Abstract
Aim
The aim of this study was to evaluate the association between periodontal parameters related with the periodontal disease severity and the presence and levels of anti-citrullinated protein antibodies (ACPAs) in rheumatoid arthritis (RA) patients.
Materials and methods
This cross-sectional study included 164 RA patients. Socio-demographics and RA disease characteristics, including ELISA-detected ACPA (anti-CCP-2), were recorded. Exposure was assessed by periodontal parameters: plaque index (PI), bleeding on probing (BoP), probing pocket depth, and clinical attachment levels (CAL). Presence and levels of ACPAs (outcome) and exposure variables were compared by both parametric and non-parametric tests and associations were evaluated by adjusted odds ratio (OR).
Results
A significant association was observed between the presence of anti-CCP antibodies and severity of periodontal outcomes such as the mean CAL (OR 1.483, p = 0.036), mean PI (OR 1.029, p = 0.012), and the number of pockets ≥ 5 mm (OR 1.021, p = 0.08). High anti-CCP antibodies levels were associated with mean CAL, mean PI, and number of pockets ≥ 5 mm with an OR of 1.593 (p = 0.043), 1.060 (p < 0.001), and 1.031 (p = 0.031), respectively. Furthermore, a significant increase of 4.45 U/mL in anti-CCP antibodies levels (p = 0.002) in RA patients was found for each pocket ≥ 5 mm after adjusting for age, gender, smoking, time of disease evolution, and RA activity.
Conclusions
In RA patients, the severity of periodontal conditions such as mean CAL, mean PI, and the number of pockets ≥ 5 mm were linearly associated with both the presence and levels of anti-CCP antibodies.
Similar content being viewed by others
Key messages
-
1)
Periodontitis severity parameters such as CAL and pockets ≥ 5 mm are associated with anti-CCP antibodies levels.
-
2)
This association was more pronounced in patients with higher levels of these antibodies.
-
3)
There is a linear correlation between periodontal parameters such as pockets ≥ 5 mm and anti-CCP titers.
Introduction
Rheumatoid arthritis (RA) is a chronic systemic autoimmune disease characterized by painful joint inflammation, disability, and increased mortality [1]. Although knowledge of the pathogenesis underlying RA has increased substantially during the last decade, its etiology is still unknown. A complex interplay of genetic, environmental, and hormonal factors seem to influence the host immune tolerance leading to the characteristic autoimmune response of RA mainly characterized by the presence of rheumatoid factor (RF) and anti-citrullinated protein antibodies (ACPAs). These factors may also affect the mucosal surfaces of lungs, gut, and/or the periodontium [2, 3].
While citrullination, a post-translational protein modification caused by the enzyme peptidyl arginine deiminase (PAD), is not an exclusive process of RA, the formation of ACPAs is mainly restricted to RA patients. In fact, evidence strongly suggests that these antibodies are markers of more aggressive disease [4,5,6]. Although other factors may be able to induce protein citrullination [7], Porphyromonas gingivalis a key pathogen associated with the pathogenesis of periodontitis by inducing dysbiotic changes in the subgingival biofilm [8, 9] releases a specific deaminase, which has been linked to protein citrullination, thereby with potential to stimulate ACPA formation in RA patients [10, 11]. In fact, in patients with chronic periodontitis, high levels of citrullinated proteins have been identified within the periodontal tissues [12] and a recent in vivo experimental study has demonstrated a positive correlation between P. gingivalis-induced periodontitis and anti-cyclic citrullinated peptide (anti-CCP) antibodies levels in rats [13]. Furthermore, recently published evidence suggests that the presence of P. gingivalis infections may precede the clinical onset of RA years in advance [14].
Nowadays, there is solid evidence supporting an epidemiological association between periodontitis and RA [15,16,17,18,19,20,21]. Recently, our group has shown a significant and consistent association between these two diseases, mainly between severe periodontitis and RA disease activity [21]. Nonetheless, the relationship between P. gingivalis, periodontitis, and the presence of ACPAs has been a controversial topic. Some authors have remarked upon the specific role of P. gingivalis in shaping autoantibody specificity in RA patients [10, 22] independently of smoking status [10]. In fact, anti-CCP antibodies have been identified in periodontitis patients without RA [23, 24]. However, there are some studies that have not supported this association between periodontitis, the presence of P. gingivalis, and seropositivity for anti-CCP antibodies [10, 25, 26].
It was, therefore, the main objective of this cross-sectional study to further investigate the link between periodontitis and its severity with the presence and levels of anti-CCP antibodies in RA patients. In addition, the possible impact of shared risk factors as tobacco habit on the presence and levels of anti-CCP antibodies was evaluated.
