Abstract
Background
Clinical remission can be maintained after the discontinuation of biological disease-modifying antirheumatic drugs (bDMARDs) in some patients with rheumatoid arthritis (RA) (bDMARD-free remission (BFR)). It is unknown which bDMARD is advantageous for achieving BFR or under which conditions BFR can be considered. This study aimed to determine the factors associated with BFR achievement in clinical practice.
Methods
Patients with RA were enrolled from a Japanese multicenter observational registry. Patients with RA who achieved clinical remission (Disease Activity Score 28—C-reactive protein < 2.3) at the time of bDMARD discontinuation were included. Serial disease activities and treatment changes were followed up. BFR was considered to have failed if the disease activity exceeded the remission cutoff value or if bDMARDs were restarted.
Results
Overall, 181 RA patients were included. BFR was maintained in 21.5% of patients at 1 year after bDMARD discontinuation. BFR was more successfully achieved after discontinuation of anti-tumor necrosis factor (TNF) monoclonal antibodies (TNFi(mAb)) (infliximab, adalimumab, and golimumab), followed by CTLA4-Ig (abatacept), soluble TNF receptor or Fab fragments against TNF fused with polyethylene glycol (etanercept and certolizumab), and anti-interleukin-6 receptor Ab (tocilizumab). After multivariate analysis, sustained remission (> 6 months), Boolean remission, no glucocorticoid use at the time of bDMARD discontinuation, and use of TNFi(mAb) or CTLA4-Ig remained as independent factors associated with BFR.
Conclusions
BFR can be achieved in some patients with RA after bDMARD discontinuation in clinical practice. Use of TNFi(mAb) or CTLA4-Ig, sustained remission, Boolean remission, and no glucocorticoid use at the time of bDMARD discontinuation are advantageous for achieving BFR.
Similar content being viewed by others
Background
Intensive treatment strategies utilizing biological disease-modifying antirheumatic drugs (bDMARDs) have revolutionized rheumatoid arthritis (RA) treatment. Remission or low disease activity is now a realistic goal for most patients. After achieving remission, it would be advantageous if remission could be maintained without using bDMARDs (bDMARDs-free remission (BFR)) because of the associated cost-effectiveness and prevention of adverse events. It would be of clinical importance to determine which bDMARD is advantageous for achieving BFR and in what conditions BFR could be successfully maintained in daily clinical practice [1].
Discontinuation of bDMARDs after remission has been attempted in previous studies, including prospective uncontrolled trials and randomized controlled trials (RCTs) [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17]. For example, discontinuation of infliximab (IFX) was attempted in patients with established RA, and low disease activity was maintained in 43% of patients at 1 year after discontinuation in the RRR study [5]. Similarly, remission was maintained in 58% of patients with established RA at 6 months after discontinuation of adalimumab (ADA) in the HONOR study [8]. The remission maintenance rate after discontinuation of a soluble tumor necrosis factor (TNF) receptor, etanercept (ETN), was low (28%) compared to that in the ETN continuation group (50 mg/week; 59%) or the ETN reduction group (25 mg/week: 69%) at 1 year in the PRESERVE study [11, 12]. However, the ENCOURAGE study showed that 54% of patients maintained clinical remission after discontinuation of ETN [13]. Certolizumab pegol (CZP) (Fab fragments against TNF fused with polyethylene glycol) was discontinued after achieving remission in early RA patients, and 42% of patients remained in remission 1 year after discontinuation in the C-OPERA study [14]. Discontinuation of bDMARDs has also been attempted for non-TNF inhibitors. For example, abatacept (ABT) was withdrawn along with concomitant methotrexate (MTX) treatment after achieving remission, and “drug-free remission” was maintained in 15% of patients in the AVERT study [15]. “Drug-free remission” was also maintained after discontinuation of the anti-interleukin (IL)-6 receptor antibody tocilizumab (TCZ) in 9% of patients in the DREAM study [16] and 14% of patients in the ACT-RAY study [17], respectively, after 1 year.
