Abstract
Background
There is a paucity of community-based data regarding the prevalence and impact of gout flares as these may often be self-managed. The aim of this study was to determine the prevalence of self-reported gout and gout flares, the use of urate-lowering therapy (ULT), and the association of gout flares with health-related quality of life (HRQoL) in a large community sample. Covariate associations with flare frequency and allopurinol use were also examined.
Methods
The South Australian Health Omnibus Survey is an annual, face-to-face population-based survey. Data collected in the 2017 survey included self-reported medically diagnosed gout, allopurinol use (first-line ULT in Australia), and gout attacks (flares) in the last 12 months, in addition to sociodemographic variables and health-related quality of life (HRQoL, SF-12). Data were weighted to the Australian Bureau of Statistics 2016 census data to reflect the South Australian population. Participants 25 years and over (n = 2778) were included in the analysis.
Results
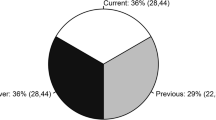
The prevalence of gout was 6.5% (95%CI 5.5, 7.5). Amongst participants with gout, 37.1% (95%CI 29.6, 45.3) reported currently using allopurinol, while 23.2% (95%CI 16.9, 21.0) reported prior use (38% discontinuation rate). Frequent flares (≥ 2 in the last year) were reported by 25% of participants with gout and were more likely with younger age, higher body mass index, and current allopurinol use (p < 0.05). The frequency of gout flares was associated with a lower physical HRQoL (p = 0.012). Current allopurinol use was reported by 51% of participants with frequent gout flares.
Conclusion
Flares were frequently reported by people with gout in the community. Gout flares were associated with reduced physical HRQoL. Almost one half of people with frequent gout flares were not receiving allopurinol, and current allopurinol use was associated with frequent gout flares, suggesting undertreated disease and suboptimal use of ULT. Determining covariate associations with flares and ineffective allopurinol use may identify means of improving treatment and reducing flares.
Similar content being viewed by others
Background
Gout is the most common inflammatory arthritis. The reported prevalence of gout is highly variable across the world, ranging from 0.1% to approximately 10%, with prevalence estimates greater than 1% in most developed countries [1]. The prevalence of gout in the UK was recently estimated as 2.5% [2], 3.9% in the USA, [3], and 5.2% in a recent Australian cohort study [4]. High baseline serum urate level and subcutaneous tophi have been linked to increased mortality, mostly attributable to cardiovascular disease [5, 6]. Furthermore, despite advances in understanding of the pathophysiology, risk factors, and therapy, gout remains a burden on the individual’s health-related quality of life (HRQoL) and on healthcare resources [7,8,9].
Current guidelines from the American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR) advise that long-term urate-lowering therapy (ULT), with the aim of maintaining serum urate levels (generally below 6 mg/dL) [10, 11], is key to effective control of gout and should be initiated in the presence of certain clinical features: for example, tophi, frequent gouty attacks (flares; two or more per year), and urate arthropathy [12].
Nonetheless, studies suggest a poor adherence to guidelines [13,14,15,16]. Reasons for this include inappropriate ULT dosing by prescribers or inadequate monitoring of serum urate levels [16, 17] and low rates of continuation of therapy when prescribed [18]. Gout flares are a clinical indicator of disease severity and the need for commencing or optimizing ULT and may continue to occur in up to one third of patients [19,20,21].
There is a paucity of community-based data regarding gout flares. One internet-based case-crossover US-based study found that 53% of enrolled participants did not consult a health care physician during an acute gout flare [22], suggesting most gout flares are self-managed in the community and that the “treatment gap” may be under-estimated.
The aim of this study was to identify the prevalence of self-reported gout flare and the frequency of allopurinol use in a representative community-based survey. In addition, sociodemographic and clinical covariates associated with flare frequency and associations with comorbidities and HRQoL were sought.
Methods
Study population
Data were obtained from the 2017 South Australian Health Omnibus Survey (HOS). The HOS is an annual, population survey, conducted by face-to-face interviews of approximately 3000 people aged 15 years and over, that obtains cross-sectional representative information on health, well-being, and related issues amongst the South Australian population living in metropolitan and rural areas. HOS has been designed to meet the highest standards of population survey methodology and is a clustered, multi-stage, systematic, self-weighting area sample [23, 24].
The 2017 HOS survey consisted of data from 2977 interviews from 5300 selected households (participation rate 65.3%) and was conducted between September and December 2017.