Methods
Study population
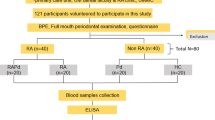
Study subjects were consecutively included from those outpatients attending the Department of Rheumatology from January to September 2016. RA patients aged 18 years and older who fulfilled the 2010 ACR/EULAR classification criteria [27] were invited to participate. Patients with less than 8 teeth [17], who had received periodontal or antibiotic treatment during the previous 6 months [28], who had undergone joint replacement(s), who were in need of antibiotic prophylaxis, or who were being treated with cyclosporine A or anticonvulsants [29] were excluded. Patients treated with oral glucocorticoids maintained a stable dose during the month prior to their periodontal assessment and none received systemic treatment with these products during this period. RA patients who received intraarticular glucocorticoids during the month prior to evaluation were excluded from this study.
All enrolled patients were informed about the objectives and characteristics of the study and signed a written informed consent that had been previously approved by the Ethics Committee of the Hospital Universitario de Canarias (code 2015_069). Additionally, all the study recordings were performed in agreement with the principles of the Declaration of Helsinki.
Study design
This was an observational, cross-sectional study of RA patients treated in a single Rheumatology Department who were assessed for the presence and severity of periodontitis.
Medical examination
All RA patients were subjected to a routine medical examination. Both the patient’s and care provider’s global assessments of disease activity were based on a 100-mm visual analog scale (VAS). Disease activity was calculated by means of 28-joint Disease Activity Score (DAS28), DAS28-C-reactive protein (CRP) [30], and Simplified Disease Activity Index (SDAI) [31] scores, and the medications used were logged from the medical files and by asking the patient during examination. Blood samples were tested for plasma RF and CRP, using an immunoturbidimetric assay (Roche/cobas® 8000 Modular Analyzer Series, Roche Diagnostics, USA), and for ACPA (anti-CCP-2) by Immunoscan CCPlus®. Euro Diagnostica with a positive value established as that exceeding 25 U/mL in both serological tests, and 3 mg/L in the CRP test. Anti-CCP antibody levels were stratified as low (between 25 and 75 U/mL), moderate (76–300 U/mL), or high (> 300 U/mL).
Periodontal examination
Full oral and periodontal examinations were carried out independently by two experienced periodontists. A kappa test showed 70% inter-examiner concordance. Full mouth probing pocket depth (PPD) and clinical attachment level (CAL) measurements were registered using an UNC-15 periodontal probe (six sites per tooth), excluding third molars and implants. Although subjects were informed of their periodontal status and advised to seek periodontal therapy when appropriate, no periodontal therapy was rendered as part of this investigation.
Study variables
To evaluate RA, the following parameters were recorded: DAS28 using the erythrocyte sedimentation rate (ESR) (DAS28) or C-reactive protein (DAS28-CRP) [30], SDAI [31], RF, and ACPA presence and titers. Patients were categorized as being in remission, or having low, moderate, and high disease activity when at least two of the DAS28, DAS28-CRP, and SDAI scores were in agreement with the level of disease activity [21]. According to the time of disease evolution, patients with less than 2 years were classified as early RA.
Patient periodontal status was assessed using the following parameters: Full mouth plaque index (PI) and bleeding on probing (BoP) [32] were reported as mean percent of plaque and gingivitis, respectively [33], PPD, mean clinical attachment level (CAL), and tooth loss (dental implants and third molars were excluded). Based on these parameters, patients were categorized using two different case definition criteria for periodontitis: the 2005 Tonetti’s definition [34], which establishes 3 levels—level 0, individuals with a healthy periodontium; level 1, presence of proximal attachment loss ≥ 3 mm in ≥ 2 nonadjacent teeth; and level 2, presence of proximal attachment loss ≥ 5 mm in ≥ 30% of teeth—and the 2018 Tonetti’s definition [35], which grades periodontitis mainly based on the interdental CAL at the site of greatest loss and also on radiographic bone loss and on tooth loss, over 5 stages—stage 0, individuals with a healthy periodontium; stage I, initial; stage II, moderate; stage III, severe; and stage IV, advanced periodontitis.
The presences of co-morbidities, such as diabetes mellitus, osteoporosis, myocardial infarction, or dyslipidemia were recorded, as were anthropometric and socioeconomic variables. These included body mass index (BMI); smoking status (none, former and current); stress via the Perceived Stress Scale (PSS-14), categorized as high stress: yes (> 28 points) or no (≤ 28 points) [36, 37]; and social welfare indicators using the Graffar Scale Questionnaire [38]. History of therapy involving glucocorticoids, synthetic disease-modifying antirheumatic drugs (sDMARDs), and biologic DMARDs (bDMARDs) were also recorded.
Statistical analyses
Descriptive data are presented as means, standard deviations (SD), and frequency distributions. t test and one-way ANOVA were used in continuous variables to analyze inter-group comparisons. Chi-square tests were used to compare categorical variables and when expected values were less than 5; the Fisher’s exact test in 2 × 2 tables was used.