However, the results of these clinical trials cannot be compared because each clinical trial was conducted under different conditions, with different patient backgrounds (early or established RA), study designs (prospective uncontrolled trials or RCTs), protocols (bDMARD free or drug free), and failure outcomes (remission, low disease activity, or restart of bDMARDs) [1, 18]. BFR achievability may vary depending on the type of bDMARDs, which have different modes of action (TNF inhibitors (TNFi), CTLA4-Ig (ABT), and IL-6R inhibitors (IL-6Ri)). In addition to the typical classification of bDMARDs according to target molecules, TNFi can be classified into two groups: fully functional monoclonal antibodies with an immunoglobulin Fc portion (TNFi(mAb)), such as IFX, ADA, and golimumab (GLM); and soluble TNF absorption molecules (TNFi(R/P)), such as soluble TNF receptor (ETN) or Fab fragments against TNF fused with polyethylene glycol (CZP). It is possible that TNFi(mAb) (IFX, ADA, and GLM) might be more advantageous for achieving BFR than TNFi(R/P) (ETN and CZP) because anti-TNF monoclonal antibodies have higher cytotoxic activity against transmembrane TNF-expressing cells via complement-dependent and antibody-dependent cell-mediated cytotoxicity and inhibit granulomatous inflammation [19, 20]. However, this hypothesis cannot be tested by clinical trials that use totally different protocols. Therefore, the data from these clinical trials are not sufficient for determining how and when BFR can be successfully achieved in typical clinical practice.
Observational data from registries of patients in typical clinical practice could potentially contribute to answering these questions and providing real-world data that could be applied in daily clinical practice [21]. The Kansai Consortium for Well-being of Rheumatic Disease Patients (ANSWER) cohort was an observational multicenter registry of patients with RA in the Kansai district in Japan [22]. The data of patients at six universities (Kyoto University, Osaka University, Osaka Medical University, Kansai Medical University, Kobe University, and Nara Medial University) and associated hospitals were included. From 2011 to 2016, 4461 patients with RA were registered, and 52,654 serial disease activities were available from the database.
With the aforementioned in mind, the aim of this study was to determine which bDMARD is advantageous for achieving BFR and in what conditions BFR can be successfully achieved in typical clinical practice by utilizing the data from this multicenter observational cohort.
Methods
Study design and participants
We retrospectively analyzed the data for the ANSWER cohort from 2011 to 2016. Patients with RA fulfilled the 2010 ACR/European League Against Rheumatism (EULAR) criteria. In this study, we included all RA patients with Disease Activity Score 28—C-reactive protein (DAS28-CRP) < 2.3 (remission) at the time of bDMARD discontinuation to those with serial disease activity and treatment records that were fully available before and after bDMARD discontinuation. We used a DAS28-CRP remission cutoff value of 2.3, which has been validated in Japanese patients [23]. The study was approved by the ethics committee of Kyoto University (approval number R0357) as well as the ethics committees of all six institutions (Osaka University, Osaka Medical College, Kansai Medical University, Kobe University, Nara Medical University, and Osaka Red Cross Hospital). The study was conducted in accordance with the Declaration of Helsinki and written informed consent was obtained from all participants.
Treatments
In this study, the following bDMARDs were used: IFX, ADA, GLM, ETN, CZP, ABT, and TCZ. These were categorized into four groups based on their mode of action: TNFi(mAb) (IFX, ADA, and GLM); TNFi(R/P) (ETN and CZP); CTLA4-Ig (ABT); and anti-IL-6Ri antibodies (TCZ). Other bDMARDs such as rituximab or targeted synthetic DMARDs such as JAK inhibitors were not permitted for use in patients with RA in Japan during the study period. The reasons for the discontinuation of bDMARDs were remission, inefficiency, toxic adverse events, and nontoxic reasons [22]. The conventional synthetic DMARDs (csDMARDs) used in this study were MTX, sulfasalazine, bucillamine, tacrolimus, leflunomide, iguratimod, and gold compounds. The glucocorticoids used in this study were prednisolone, methylprednisolone, and betamethasone; these were converted into prednisolone-equivalent doses.