Outcomes
Within the survey interview, three gout-related questions were asked, “Have you even been told by a doctor that you have gout?” with the response options of “Yes,” “No,” or “Do not know/refused.” To determine allopurinol use, the respondents were asked “Do you currently take/have you taken allopurinol for gout?” with the response options of “No, never taken” (never), “No, previously taken” (prior), or “Yes, still taking” (current), with a list of the current brand names of allopurinol available to the interviewees. Febuxostat was not included because it only became available on the Australian Pharmaceutical Benefits Scheme in 2015 as a second-line option if allopurinol was contraindicated or not tolerated [25]. A recent US study reported little change to gout therapy since the introduction of febuxostat to the market (prescribed to only 3% of gout study population) [26].
To determine frequency of flares, respondents were asked, “If you have gout, how many gout attacks have you had over the last 12 months?” with the response options comprising of “None,” “One,” “Two,” “Three,” “Four,” or “Five or more.”
Covariates
Sociodemographic data collected included age, gender, and socioeconomic status (SES). SES was determined using the Index of Relative Socioeconomic Advantage and Disadvantage (IRSAD) [27], which is normalized to a mean of 1000 and standard deviation of 100, and where a low index score suggests relative disadvantage, and a higher index score represents relative advantage.
Body mass index (BMI) was based on self-reported height and weight, calculated according to standard formula, and was classified according to World Health Organization (WHO) criteria [28]. Information on comorbidities was obtained from the questions “Has a doctor ever told you that you have: (a) a heart attack/angina or did you undergo a heart procedure to unblock blocked vessels in your heart (called angioplasty or stenting), (b) Stroke, (c) High blood pressure, (d) Diabetes/high blood sugar, (e) High cholesterol levels”.
HRQoL was measured by the SF-12 v1 (US version) with Physical Component Scores (PCS) and Mental Component Scores (MCS) computed as norm-based t-scores with a mean of 50 and a standard deviation of 10 [29].
Statistical analyses
Analyses were performed using Stata v15.1 (StataCorp LLC, Texas, USA), and all tabulations, descriptive statistics, and regression models utilized appropriate survey weights. Only participants aged 25 and over were included in the analysis because gout is a disease of older adults. Prior to analysis, flares were grouped into three categories: none, 1, and ≥ 2 because, according to the ACR, ULT is indicated in patients with two or more gout flares/year [12].
Data were weighted by the inverse of the individual’s probability of selection, as well as the response rate in metropolitan and country regions and then re-weighted to benchmarks derived from the Australian Bureau of Statistics 2016 Census data (age and sex). These person weights adjust the data to better align each case (individual) with the age, gender, and geographic location distribution in the total South Australian population. Survey-weighted logistic (gout prevalence), multinomial logistic (allopurinol use and frequency of gout flares per year), and linear (HRQoL) regression models were used to analyze relationships with relevant predictor variables. All models included the sociodemographic variables of age, gender, SES (IRSAD score), and BMI, with both linear and quadratic regression terms to allow for non-linearity in any relationship with the response variable. To enable interpretation of regression models, Stata post estimation commands were used to express results for each outcome as adjusted population-weighted marginal proportions/probabilities (for logistic or multinomial models), or predicted means (for linear regression), for different levels of each predictor variable, and to determine the effect size (the derivative or change in the marginal outcome with a change in the predictor variable) averaged over other covariates. For multinomial outcomes (flares and allopurinol use), Helmert contrasts of the outcomes were used to define meaningful comparisons, and joint p values were reported.
Results
Gout prevalence and relationship to sociodemographic variables
Of the 2977 interviews conducted, 2778 participants, aged 25 years and over, were included in the analysis, with 71.4% (95% CI 69.3, 73.4) born in Australia and 10.0% (95% CI 8.9, 11.2) born in the UK. The estimated gout prevalence was 6.5% (95% CI 5.5, 7.5).
Comparison of sociodemographic variables (sex, age, and SES), BMI, and comorbidities between the general South Australian population and group with gout are presented in Table 1. Participants with gout were more likely to be male (p < 0.001), were older (p < 0.001), have a higher BMI (p < 0.001), and have a lower SES (p = 0.022, Additional file 1: Table S1). There was a high burden of comorbidities in participants with gout, including heart disease (24%), diabetes (33%), high blood pressure (54%), and high cholesterol (40%), which is consistent with their sociodemographic profile. The prevalence of gout by sex, by age group (decades), and by BMI (WHO classification) is reported in Additional file 1: Table S2, and the prevalence breakdown by sex and age group is reported in Additional file 1: Table S3.
