expression level of TET1 in CD4+ T cells of SSc patients
Since we found that the overexpression of OASL and TET1 commonly increase the hydroxymethylation level of CD4+ T cells, we wondered whether OASL could regulate the expression of TET1. We first silenced OASL expression to observe whether it influenced the expression of TET1. The results showed that the expression level of TET1 decreased after OASL-siRNA treatment of CD4+ T cells of SSc patients (Fig. 4a, b), while the overexpression of OASL increased TET1 expression levels in normal CD4+ T cells (Fig. 4c–e).
OASL upregulates expression level of TET1 via IRF1 signaling
To explore the exact mechanism of how OASL regulates TET1, we selected several possible immune-related transcription factors (PAX5, STAT4, IRF1, IRF2, and FOXP3) by analyzing the 5′-UTR region of Tet1. We then detected the expression levels of TET1 after interfering with these transcription factors in normal CD4+ T cells transfected with an OASL-Flag recombinant plasmid. The results showed that the ability of OASL to upregulate TET1 expression was significantly weakened only after IRF1 was silenced in normal CD4+ T cells transfected with the OASL-Flag recombinant plasmid (Fig. 5a, b). These results were consistent with our expression profile chip in which IRF1 is upregulated in CD4+ T cells of SSc. Then, to explore the exact role of IRF1 in the mechanism by which OASL regulates TET1 expression, we transfected an IRF1-myc recombinant plasmid in normal human CD4+ T cells. The results showed that the expression levels of Tet1 mRNA and protein were significantly increased in normal human CD4+ T cells transfected with the IRF1-myc recombinant plasmid; however, the expression of OASL was not influenced (Fig. 5c–e). We used the transcription start site as the 0 position and constructed a full-length 600 bp deletion in the 5’-UTR region of Tet1 (plz1), a 0 to +100 deletion (plz2), a − 500 to − 300 deletion (plz3), a 0 to +100 deletion combined with a − 500 to − 300 segment deletion (plz4), and a − 300 to 0 deletion (plz5). These plasmids and IRF1-myc plasmid were co-transformed into HEK293 cells. The dual-luciferase reporter assay shows that plz1-4 could obviously generate fluorescent signals, while the plz5 plasmid lacking the −300 to 0 fragment did not generate clear fluorescent signals (Fig. 5f, g). These results indicate that IRF1 could upregulate TET1 by binding to the promoter region of TET1.
OASL upregulated TET1 via stimulating IRF1 signaling. a, b The mRNA expression level of Tet1 after transcription factors silenced in normal CD4+T cells transfected with OASL-Flag recombinant plasmid. c–e The expression level of TET1 in normal CD4+T cells after transfected with IRF1-Flag recombinant plasmid. f Conservation analysis of IRF1 binding sites in TET1 promoter. g Dual-luciferase reporter assay detected relative luciferase activities after cotransfection of five truncated IRF1 promoters with pcDNA3.1 vector in HEK293T cells (n=3)
Discussion
Our previous study confirmed that CD4+ T cells of SSc patients have global hypomethylation [20], and we further found that DNA hypomethylation in the promoter regions of immune-related genes, such as CD40L and CD70, contribute to their hyperexpression and promote abnormal activation of CD4+ T cells [17, 19, 20]. However, the exact mechanism by which CD4+ T cells acquire pathological hypomethylation is not fully known. TET1 is a member of the TET family, which consists of enzymes that oxidize 5mC to 5hmC, eventually leading to DNA demethylation. Lu et al. found that the overexpression of TET2 and TET3 promotes global DNA hydroxymethylation, thus inducing the overexpression of many immune-related genes in CD4+ T cells of SLE patients [34]. Hattori et al. have shown that TET1, but not TET2 or TET3, is upregulated in skin dermal fibroblasts of SSc patients and contributes to global DNA hypomethylation [35]. These data indicate that TET proteins participate in the pathogenesis of autoimmune diseases through DNA methylation [36,37,38]. We wondered whether the DNA demethylase TET1 plays a pathogenic role in the aberrant activation of CD4+ T cells in SSc. Our study supports the above findings and provides further molecular details. In the present research, we found that upregulation of TET1 enhances global DNA hydroxymethylation and increases the expression of immune-related genes CD40L and CD70, eventually promoting the aberrant activation of CD4+ T cells in SSc patients.