To study the association between the presence of periodontitis (exposure) and anti-CCP antibody presence (outcome), a logistic regression model was constructed calculating the odds by means of odds ratios (OR), and 95% confidence intervals. Each analysis was adjusted for possible confounders (covariates) such as age, sex, tobacco use, time of disease evolution, and disease activity.
In addition, a logistic regression model with the backward Wald variable selection method was used to analyze the influence of periodontal variables over the presence of anti-CCP antibody, adjusting for the same covariates. Hence, ROC (receiver operating characteristic) curves and the areas under those curves (AUC-ROC) were analyzed.
In order to investigate the relationship between periodontal parameters as mean PI (in %), number of pocket ≥ 5 mm, BoP, and mean CAL (exposure) and anti-CCP antibody levels (multinomial outcome), an ordinal logistic regression model adjusted by age, sex, time of RA evolution, and RA disease activity and tobacco was carried out. Afterwards, an analysis of the covariates (ANCOVA) was performed to elucidate the association between anti-CCP antibody quantification and the number of pockets ≥ 5 mm, controlling for the same confounders.
Finally, adjusted OR calculations were carried out to determine the association between tobacco consumption and periodontitis. An ordinal logistic regression model was applied to assess the relationship between periodontitis and tobacco consumption (exposures) and anti-CCP antibody levels (outcome), including the interactions between them. The statistical analyses were performed using the statistical package SPSS 25 (IBM SPSS, Armonk, NY).
Results
Characteristics of RA patients
In total, 164 RA patients, 77% women, with a mean age of 54.1 ± 10.46 years and mean disease duration of 8.3 ± 7.23 years were included in this study. Positivity for anti-CCP antibodies (> 25 UI/ml) was detected in 109 patients (66.5%), being distributed according to the antibody level titers in low (28%), moderate (42%), and high (30%). Demographic characteristics and the presence of co-morbidities in this RA population are shown in Table 1, being described for the total RA patient population, and those with presence or absence of anti-CCP antibodies. Presence of anti-CCP antibodies was not associated with age, gender, race, CRP, BMD, smoking, dyslipidemia, hypertension, BMI, or myocardial infarction, while a significant association was demonstrated with higher stress levels, ESR, and overweight. There was a tendency for an association between presence of anti-CCP antibodies and reduced attendance to oral preventive interventions and lower socioeconomic status.
Table 2 depicts the disease characteristics in patients with positive and negative anti-CCP antibodies. There were statistical differences between anti-CCP antibody negative and positive patients in terms of RF seropositivity (46% vs 87%, p < 0.001), RF titers (63 ± 90.48 vs 234.5 ± 392.73, p < 0.001), DAS28 (ESR) (3.47 ± 1.31 vs 3.98 ± 1.34, p = 0.023), and SDAI (12.16 ± 8.97 vs 15.77 ± 11.77, p = 0.043). The same tendency was observed when disease activity was assessed by DAS28 (CRP) (2.95 ± 1.15 vs 3.30 ± 1.22, p = 0.069). However, when patients were categorized by disease activity (remission, low, moderate, or high activity), no significant differences were observed between anti-CCP antibodies positive and negative patients. Nor were any differences noted between the two groups with respect to treatment with glucocorticoids, sDMARDs, or bDMARDs.
Association between the presence and levels of anti-CCP antibodies and periodontal status in RA patients
Using the 2005 definition [34], the association between periodontitis and the presence of anti-CCP antibodies was not significant (p = 0.276), with an adjusted OR of 1.228 (95% CI 0.628–2.40, p = 0.549). When the 2018 definition was used [35], although the percentage of patients with severe periodontitis (stage III+IV) was higher in positive, versus negative, anti-CCP antibody patients (51 vs 47%, respectively), the association between periodontitis and the presence of anti-CCP antibodies was also not significant (p = 0.370), with an adjusted OR of 1.222 (95% CI 0.628–2.379, p = 0.555); indeed, it proved quite similar to the 2005 [34] periodontitis case definition. Therefore, the 2018 classification [35] of periodontitis was used for the following comparisons.
When periodontal outcome parameters were used instead of case definitions, anti-CCP positive patients showed significantly higher CAL (4.16 ± 1.43 vs 3.72 ± 0.85, p = 0.015), higher numbers (16.93 ± 19.63 vs 11.64 ± 11.02, p = 0.029) and percentages of pockets ≥ 5 mm (0.14 ± 0.16 vs 0.09 ± 0.09, p = 0.014), and higher mean PI (31.0 ± 19.7 vs 22.4 ± 13.3, p < 0.001), compared to their anti-CCP antibody negative counterparts.