Allopurinol use by participants with gout
Current allopurinol was reported by 37.1% of the participants with gout, prior use by 23.2% (discontinuation rate of 38%), and 39.7% reported never using allopurinol (Table 2). Current allopurinol use amongst participants with gout was not associated with SF-12 PCS (Table 3) nor MCS.
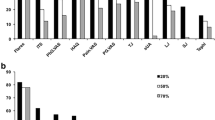
Sociodemographic and clinical covariates of allopurinol use were analyzed by multinomial logistic regression (Additional file 1: Table S4), and the predicted outcome probabilities and effects sizes are depicted in Fig. 1. Age (p = 0.009) and sex (p < 0.001) were associated with allopurinol use, whereas BMI (p = 0.63) and SES (IRSAD, p = 0.51) were not. Older age was associated with a higher probability of allopurinol never-use (contrast 0.008, 95% CI 0.001, 0.015, p = 0.018), yet amongst allopurinol ever-users, a lower probability of discontinuation was observed (contrast − 0.013, 95% CI − 0.021, − 0.005, p = 0.002). Females were also associated with a higher probability of allopurinol never-use (contrast 0.45, 95% CI 0.16, 0.74, p = 0.002), as well as a higher probability of discontinuation (contrast 0.30, 95% CI 0.03, 0.56, p = 0.029) amongst allopurinol ever-users.
Covariates associated with allopurinol use in participants with gout. Legend: a Predicted, population-averaged marginal probabilities for each category of allopurinol use (classified as never, prior, current) use by covariates (stacked bar charts) and b risk difference effect sizes (outcome Helmert contrasts) for covariate associations with allopurinol use, with vertical bars representing 95% confidence intervals. Analysis was performed by multinomial logistic regression
Flares in participants with gout
A majority of the participants with gout reported no flares in the last 12 months (58%); however, nearly 25% reported having two or more flares during this time (Table 2). The adverse impact of flares on HRQoL is illustrated by an inverse ordinal relationship between increasing number of flares and decreasing SF-12 PCS (Table 3, plinear trend 0.003). There was no such trend for SF-12 MCS (p = 0.74).
Only 51% (12.5/24.6) of participants with two or more flares/year were currently taking allopurinol (Table 2).
The relationships between allopurinol use, sociodemographic and clinical variables, and flares were analyzed by multinomial logistic regression (Additional file 1: Table S5), and the predicted outcome probabilities and effect sizes are depicted in Fig. 2. Age (p = 0.002), BMI (p = 0.005), and allopurinol use (p = 0.031) were the most important covariates associated with flares. Older age was most strongly associated with a decreased probability of flares (contrast − 0.013, 95% − 0.021, − 0.006, p = 0.001). Although sex did not reach overall statistical significance (p = 0.10), possibly due to the relatively low proportion of females with gout, there was a trend that females were more likely to suffer from flares (contrast 0.27, 95% CI − 0.01, 0.55, p = 0.054). A higher BMI was associated with an increased probability of flares (contrast 0.025, 95% CI 0.003, 0.046, p = 0.027), and within participants with flares, a higher probability of ≥ 2 flares (contrast 0.017, 95%CI − 0.001, 0.035, p = 0.068). Current allopurinol use, within participants with flares, was most strongly associated with a higher probability of ≥ 2 flares (contrast 0.36, 95% CI 0.13, 0.60, p = 0.002).
Covariate associations with flares in participants with gout. Legend: a Predicted, population-averaged marginal probabilities for each category of flares (classified as 0, 1, ≥ 2) by covariates (stacked bar charts) and b Risk difference effect sizes (outcome Helmert contrasts) for covariate associations with flares, with vertical bars representing 95% confidence intervals. Analysis was performed by multinomial logistic regression
Discussion
This study is the first representative population-based study of gout flares. Nearly a quarter of all participants with gout reported two or more flares in the last 12 months, and, contrary to current guidelines, almost half of these participants were not on ULT. Frequent gout flares had a negative effect on physical HRQoL, comparable to that seen with a range of chronic health conditions [30, 31]. The prevalence of ULT (37.1% current, 23% previous use) was consistent with previous studies reporting 28–51% current ULT [7, 9, 22, 32]. Despite the established role of ULT in reducing flares [21], participants on ULT were more likely to experience frequent gout flares, suggesting suboptimal use. Furthermore, the ULT discontinuation rate was nearly 40%. Collectively, these results are consistent with suboptimal management of gout, as has been identified in previous studies [16, 22, 32].