On this basis, we further explored factors upstream of TET1 to identify DEGs that might regulate it in CD4+ T cells in SSc patients. We identified OASL as significantly upregulated in CD4+ T cells of SSc patients by using expression profiling chips. OASL is a 2′-5′ oligoadenylate synthase (OAS) family member that is a key antiviral factor induced by IFNs [23, 39, 40]. OASL is essential to fight viral infections in the innate and adaptive immune responses, usually via IFN signaling [23], and has recently been shown to be a biomarker or predictor of therapeutic responses in autoimmune diseases. Some studies have suggested that OASL and OAS2 are upregulated in PBMCs and skin tissue of SSc patients [41], and OAS family genes are also increased in CD31+/CD102+ lung microvascular endothelial cells from SSc patients with end-stage interstitial lung disease [42]. However, these studies have not clarified the mechanism by which OASL is involved in the pathogenesis of SSc. We revealed that OASL promoted global DNA hydoxymethylation levels, enhanced CD40L and CD70 expression levels, and induced the activation of CD4+ T cells. Moreover, we also found that OASL overexpression could upregulate TET1 in normal CD4+ T cells and that OASL silencing inhibited TET1 expression in CD4+ T cells of SSc patients, which suggests that OASL may play a pathogenic role in the activation of CD4+T cells in SSc through DNA hydroxymethylation mediated by TET1.
In addition to OASL, most of the DEGs screened out through our expression profiling chips in CD4+T cells were enriched in the I-IFN pathway (see Fig. 1c). Interferon regulatory factors (IRFs) are transcription regulators that contain a conserved helix-turn-helix DNA binding motif that recognizes IFN-stimulated response elements (ISRE) in the promoter regions of other genes that participate in many cellular processes, including proliferation, response to tumors and viruses, and immune regulation [43, 44]. IRF1 is an IRF that is a master regulator of type I IFN signaling, which could be regulated by OASL through the RIG-1 pathway [23, 45, 46]. Recently, a GWAS study identified IRF1 as a susceptibility gene in SSc [47]. Our findings also demonstrated that the expression level of IRF1 is enhanced in CD4+ T cells of SSc. Interestingly, there is a predicted IRF1 binding site in the TET1 promoter region. To determine whether OASL upregulates TET1 expression via IRF1, we further explored the functional relationship between TET1 and IRF1. Our data showed that overexpression of IRF1 could significantly stimulate the expression of TET1 in normal CD4+ T cells. In addition, a high expression level of IRF1 was verified in CD4+ T cells of SSc patients. Moreover, IRF1 silencing reversed the overexpression of TET1, but not OASL, in CD4+ T cells of SSc patients. Importantly, the dual-luciferase reporter assay confirmed that IRF1 can bind to the promoter region of TET1. These observations demonstrate the importance of OASL-IRF1 induced abnormal immune responses in SSc. To the best of our knowledge, this is the first study to identify the regulatory role of IFN signaling in CD4+ T cells with aberrant activation via DNA methylation in SSc.
Conclusions
In this study, we demonstrated that OASL, IRF1, and the hypomethylation-related gene TET1 were significantly upregulated in CD4+ T cells of SSc patients. OASL upregulates TET1 to increase the global DNA hydroxymethylation level, enhancing CD40L and CD70 expression levels and inducing aberrant activation of CD4+ T cells. Importantly, we identified IRF1 as a key transcriptional regulator between OASL and TET1. We found that OASL could upregulate TET1 expression via IRF1 signaling activation, and IRF1 knockdown ameliorated the TET1 expression level, but did not influence OASL. These results demonstrate that OASL mediates global hydoxymethylation and induces abnormal activation of CD4+ T cells in SSc, which is thought to upregulate TET1 expression through IRF1 signaling activation.
Taken together, these data reveal a pathogenic role for OASL and its possible mechanism in the abnormal activation of CD4+T cells. We conclude that type I IFN signaling and its downstream pathway factors OASL and IRF1 are important mediators in the pathogenesis of SSc by regulating DNA methylation. OASL and IRF1 may therefore serve as potential therapeutic targets in SSc.