Regarding the severity of periodontal parameters vis-à-vis the presence of anti-CCP antibodies, a highly significant association (p = 0.007) was observed between the mean PI and anti-CCP positivity after resulting in an adjusted OR of 1.033 (95% CI, 1.009–1.058). The mean CAL also was significantly associated with anti-CCP positivity with an adjusted OR of 1.523 (95%CI 1.057–2.194, p = 0.024). In terms of the number of pockets ≥ 5 mm, a clear tendency for a positive association with anti-CCP positivity was demonstrated (adjusted OR of 1.024 95% CI 0.999–1.048, p = 0.065). After adjusting for confounders, BoP was not associated with the presence of anti-CCP antibodies. Using AUC-ROC curves, the cut-off points of mean PI (24%) and the number of pockets ≥ 5 mm (17) were established for a significant association with presence of anti-CCP antibodies after adjusting for confounders, being the respective adjusted ORs of 2.33 (95% CI 1.17–4.65, p = 0.017) and 2.03 (95% CI 1.01–4.10, p = 0.048).
When anti-CCP antibodies were stratified by level (low, moderate, and high), an ordinal logistic regression model showed a direct association between these levels and the mean CAL with an OR of 1.593 (95% CI 1.017–2.482, p = 0.043) in patients with high anti-CCP antibody titers versus non-anti-CCP RA patients (Table 3). Similarly, although with a more modest, albeit significant association was also demonstrated for patients with high anti-CCP antibody titers and the number of pockets ≥ 5 mm (Table 4, Fig. 1) and the mean PI (Table S1) with adjusted ORs of 1.031 (95%CI 1.003–1.062, p = 0.031) and 1.060 (95% CI 1.027–1.093, p < 0.001), respectively.
Using a covariance analysis (ANCOVA), a significant increase of 4.45 U/mL (95% CI 1.60–7.29, p = 0.002) of anti-CCP titers was found for each pocket ≥ 5 mm in RA patients after adjusting for age, gender, smoking, time of disease evolution, and RA activity.
Impact of tobacco consumption
Table 1 depicts the data on tobacco consumption and its possible association with anti-CCP titers. There were no significant differences (p = 0.709) between antibody-positive and antibody-negative RA patients with respect to smoking. Similarly periodontitis assessed by 2018 [35] Tonetti’s definition either independently or in combination with tobacco consumption showed a not-significant association with low, moderate, nor high titers of anti-CCP antibodies (see Supplementary Table S2). However, smoking was significantly associated with periodontitis severity (stages III or IV compared to stages 0, I, or II) in RA patients, with an adjusted OR of 2.085 (95% CI 1.100–3.951, p = 0.021 (Table 5)).
Discussion
The most important findings of this study can be summarized as follows: (1) in RA patients, periodontal conditions such as the mean CAL, number of pockets ≥ 5 mm and PI were associated with the presence of anti-CCP antibodies; (2) this association was more pronounced in patients with higher levels of these antibodies; and (3) a linear smoking-independent correlation was found between periodontal subrogate parameters such as number of pockets ≥ 5 mm, and anti-CCP titers.
The presence of citrullinated proteins and ACPA in systemically healthy periodontitis patients has been reported in recent years [12, 24]. However, in RA patients the relationship between ACPA—specifically anti-CCP antibodies, the most commonly detected ACPA in clinics—and periodontitis has remained controversial. Although some studies have shown a link between ACPA and periodontitis in RA patients [17, 39], others did not, particularly when ACPA was assessed by the presence of anti-CCP antibodies [21, 24,25,26]. In this cross-sectional study, we have tried to clarify the plausible connection of periodontitis and anti-CCP antibodies in RA patients. Our results, in agreement with a previous study [10], showed that in RA patients, the presence of anti-CCP antibodies was not significantly associated with periodontitis (diagnosed according to Tonetti’s 2005 [34] and 2018 [35] case definitions) after adjusting for confounders. Conversely, when periodontal parameters assessing the severity of periodontitis were used, such as mean CAL and number of pockets ≥ 5 mm, there was a significant association with high anti-CCP titers, which suggests that the presence of specific periodontopathogens, such as P. gingivalis or A. actinomycetemcomitans in periodontitis cases, may plays a key role in the citrullination process and anti-CCP antibody formation, as hypothesized by some studies [10, 22, 40]. At the same time, recent evidence has put forward an association between periodontitis and P. gingivalis in anti-CCP+ pre-RA patients [41], suggesting that disease duration and activity may have an impact on the association between periodontitis and anti-CCP titers in RA patients. Nonetheless, our results have demonstrated that the associations between periodontitis severity parameters, such as CAL or pockets ≥ 5 mm, and anti-CCP titers existed independently of age, gender, smoking, time of disease evolution, and RA disease activity.