This 2017 representative population-based study demonstrated a high prevalence of self-reported, medically diagnosed gout (6.5%, 95%CI 5.5%, 7.5%) in the South Australian population aged 25 and over. This prevalence is comparable to previous population-based estimates in the South Australian population [4, 33], but greater than the 1.5% prevalence reported from an Australian primary care-based study [34]. It is also higher than prevalence estimates from Europe and America, which range between 0.9 and 3.9% [1, 2, 35]. There is, however, substantial heterogeneity between gout prevalence estimates [36], with case definition identified as an important contributor to this heterogeneity [36]. We used self-reported, medically diagnosed gout for case definition in this study, which has been validated against a hospital discharge diagnosis of gout or use of a gout-specific medication in two American population-based cohorts [37], and shown to have high sensitivity and precision for case definition for gout genetic studies [38]. As case ascertainment through medical records is contingent on both the patient seeking treatment and accurate recording of current and previous diagnoses, case definition by self-reported, medically diagnosed gout will capture a wider spectrum of patients.
There are known difficulties in optimizing ULT for the management of gout. Current rheumatology guidelines recommend a treat-to-target approach, requiring regular serum urate monitoring and slow up-titration of the dose until target serum urate levels are achieved [10,11,12]. Flares can be precipitated by an initial sudden decrease in serum urate levels and may still occur until all tophi have resolved, which may be some time after the target serum urate level has been reached [39]. While concomitant prophylaxis may prevent this, prescribing is not always appropriate or effective [22]. Subsequently, patients may perceive therapy to be ineffective and continuation rates can be poor [16, 32]. A lack of education for both medical practitioners and patients has been identified as a key barrier for success in establishing and maintaining ULT [13].
We found that younger participants with gout had lower rates of allopurinol continuation and were more likely to have flares, findings that are comparable to those from two retrospective UK general practice database studies of people with incident gout [40, 41]. Importantly, there was no evidence that low SES was a factor in either flares or ULT use; the predominantly public health care system in Australia may mean that this finding is not generalizable to countries with privatized health care systems. However, the roles of BMI and female gender in the management of gout warrant further consideration. In this study, higher BMI was associated with an increased prevalence of frequent flares, yet these patients were no more likely to receive ULT. Higher BMI has been causally linked to increased serum urate levels using a bidirectional Mendelian randomization approach [42], and a predictive model for allopurinol maintenance dose necessary to achieve serum urate target was highly dependent on body weight [43]. Interestingly, a prospective observational study from the US Multiple Risk Factor Intervention Trial database has demonstrated that, in individual patients with gout, there is a positive relationship between changes in BMI and the risk of recurrent gout flares [44], and therefore, weight loss may potentially contribute to gout management.
Although gout predominantly affects men, women were less likely to commence or adhere to ULT and experienced a greater number of flares in our study. Other studies have identified that women with gout have more severe disease with a greater burden of comorbid conditions [45] and poorer ULT adherence [41]. Gender bias in the diagnosis, management, and treatment of chest pain and cardiovascular disease may contribute to poorer outcomes in women (reviewed in [46]). Further research is required as there are limited data about the effect of gender on gout and its influence on management.
There are several limitations of this study. In addition to the use of self-reported, doctor-diagnosed gout, which has been validated for gout case definition [37, 38], flares were also self-reported. Flares may be subject to recall bias, and lower functional health literacy, identified in self-reported medically diagnosed arthritis, including gout, may also affect the responses obtained [47]. A tool for the definition of gout flare for clinical research, which utilizes patient reported flare as one of the criteria, has been validated and published following our data collection [48], so was not used for this study. Dosage and duration of allopurinol use were not quantified, nor were serum urate levels; therefore, adherence and optimization of treatment were only indirectly assessed.
Conclusion
This is the first community-based study of gout flare, which utilized rigorous sampling methodology to ensure that the data was representative of the general population. We conclude that gout continues to be a prevalent and poorly managed disease, despite readily available treatment. A quarter of participants with gout reported frequent flares that were associated with reduced physical HRQoL. Current allopurinol use was reported by only 51% of participants with frequent gout flares, suggesting undertreated disease and suboptimal use of ULT.