Availability of data and materials
The analyzed data sets generated during the present study are available from the corresponding author on reasonable request.
Abbreviations
- SSc:
-
Systemic sclerosis
- TET:
-
Ten-eleven translocation methylcytosine dioxygenase
- IFN:
-
Type I interferon
- OASL:
-
2′-5′-oligoadenylate synthetase like
- HRP:
-
Horseradish peroxidase
- DEGs:
-
Differentially expressed genes
- FDR:
-
False discovery rate
- 5mC:
-
5-methylcytosine
- 5hmC:
-
5-hydroxymethylcytosine
- IRFs:
-
Interferon regulatory factors
References
Denton CP, Khanna D. Systemic sclerosis. Lancet. 2017;390(10103):1685–99.
Brown M, O’Reilly S. The immunopathogenesis of fibrosis in systemic sclerosis. Clin Exp Immunol. 2019;195(3):310–21.
Stern EP, Denton CP. The pathogenesis of systemic sclerosis. Rheum Dis Clin North Am. 2015;41(3):367–82.
Chizzolini C, Brembilla NC, Montanari E, Truchetet ME. Fibrosis and immune dysregulation in systemic sclerosis. Autoimmun Rev. 2011;10(5):276–81.
Luo Y, Wang Y, Shu Y, Lu Q, Xiao R. Epigenetic mechanisms: an emerging role in pathogenesis and its therapeutic potential in systemic sclerosis. Int J Biochem Cell Biol. 2015;67:92–100.
Frantz C, Auffray C, Avouac J, Allanore Y. Regulatory T cells in systemic sclerosis. Front Immunol. 2018;9:2356.
Zhang M, Zhang S. T cells in fibrosis and fibrotic diseases. Front Immunol. 2020;11:1142.
Auclair G, Weber M. Mechanisms of DNA methylation and demethylation in mammals. Biochimie. 2012;94(11):2202–11.
Hendrich B, Tweedie S. The methyl-CpG binding domain and the evolving role of DNA methylation in animals. Trends Genet. 2003;19(5):269–77.
Ross SE, Bogdanovic O. TET enzymes, DNA demethylation and pluripotency. Biochem Soc Trans. 2019;47(3):875–85.
Rasmussen KD, Helin K. Role of TET enzymes in DNA methylation, development, and cancer. Genes Dev. 2016;30(7):733–50.
Johnson ND, Conneely KN. The role of DNA methylation and hydroxymethylation in immunosenescence. Ageing Res Rev. 2019;51:11–23.
Johnson ND, Huang L, Li R, Li Y, Yang Y, Kim HR, et al. Age-related DNA hydroxymethylation is enriched for gene expression and immune system processes in human peripheral blood. Epigenetics. 2020;15(3):294–306.
Chen H-Q, Chen D-J, Li Y, Yuan W-B, Fan J, Zhang Z, et al. Epigenetic silencing of TET1 mediated hydroxymethylation of base excision repair pathway during lung carcinogenesis. Environ Pollut. 2021;268(Pt B):115860.
Surace AEA, Hedrich CM. The role of epigenetics in autoimmune/inflammatory disease. Front Immunol. 2019;10:1525.
Lian X, Xiao R, Hu X, Kanekura T, Jiang H, Li Y, et al. DNA demethylation of CD40L in CD4+ T cells from women with systemic sclerosis: a possible explanation for female susceptibility. Arthritis Rheum. 2012;64(7):2338–45.
Jiang H, Xiao R, Lian X, Kanekura T, Luo Y, Yin Y, et al. Demethylation of TNFSF7 contributes to CD70 overexpression in CD4+ T cells from patients with systemic sclerosis. Clin Immunol. 2012;143(1):39–44.
Wang YY, Wang Q, Sun XH, Liu RZ, Shu Y, Kanekura T, et al. DNA hypermethylation of the forkhead box protein 3 (FOXP3) promoter in CD4+ T cells of patients with systemic sclerosis. Br J Dermatol. 2014;171(1):39–47.