There is increasing evidence linking periodontitis to other non-anti-CCP ACPAs [24, 26]. In a group of 248 RA patients and 85 healthy controls, Lee et al. demonstrated a positive correlation between antibodies against human α-enolase (ENO1) and PPD, BoP, and CAL [26]. In this work, anti-ENO1 antibodies significantly correlated with anti-CCP antibody titers and RA parameters, such as DAS28 [26]. Further studies, including anti-CCP and anti-ENO1 antibodies, in addition to other types of ACPAs such as anti-fibrinogen, anti-vimentin, or anti-CEP-1 antibodies could help to clarify the role of periodontitis and periodontal pathogens, such as P. gingivalis and A. actinomycetemcomitans in ACPA formation in RA patients, as well as their relationship with rheumatoid clinical variables.
In regard to periodontal variables and anti-CCP antibody titers, there is some evidence that links BoP, PI, and PPD to various ACPAs, including anti-CCP antibodies [10, 25, 26]. Interestingly, Mikuls et al. [10] reported a higher incidence of pockets ≥ 5 mm in anti-CCP-positive patients than controls, which remained true independently of smoking habit. Our results have also shown significantly higher levels of mean CAL, numbers and percentages of pockets ≥ 5 mm, mean PI, and mean PPD, in anti-CCP antibody-positive versus antibody-negative patients. Using logistic regression analysis and after adjusting for confounders, we identified a gradient effect in the association between mean CAL, mean PI, and the number of pockets ≥ 5 mm with anti-CCP antibody titers. In fact, all those periodontal parameters reached statistical significance in patients with high anti-CCP antibody titers (> 300). In addition, using AUC-ROC curves, we established cut-off points for mean PI and the number of pockets ≥ 5 mm vis-à-vis risk for the presence of anti-CCP antibodies in RA patients. RA patients with more than 17 pockets ≥ 5 mm, a situation characteristic of severe periodontitis, or a mean PI of 24% or higher, a finding that denotes poor oral hygiene, doubles the likelihood of being anti-CCP positive than those with periodontal parameters below those levels. Thus, in regard to pockets, we have established a previously unreported linear relationship between the number of pockets ≥ 5 mm and the quantity of anti-CCP antibodies. After adjusting for age, gender, smoking habit, time of disease evolution, and RA disease activity, we found in our group of RA patients that for every additional pocket ≥ 5 mm, the concentration of anti-CCP antibodies increased by 4.45 U/ml in RA patients. This relationship could reflect the potential role of P. gingivalis and A. actinomycetemcomitans in protein citrullination and ACPA formation, due to the fact that these periodontal pathogens are likely present in pockets ≥ 5 mm [42,43,44]. However, adequate studies using microbiological data are needed to correlate the presence of such bacteria, ACPA titers, and RA clinical variables.
Apart from periodontitis and presence of key periodontal pathogens as sources of citrullination leading to ACPA formation [10, 13, 15, 23, 26, 40], smoking may also contribute to citrullination and ACPA formation [7, 45]. Likewise, tobacco use is also a risk factor for periodontitis in both healthy [46] and RA patients [47]. Some authors have hypothesized that the impact of smoking may be so strong that it may mask the effects of P. gingivalis and periodontitis on citrullination and ACPA formation in RA patients [48], although there is also data associating periodontitis and anti-CCP antibody titers, independently from tobacco consumption in non-RA patients [23]. Our results have shown that tobacco use was associated with periodontitis (according to Tonetti’s case definitions) in RA patients, and at the same time, subrogate parameters of periodontitis severity such as CAL or PPD ≥ 5 mm were significantly associated with anti-CCP antibody titers after adjusting by age, gender, and smoking. However, both tobacco and periodontitis, either independently or in combination, did not show an association with anti-CCP antibodies titers in our series of RA patients. These results may be explained by the lack of microbiological data in our study, due to the fact that P. gingivalis and A. actinomycetemcomitans concentrations may play significant roles in ACPA formation, independently of tobacco consumption [10, 22, 40].
There are some limitations in this study. The design of our study does not address causality and the lack of microbiological data does not allow for assessing the possible association between pathogens and anti-CCP titers. The correlation between periodontitis pathogens, including P. gingivalis and A. actinomycetemcomitans and anti-CCP antibodies, should be the focus of future research to elucidate their impact on the pathogenesis of RA, as has been previously mentioned. Future research, including interventional studies focused on the influence of periodontal status and the bacteria responsible for periodontitis on both the presence of anti-CCP antibodies and disease activity, would help us better understand the causal connections between periodontitis and RA.