Availability of data and materials
Data and material available for this study would require further approval upon request from the corresponding author.
Abbreviations
- ACR:
-
American College of Rheumatology
- BMI:
-
Body mass index
- EULAR:
-
European League Against Rheumatism
- HOS:
-
Health Omnibus Survey
- HRQoL:
-
Health-Related Quality of Life
- IRSAD:
-
Index of Relative Socioeconomic Advantage and Disadvantage
- MCS:
-
Mental Component Score
- PCS:
-
Physical Component Score
- SES:
-
Socioeconomic status
- ULT:
-
Urate-lowering therapy
- WHO:
-
World Health Organization
References
Kuo CF, Grainge MJ, Zhang W, Doherty M. Global epidemiology of gout: prevalence, incidence and risk factors. Nat Rev Rheumatol. 2015;11(11):649–62.
Kuo CF, Grainge MJ, Mallen C, Zhang W, Doherty M. Rising burden of gout in the UK but continuing suboptimal management: a nationwide population study. Ann Rheum Dis. 2015;74(4):661–7.
Chen-Xu M, Yokose C, Rai SK, Pillinger MH, Choi HK: Contemporary prevalence of gout and hyperuricemia in the United States and decadal trends: the National Health and nutrition examination survey, 2007-2016. Arthritis Rheumatol 2019. https://doi.org/10.1002/art.40807. [Epub ahead of print]
Ting K, Hill C, Gill T, Tucker G. Prevalence and associations of gout and hyperuricaemia: results from an Australian population-based study. Intern Med J. 2015;45:5.
Bardin T, Richette P. Impact of comorbidities on gout and hyperuricaemia: an update on prevalence and treatment options. BMC Med. 2017;15(1):123.
Perez-Ruiz F, Martinez-Indart L, Carmona L, Herrero-Beites AM, Pijoan JI, Krishnan E. Tophaceous gout and high level of hyperuricaemia are both associated with increased risk of mortality in patients with gout. Ann Rheum Dis. 2014;73(1):177–82.
Roddy E, Zhang W, Doherty M. Is gout associated with reduced quality of life? A case-control study. Rheumatol. 2007;46(9):1441–4.
Bardin T, Voshaar MA, van de Laar MA. The human and economic burden of difficult-to-treat gouty arthritis. Joint Bone Spine. 2015;82(Suppl 1):eS2–8.
Khanna PP, Nuki G, Bardin T, Tausche AK, Forsythe A, Goren A, Vietri J, Khanna D. Tophi and frequent gout flares are associated with impairments to quality of life, productivity, and increased healthcare resource use: results from a cross-sectional survey. Health Qual Life Outcomes. 2012;10:117.
Khanna D, Fitzgerald JD, Khanna PP, Bae S, Singh MK, Neogi T, Pillinger MH, Merill J, Lee S, Prakash S, et al. 2012 American college of rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res. 2012;64(10):1431–46.
Richette P, Doherty M, Pascual E, Barskova V, Becce F, Castañeda-Sanabria J, Coyfish M, Guillo S, Jansen TL, Janssens H, et al. 2016 updated EULAR evidence-based recommendations for the management of gout. Ann Rheum Dis. 2017;76(1):29–42.
Khanna D, Khanna PP, Fitzgerald JD, Singh MK, Bae S, Neogi T, Pillinger MH, Merill J, Lee S, Prakash S, et al. 2012 American college of rheumatology guidelines for management of gout. Part 2: therapy and antiinflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res. 2012;64(10):1447–61.
Vaccher S, Kannangara DR, Baysari MT, Reath J, Zwar N, Williams KM, Day RO. Barriers to care in gout: from prescriber to patient. J Rheumatol. 2016;43(1):144–9.
Horsburgh S, Norris P, Becket G, Arroll B, Crampton P, Cumming J, Keown S, Herbison P. Allopurinol use in a New Zealand population: prevalence and adherence. Rheumatol Int. 2014;34(7):963–70.
Keenan RT. Limitations of the current standards of Care for Treating Gout and Crystal Deposition in the primary care setting: a review. Clin Ther. 2017;39(2):430–41.
Sarawate CA, Brewer KK, Yang W, Patel PA, Schumacher HR, Saag KG, Bakst AW. Gout medication treatment patterns and adherence to standards of care from a managed care perspective. Mayo Clin Proc. 2006;81(7):925–34.