Wang Y, Shu Y, Xiao Y, Wang Q, Kanekura T, Li Y, et al. Hypomethylation and overexpression of ITGAL (CD11a) in CD4(+) T cells in systemic sclerosis. Clin Epigenetics. 2014;6(1):25.
Lei W, Luo Y, Lei W, Luo Y, Yan K, Zhao S, et al. Abnormal DNA methylation in CD4+ T cells from patients with systemic lupus erythematosus, systemic sclerosis, and dermatomyositis. Scand J Rheumatol. 2009;38(5):369–74.
Hall JC, Rosen A. Type I interferons: crucial participants in disease amplification in autoimmunity. Nat Rev Rheumatol. 2010;6(1):40–9.
Jiang J, Zhao M, Chang C, Wu H, Lu Q. Type I interferons in the pathogenesis and treatment of autoimmune diseases. Clin Rev Allergy Immunol. 2020;59(2):248–72.
Ghosh A, Shao L, Sampath P, Zhao B, Patel NV, Zhu J, et al. Oligoadenylate-synthetase-family protein OASL inhibits activity of the DNA sensor cGAS during DNA virus infection to limit interferon production. Immunity. 2019;50(1):51–63.e5.
Gao F, Tan Y, Luo H. MALAT1 is involved in type I IFNs-mediated systemic lupus erythematosus by up-regulating OAS2, OAS3, and OASL. Braz J Med Biol Res. 2020;53(5):e9292.
Wu M, Assassi S. The role of type 1 interferon in systemic sclerosis. Front Immunol. 2013;4:266.
Eloranta M-L, Franck-Larsson K, Lövgren T, Kalamajski S, Rönnblom A, Rubin K, et al. Type I interferon system activation and association with disease manifestations in systemic sclerosis. Ann Rheum Dis. 2010;69(7):1396–402.
van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, et al. 2013 classification criteria for systemic sclerosis: an American college of rheumatology/European league against rheumatism collaborative initiative. Ann Rheum Dis. 2013;72(11):1747–55.
Ishizu A, Tomaru U, Masuda S, Sada K-E, Amano K, Harigai M, et al. Prediction of response to remission induction therapy by gene expression profiling of peripheral blood in Japanese patients with microscopic polyangiitis. Arthritis Res Ther. 2017;19(1):117.
Juárez-Vicuña Y, Pérez-Ramos J, Adalid-Peralta L, Sánchez F, Martínez-Martínez LA, Ortiz-Segura MDC, et al. Interferon Lambda 3/4 (IFNλ3/4) rs12979860 polymorphisms is not associated with susceptibility to systemic lupus erythematosus, although it regulates OASL expression in patients with SLE. Front Genet. 2021;12:647487.
Al-Nashmi M, Taha S, Alsharoqi I, Bakhiet M. Interleukin 1 receptor antagonist and 2’-5’-oligoadenylate synthetase-like molecules as novel biomarkers for multiple sclerosis patients in Bahrain. Mult Scler Relat Disord. 2017;18:1–7.
Zhang Y, Wang H, Mao X, Guo Q, Li W, Wang X, et al. A novel gene-expression-signature-based model for prediction of response to Tripterysium glycosides tablet for rheumatoid arthritis patients. J Transl Med. 2018;16(1):187.
Jeschke J, Collignon E, Fuks F. Portraits of TET-mediated DNA hydroxymethylation in cancer. Curr Opin Genet Dev. 2016;36:16–26.
Branco MR, Ficz G, Reik W. Uncovering the role of 5-hydroxymethylcytosine in the epigenome. Nat Rev Genet. 2011;13(1):7–13.
Zhao M, Wang J, Liao W, Li D, Li M, Wu H, et al. Increased 5-hydroxymethylcytosine in CD4(+) T cells in systemic lupus erythematosus. J Autoimmun. 2016;69:64–73.
Hattori M, Yokoyama Y, Hattori T, Motegi S, Amano H, Hatada I, et al. Global DNA hypomethylation and hypoxia-induced expression of the ten eleven translocation (TET) family, TET1, in scleroderma fibroblasts. Exp Dermatol. 2015;24(11):841–6.