Conclusions
There is a link between anti-CCP antibody titers and periodontitis, in which worsening periodontal conditions, in terms of mean CAL, mean PI, and number of pockets > 5 mm, were statistically associated with both anti-CCP antibody positivity and higher levels of anti-CCP antibody titers. However, tobacco consumption and periodontitis (assessed by Tonetti’s definition) does not seem to be associated with levels of anti-CCP antibodies in our RA patient series.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author, who has the ORCID identifier 0000-0002-4139-9295, on reasonable request.
Abbreviations
- ACPAs:
-
Anti-citrullinated peptide antibodies
- ANCOVA:
-
Analysis of the covariates
- anti-CCP:
-
Anti-cyclic citrullinated peptide
- AUC-ROC:
-
Areas under ROC curves
- bDMARD:
-
Biologic disease-modifying antirheumatic drug
- BMI:
-
Body mass index
- BoP:
-
Bleeding of probing
- CAL:
-
Clinical attachment level
- CDAI:
-
Clinical Disease Activity Index
- CRP:
-
C-reactive protein
- DAS28:
-
28-joint Disease Activity Score
- DMARD:
-
Disease-modifying antirheumatic drug
- ERA:
-
Early rheumatoid arthritis
- ESR:
-
Erythrocyte sedimentation rate
- OD:
-
Odds ratio
- PAD:
-
Peptidil arginine deiminase
- PI:
-
Plaque index
- PPD:
-
Proving pocket depth
- PSS-14:
-
Perceived Stress Scale
- RA:
-
Rheumatoid arthritis
- RF:
-
Rheumatoid factor
- ROC:
-
Receiver operating characteristic
- SD:
-
Standard deviation
- sDMARD:
-
Synthetic disease-modifying antirheumatic drug
References
Aviña-Zubieta JA, Choi HK, Sadatsafavi M, Etminan M, Esdaile JM, Lacaille D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690–7.
de Pablo P, Chapple ILC, Buckley CD, Dietrich T. Periodontitis in systemic rheumatic diseases. Nat Rev Rheumatol. 2009;5:218–24.
Potempa J, Mydel P, Koziel J. The case for periodontitis in the pathogenesis of rheumatoid arthritis. Nat Rev Rheumatol. 2017;13:606–20.
Brink M, Hansson M, Mathsson L, Jakobsson P-J, Holmdahl R, Hallmans G, et al. Multiplex analyses of antibodies against citrullinated peptides in individuals prior to development of rheumatoid arthritis. Arthritis Rheum. 2013;65:899–910.
Rakieh C, Rakieh C, Nam JL, Nam JL, Hunt L, Hunt L, et al. Predicting the development of clinical arthritis in anti-CCP positive individuals with non-specific musculoskeletal symptoms: a prospective observational cohort study. Ann Rheum Dis. 2015;74:1659–66.
Katchamart W, Koolvisoot A, Aromdee E, Chiowchanwesawakit P, Muengchan C. Associations of rheumatoid factor and anti-citrullinated peptide antibody with disease progression and treatment outcomes in patients with rheumatoid arthritis. Rheumatol Int. 2015;35:1693–9.
Lee DM, Phillips R, Hagan EM, Chibnik LB, Costenbader KH, Schur PH. Quantifying anti-cyclic citrullinated peptide titres: clinical utility and association with tobacco exposure in patients with rheumatoid arthritis. Ann Rheum Dis. 2009;68:201–8.
Roberts FA, Darveau RP. Microbial protection and virulence in periodontal tissue as a function of polymicrobial communities: symbiosis and dysbiosis. Periodontol 2000. 2015;69:18–27.
Hajishengallis G. Immunomicrobial pathogenesis of periodontitis: keystones, pathobionts, and host response. Trends Immunol. 2014;35:3–11.
Mikuls TR, Payne JB, Yu F, Thiele GM, Reynolds RJ, Cannon GW, et al. Periodontitis and porphyromonas gingivalisin patients with rheumatoid arthritis. Arthritis Rheum. 2014;66:1090–100.
Ziebolz D, Pabel SO, Lange K, Krohn-Grimberghe B, Hornecker E, Mausberg RF. Clinical periodontal and microbiologic parameters in patients with rheumatoid arthritis. J Periodontol. 2011;82:1424–32.
Nesse W, Nesse W, Westra J, Westra J, van der Wal JE, van der Wal JE, et al. The periodontium of periodontitis patients contains citrullinated proteins which may play a role in ACPA (anti-citrullinated protein antibody) formation. J Clin Periodontol. 2012;39:599–607.
Courbon G, Rinaudo-Gaujous M, Blasco-Baque V, Auger I, Caire R, Mijola L, et al. Porphyromonas gingivalis experimentally induces periodontis and an anti-CCP2-associated arthritis in the rat. Ann Rheum Dis. 2019;78:594–99.