Mikuls TR, Farrar JT, Bilker WB, Fernandes S, Saag KG. Suboptimal physician adherence to quality indicators for the management of gout and asymptomatic hyperuricaemia: results from the UK general practice research database (GPRD). Rheumatol. 2005;44(8):1038–42.
Solomon DH, Avorn J, Levin R, Brookhart MA. Uric acid lowering therapy: prescribing patterns in a large cohort of older adults. Ann Rheum Dis. 2008;67(5):609–13.
Jackson R, Shiozawa A, Buysman EK, Altan A, Korrer S, Choi H. Flare frequency, healthcare resource utilisation and costs among patients with gout in a managed care setting: a retrospective medical claims-based analysis. BMJ Open. 2015;5(6):e007214.
Wu EQ, Patel PA, Mody RR, Yu AP, Cahill KE, Tang J, Krishnan E. Frequency, risk, and cost of gout-related episodes among the elderly: does serum uric acid level matter? J Rheumatol. 2009;36(5):1032–40.
Halpern R, Fuldeore MJ, Mody RR, Patel PA, Mikuls TR. The effect of serum urate on gout flares and their associated costs: an administrative claims analysis. J Clin Rheumatol. 2009;15(1):3–7.
Neogi T, Chen C, Chaisson CE, Hunter DJ, Choi H, Zhang Y. Management of gout attacks in the community. Arthritis Rheum. 2012;64:S66.
Population Research and Outcome Studies (PROS). The Health Omnibus Survey (HOS) methodology. Brief report no. 2002-04. Adelaide: South Australian Department of Health; 2002.
Population Research and Outcome Studies Unit (PROS). Health omnibus survey (HOS) evaluation report 2006. Adelaide: South Australian Department of Health; 2006.
Febuxostat [http://www.pbs.gov.au/medicine/item/10445R].
Castro KE, Corey KD, Raymond DL, Jiroutek MR, Holland MA. An evaluation of gout visits in the United States for the years 2007 to 2011. BMC Rheumatology. 2018;2(1):14.
Australian Bureau of Statistics Technical Paper: Socio-Economic Indexes for Areas (SEIFA). 2033055001 2011.
World Health Organisation: Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organization technical report series 1995, 854:1–452.
Ware JE. SF-12 : how to score the SF-12 physical and mental health summary scales. 3rd ed. Lincoln, Boston: Quality Metric Inc. and the Health Assessment Lab; 1998.
Avery J, Dal Grande, E., Taylor, A.: Quality of life in South Australia as measured by the SF12 Health Status Questionnaire. In Edited by Services DoH South Australia: South Australian Department of Human Services; 2004.
Gonzalez-Chica DA, Hill CL, Gill TK, Hay P, Haag D, Stocks N. Individual diseases or clustering of health conditions? Association between multiple chronic diseases and health-related quality of life in adults. Health Qual Life Outcomes. 2017;15(1):244.
Roddy E, Zhang W, Doherty M. Concordance of the management of chronic gout in a UK primary-care population with the EULAR gout recommendations. Ann Rheum Dis. 2007;66(10):1311–5.
Pisaniello H, Lester S, Gonzalez-Chica D, Longo M, Sharplin G, Dal Grande E, Gill T, Whittle S, Hill C. Prevalence and predictors of gout and allopurinol use, and their association with risk perception: results from a south Australian population-based study. Intern Med J. 2017;47:13–4.
Robinson P, Taylor W, Dalbeth N. Gout prevalence and quality of care in an australian general practice population. Intern Med J. 2015;45:13.
Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011;63(10):3136–41.
Wijnands JM, Viechtbauer W, Thevissen K, Arts IC, Dagnelie PC, Stehouwer CD, van der Linden S, Boonen A. Determinants of the prevalence of gout in the general population: a systematic review and meta-regression. Eur J Epidemiol. 2015;30(1):19–33.
McAdams MA, Maynard JW, Baer AN, Kottgen A, Clipp S, Coresh J, Gelber AC. Reliability and sensitivity of the self-report of physician-diagnosed gout in the campaign against cancer and heart disease and the atherosclerosis risk in the community cohorts. J Rheumatol. 2011;38(1):135–41.
Cadzow M, Merriman TR, Dalbeth N. Performance of gout definitions for genetic epidemiological studies: analysis of UK biobank. Arthritis Res Ther. 2017;19(1):181.
Robinson PC, Stamp LK. The management of gout: much has changed. Aust Fam Physician. 2016;45(5):299–302.