Li J, Li L, Sun X, Deng T, Huang G, Li X, et al. Role of Tet2 in regulating adaptive and innate immunity. Front Cell Dev Biol. 2021;9:665897.
Tanaka S, Ise W, Inoue T, Ito A, Ono C, Shima Y, et al. Tet2 and Tet3 in B cells are required to repress CD86 and prevent autoimmunity. Nat Immunol. 2020;21(8):950–61.
Lagos C, Carvajal P, Castro I, Jara D, González S, Aguilera S, et al. Association of high 5-hydroxymethylcytosine levels with Ten Eleven Translocation 2 overexpression and inflammation in Sjögren's syndrome patients. Clin Immunol. 2018;196:85–96.
Zhu J, Ghosh A, Sarkar SN. OASL-a new player in controlling antiviral innate immunity. Curr Opin Virol. 2015;12:15–9.
Choi UY, Kang J-S, Hwang YS, Kim Y-J. Oligoadenylate synthase-like (OASL) proteins: dual functions and associations with diseases. Exp Mol Med. 2015;47:e144.
de Freitas Almeida GM, Oliveira DB, Botelho LM, Silva LKS, Guedes ACM, Santos FPST, et al. Differential upregulation of human 2’5’OAS genes on systemic sclerosis: detection of increased basal levels of OASL and OAS2 genes through a qPCR based assay. Autoimmunity. 2014;47(2):119–26.
Piera-Velazquez S, Mendoza FA, Addya S, Pomante D, Jimenez SA. Increased expression of interferon regulated and antiviral response genes in CD31+/CD102+ lung microvascular endothelial cells from systemic sclerosis patients with end-stage interstitial lung disease. Clin Exp Rheumatol. 2021;39(6):1298–306.
Yang M-Q, Du Q, Varley PR, Goswami J, Liang Z, Wang R, et al. Interferon regulatory factor 1 priming of tumour-derived exosomes enhances the antitumour immune response. Br J Cancer. 2018;118(1):62–71.
Nakaoka H, Mogi M, Suzuki J, Kan-No H, Min L-J, Iwanami J, et al. Interferon regulatory factor 1 attenuates vascular remodeling; roles of angiotensin II type 2 receptor. J Am Soc Hypertens. 2016;10(10):811–8.
Schoggins JW, Wilson SJ, Panis M, Murphy MY, Jones CT, Bieniasz P, et al. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature. 2011;472(7344):481–5.
Zhu J, Zhang Y, Ghosh A, Cuevas RA, Forero A, Dhar J, et al. Antiviral activity of human OASL protein is mediated by enhancing signaling of the RIG-I RNA sensor. Immunity. 2014;40(6):936–48.
González-Serna D, Ochoa E, López-Isac E, Julià A, Degenhardt F, Ortego-Centeno N, et al. A cross-disease meta-GWAS identifies four new susceptibility loci shared between systemic sclerosis and Crohn’s disease. Sci Rep. 2020;10(1):1862.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science, Foundation of China (NO.81371744, No.81773333, No.82073449, No.82003363, No.82001738), Hunan Development and Reform Commission Innovation Project (2019412), Fundamental Research Funds for the Central Universities of Central South University (No.2020zzts290).
Author information
Authors and Affiliations
Contributions
ZZ: investigation, formal analysis, project administration; YW: methodology, resources; YX: methodology, data curation, resources; J Z: data curation, software; RL: formal analysis, methodology; XH: methodology, resources, data curation, resources; JY: software; BT: software, data curation; XQ: methodology; RT: data curation; RX: writing e review and editing, project administration; YS: writing the original draft, formal analysis. The author(s) read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Institutional Review Board of Central South University and all of the subjects provided informed consent.
Consent for publication
The results presented in this paper have not been published previously in whole or in part.
Competing interests
The authors declare that they have no known competing, financial interests, nor personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zeng, Z., Wang, Y., Xiao, Y. et al. Overexpression of OASL upregulates TET1 to induce aberrant activation of CD4+ T cells in systemic sclerosis via IRF1 signaling. Arthritis Res Ther 24, 50 (2022). https://doi.org/10.1186/s13075-022-02741-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13075-022-02741-w