Johansson L, Sherina N, Kharlamova N, Potempa B, Larsson B, Israelsson L, et al. Concentration of antibodies against Porphyromonas gingivalis is increased before the onset of symptoms of rheumatoid arthritis. Arthritis Res Ther. 2016;18:201.
Mikuls TR, Thiele GM, Deane KD, Payne JB, O'Dell JR, Yu F, et al. Porphyromonas gingivalis and disease-related autoantibodies in individuals at increased risk of rheumatoid arthritis. Arthritis Rheum. 2012;64:3522–30.
Demmer RT, Molitor JA, Jacobs DR Jr, Michalowicz BS. Periodontal disease, tooth loss and incident rheumatoid arthritis: results from the First National Health and Nutrition Examination Survey and its epidemiological follow-up study. J Clin Periodontol. 2011;38:998–1006.
Dissick A, Redman RS, Jones M, Rangan BV, Reimold A, Griffiths GR, et al. Association of periodontitis with rheumatoid arthritis: a pilot study. J Periodontol. 2010;81:223–30.
de Pablo P, Dietrich T, McAlindon TE. Association of periodontal disease and tooth loss with rheumatoid arthritis in the US population. J Rheumatol. 2008;35:70–6.
Kaur S, White S, Bartold PM. Periodontal disease and rheumatoid arthritis: a systematic review. J Dent Res. 2013;92:399–408.
Kim J-H, Choi IA, Lee JY, Kim K-H, Kim S, Koo K-T, et al. Periodontal pathogens and the association between periodontitis and rheumatoid arthritis in Korean adults. J Periodontal Implant Sci. 2018;48:347–59.
Rodríguez-Lozano B, González-Febles J, Garnier-Rodríguez JL, Dadlani S, Bustabad-Reyes S, Sanz M, et al. Association between severity of periodontitis and clinical activity in rheumatoid arthritis patients: a case-control study. Arthritis Res Ther. 2019;21:27.
de Smit M, Westra J, Vissink A, Doornbos-van der Meer B, Brouwer E, van Winkelhoff AJ. Periodontitis in established rheumatoid arthritis patients: a cross-sectional clinical, microbiological and serological study. Arthritis Res Ther. 2012;14:R222.
Lappin DF, Apatzidou D, Quirke A-M, Oliver-Bell J, Butcher JP, Kinane DF, et al. Influence of periodontal disease, Porphyromonas gingivalis and cigarette smoking on systemic anti-citrullinated peptide antibody titres. J Clin Periodontol. 2013;40:907–15.
de Pablo P, Dietrich T, Chapple ILC, Milward M, Chowdhury M, Charles PJ, et al. The autoantibody repertoire in periodontitis: a role in the induction of autoimmunity to citrullinated proteins in rheumatoid arthritis? Ann Rheum Dis. 2014;73:580–6.
Gonzalez SM, Payne JB, Yu F, Thiele GM, Erickson AR, Johnson PG, et al. Alveolar bone loss is associated with circulating anti-citrullinated protein antibody (ACPA) in patients with rheumatoid arthritis. J Periodontol. 2015;86:222–31.
Lee JY, Lee JY, Choi IA, Choi IA, Kim J-H, Kim J-H, et al. Association between anti-Porphyromonas gingivalis or anti-α-enolase antibody and severity of periodontitis or rheumatoid arthritis (RA) disease activity in RA. BMC Musculoskelet Disord. 2015;16:190.
Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–81.
Herrera D, Sanz M, Jepsen S, Needleman I, Roldán S. A systematic review on the effect of systemic antimicrobials as an adjunct to scaling and root planing in periodontitis patients. J Clin Periodontol. 2002;29:136–59.
Mavrogiannis M, Mavrogiannis M, Ellis JS, Ellis JS, Thomason JM, Thomason JM, et al. The management of drug-induced gingival overgrowth. J Clin Periodontol. 2006;33:434–9.
Prevoo ML, van 't Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–8.
Smolen JS, Breedveld FC, Schiff MH, Kalden JR, Emery P, Eberl G, et al. A simplified disease activity index for rheumatoid arthritis for use in clinical practice. Rheumatology (Oxford). 2003;42:244–57.
Silness J, Löe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1964;22:121–35.
Ainamo J, Bay I. Problems and proposals for recording gingivitis and plaque. Int Dent J. 1975;25:229–35.
Tonetti MS, Claffey N, Claffey N, European Workshop in Periodontology group C, European Workshop in Periodontology group C. Advances in the progression of periodontitis and proposal of definitions of a periodontitis case and disease progression for use in risk factor research. Group C consensus report of the 5th European Workshop in Periodontology. J Clin Periodontol. 2005;32(Suppl 6):210–3.
Tonetti MS, Greenwell H, Kornman KS. Staging and grading of periodontitis: framework and proposal of a new classification and case definition. J Clin Periodontol. 2018;45:S149–61.
Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–96.