Rothenbacher D, Primatesta P, Ferreira A, Cea-Soriano L, Rodriguez LA. Frequency and risk factors of gout flares in a large population-based cohort of incident gout. Rheumatol. 2011;50(5):973–81.
Scheepers L, Burden AM, Arts ICW, Spaetgens B, Souverein P, de Vries F, Boonen A. Medication adherence among gout patients initiated allopurinol: a retrospective cohort study in the clinical practice research datalink (CPRD). Rheumatol. 2018;57(9):1641–50.
Lyngdoh T, Vuistiner P, Marques-Vidal P, Rousson V, Waeber G, Vollenweider P, Bochud M. Serum uric acid and adiposity: deciphering causality using a bidirectional Mendelian randomization approach. PLoS One. 2012;7(6):e39321.
Wright DF, Duffull SB, Merriman TR, Dalbeth N, Barclay ML, Stamp LK. Predicting allopurinol response in patients with gout. Br J Clin Pharmacol. 2016;81(2):277–89.
Nguyen UD, Zhang Y, Louie-Gao Q, Niu J, Felson DT, LaValley MP, Choi HK. Obesity paradox in recurrent attacks of gout in observational studies: clarification and remedy. Arthritis Care Res. 2017;69(4):561–6.
Harrold LR, Etzel CJ, Gibofsky A, Kremer JM, Pillinger MH, Saag KG, Schlesinger N, Terkeltaub R, Cox V, Greenberg JD. Sex differences in gout characteristics: tailoring care for women and men. BMC Musculoskelet Disord. 2017;18(1):108.
Mackey C, Diercks DB. Gender bias in the management of patients still exists. Acad Emerg Med Off J Soc Acad Emerg Med. 2018.
Hill CL, Appleton SL, Black J, Hoon E, Rudd RE, Adams RJ, Gill T. Role of health literacy in self-reported musculoskeletal disorders. Arthritis. 2015;2015:607472.
Gaffo AL, Dalbeth N, Saag KG, Singh JA, Rahn EJ, Mudano AS, Chen YH, Lin CT, Bourke S, Louthrenoo W, et al. Validation of a definition of flare in patients with established gout. Arthritis Rheumatol. 2018;70(3):462-7.
Acknowledgements
We would like to thank the participants of the Health Omnibus Survey 2017.
Funding
No funding involved in this study.
Author information
Authors and Affiliations
Contributions
All authors involved in this study made significant contributions. CP was involved in the conception and design of this study, data analysis and interpretation, and drafting of this manuscript. SL was involved in the conception and design of this study, data acquisition and analysis, data interpretation, and critical appraisal of the drafts of this manuscript. DGC and TG were involved in data acquisition and critical appraisal of the drafts of this manuscript. CH and ND was involved in the conception and design of this study, data acquisition, and analysis, data interpretation, and critical appraisal of the drafts of this manuscript. All authors read and approved the final manuscript.
Authors’ information
Not applicable
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The questionnaire and methodology for this study was approved by the Human Research Ethics committee of the University of Adelaide (Ethics number approval number: H-097-2010). Verbal informed consent was obtained prior to the interview.
Consent for publication
No individual data were presented in any form in this study, and therefore, consent to publish is not applicable.
Competing interests
Professor Dalbeth: research grant funding from Amgen and AstraZeneca/Ironwood, speaker fees from Pfizer, Janssen, and Abbvie, and consulting fees from Horizon and Kowa.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:
Table S1. Sociodemographic variables as predictors of gout. Analysis was performed by survey-weighted logistic regression, and coefficients represent log-odds ratios. Table S2. Prevalence of gout by gender, age, and BMI. Table S3. Prevalence of gout % (95%CI) by age × gender. Table S4. Sociodemographic variables as predictors of allopurinol use (within gout participants). Analysis was performed by survey-weighted multinomial logistic regression, and coefficients represent log-odds ratios. Table S5. Sociodemographic variables and allopurinol use as predictors of the number of gout flares in the preceding year (within gout participants). Analysis was performed by survey-weighted multinomial logistic regression, and coefficients represent log-odds ratios. (PDF 139 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Proudman, C., Lester, S.E., Gonzalez-Chica, D.A. et al. Gout, flares, and allopurinol use: a population-based study. Arthritis Res Ther 21, 132 (2019). https://doi.org/10.1186/s13075-019-1918-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13075-019-1918-7