Remor E. Psychometric properties of a European Spanish version of the Perceived Stress Scale (PSS). Span J Psychol. 2006;9:86–93.
Graffar M. Une methode de classification sociales d’echantillons de population. Courrier. 1956;6:455–9.
Molitor JA, Alonso A, Wener MH, Michalowics BS, Gersuk VH. Moderate to severe periodontitis increases risk of rheumatoid arthritis in non-smokers and is associated with elevated ACPA titers: The ARIC Study. Arthritis Rheum. 2009;60:1160.
Konig MF, Abusleme L, Reinholdt J, Palmer RJ, Teles RP, Sampson K, et al. Aggregatibacter actinomycetemcomitans-induced hypercitrullination links periodontal infection to autoimmunity in rheumatoid arthritis. Sci Transl Med. 2016;8:369ra176.
Mankia K, Cheng Z, Do T, Hunt L, Meade J, Kang J, et al. Prevalence of periodontal disease and periodontopathic bacteria in anti-cyclic citrullinated protein antibody-positive at-risk adults without arthritis. JAMA Netw Open. 2019;2:e195394.
Henderson B, Ward JM, Ready D. Aggregatibacter (Actinobacillus) actinomycetemcomitans: a triple A* periodontopathogen? Periodontol 2000. 2010;54:78–105.
Farias BC, Souza PRE, Ferreira B, Melo RSA, Machado FB, Gusmão ES, et al. Occurrence of periodontal pathogens among patients with chronic periodontitis. Braz J Microbiol. 2012;43:909–16.
Polak D, Polak D, Wilensky A, Wilensky A, Shapira L, Halabi A, et al. Mouse model of experimental periodontitis induced by Porphyromonas gingivalis/Fusobacterium nucleatuminfection: bone loss and host response. J Clin Periodontol. 2009;36:406–10.
Makrygiannakis D, Hermansson M, Nicholas AP, Zendman AJW, Eklund A, Grunewald J, et al. Smoking increases peptidylarginine deiminase 2 enzyme expression in human lungs and increases citrullination in BAL cells. Ann Rheum Dis. 2008;67:1488–92.
Martinez-Canut P, Llobell A, Romero A. Predictors of long-term outcomes in patients undergoing periodontal maintenance. J Clin Periodontol. 2017;44:620–31.
Beyer K, Zaura E, Brandt BW, Buijs MJ, Brun JG, Crielaard W, et al. Subgingival microbiome of rheumatoid arthritis patients in relation to their disease status and periodontal health. PLoS One. 2018;13:e0202278–18.
Seror R, Seror R, Le Gall-David S, Le Gall-David S, Bonnaure-Mallet M, Bonnaure-Mallet M, et al. Association of anti-porphyromonas gingivalis antibody titers with nonsmoking status in early rheumatoid arthritis: results from the prospective French cohort of patients with early rheumatoid arthritis. Arthritis Rheumatol. 2015;67:1729–37.
Acknowledgments
The authors would like to thank to all members of the Department of Rheumatology of Hospital Universitario de Canarias for their helpfulness in the patients’ recruitment.
Funding
This work was partially supported by a grant from the Spanish Ministry of Health (Fondo de Investigaciones Sanitarias Carlos III) to FD-G [15/01810] and cofinanced by the European Regional Development Fund. A part of this study was also supported by REUNINVES Asociación.
Author information
Authors and Affiliations
Contributions
JG-F designed the trial, collected periodontal data, interpreted results, and co-wrote the manuscript. BR-L designed the trial, collected clinical data, interpreted results, and participated in manuscript writing. CS-P interpreted results and performed statistical analysis. JLG-R collected periodontal data and interpreted results. SB collected clinical data and interpreted results. MH-G collected clinical data and interpreted results. EG-D interpreted results and performed statistical analysis (ordinal logistic regression). MS participated in the trial design and interpreted results. FD-G designed the trial, interpreted results, and wrote the manuscript. All authors revised the final version of the manuscript. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by an independent ethics committee and institutional review board from Hospital Universitario de Canarias (Spain) (code 2015_06), and all subjects provided written informed consent.
Consent for publication
In this manuscript, individual patient data are not presented.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Table S1.
Association between mean PI and anti-CCP antibody levels (referred to its absence): ordinal logistic regression model.
Additional file 2: Table S2.
Association between tobacco and periodontitis with anti-CCP antibody levels (referred to its absence): ordinal logistic regression model.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
González-Febles, J., Rodríguez-Lozano, B., Sánchez-Piedra, C. et al. Association between periodontitis and anti-citrullinated protein antibodies in rheumatoid arthritis patients: a cross-sectional study. Arthritis Res Ther 22, 27 (2020). https://doi.org/10.1186/s13075-020-2121-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13075-020-2121-6