Outcomes
BFR failure was defined if DAS28-CRP exceeded 2.3 or if bDMARDs were restarted (including previous biologics or introduction of new bDMARDs). Changes in concomitant csDMARDs (including MTX) or glucocorticoids were not regarded as failures. If the disease activity record was not available for more than 6 months, then the case was regarded as censored at the date of the last disease activity record.
Statistical analysis
The Kaplan–Meier method was used to estimate the median time to BFR failure from bDMARD discontinuation. A Cox proportional hazard model was used to investigate the factors associated with BFR and to obtain hazard ratios (HRs) with 95% confidence intervals (CIs). The following variables were included in univariate analysis: age, sex, disease duration, type of bDMARD, disease duration, anti-cyclic citrullinated peptide (CCP) antibody and rheumatoid factor (RF) status, bDMARD status (naïve or switched), reason for bDMARD discontinuation (physician’s intentional bDMARD discontinuation due to remission induction or not), remission maintenance period before bDMARD discontinuation, achievement of Boolean remission at the time of discontinuation, use and dosage of MTX at the time of bDMARD discontinuation, and use and dosage of glucocorticoids at the time of bDMARD discontinuation. The variables included in the multivariate analysis were selected based on the results of the univariate analysis and clinical meaningfulness. Survival curves based on the Cox proportional hazard model were evaluated for patients in each category after adjustment for covariates using direct adjusted survival estimation [24]. Two-sided p < 0.05 was considered statistically significant. All statistical analyses were performed using SAS statistical software, version 9.4 (SAS Institute, Cary, NC, USA).
Results
Patient characteristics
From 2011 to 2016, bDMARDs were used for 1307 cases in the ANSWER cohort, and serial disease activity was available for 572 cases. Based on the inclusion criteria, 181 patients with disease activity under the DAS28-CRP remission cutoff value (< 2.3) at the time of bDMARD discontinuation were included in the study and serial disease activity and treatment changes were followed up after bDMARD discontinuation.
At the time of bDMARD discontinuation, the study participants were 49 years old on average and had a disease duration of 7.6 years (Table 1). The bDMARDs used in the patients were IFX (n = 40), ADA (n = 25), GLM (n = 26), ETN (n = 22), CZP (n = 10), ABT (n = 12), and TCZ (n = 27). In 65.2% of patients, bDMARDs were the first-ever bDMARDs used (bDMARD-naïve). In 18.8% of patients, bDMARDs were intentionally discontinued by physicians owing to remission induction. At the time of bDMARD discontinuation, 78.5% and 42.5% of patients received MTX and glucocorticoids, respectively. All patients were treated with some DMARDs after bDMARD discontinuation, and none of them achieved drug-free remission. Patients’ baseline characteristics according to the different types of bDMARDs administered are presented in Table 1.
Maintenance of BFR
After bDMARD discontinuation, the BFR maintenance rates were 21.5% and 12.2% at 1 and 2 years, respectively, based on the Kaplan–Meier method. The median duration until BFR failure was 70 days (range 58–93 days). BFR failed because of disease activity flares and reinitiation of bDMARDs in 61.2% and 48.8% of patients, respectively.
Types of bDMARDs and maintenance of BFR
First, we analyzed the association between the types of bDMARDs and BFR maintenance. The BFR rates according to each bDMARD are shown in Additional file 1: Figure S1. Interestingly, among multiple TNF inhibitors, the BFR rate was clearly different between patients administered TNFi(mAb) (IFX, ADA, GLM) and those administered TNFi(R/P) (ETN and CZP) (Fig. 1). The longest median BFR maintenance period was after TNFi(mAb) administration, followed by CTLA4Ig, TNFi(R/P), and IL-6Ri (Table 2). TNFi(mAb) use was associated with a decreased risk of BFR failure compared with TNFi(R/P) and IL-6Ri use (Table 2). CTLA4-Ig use was associated with a decreased risk of BFR failure compared to IL-6Ri use (Table 2).
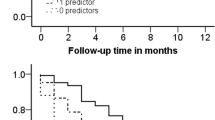
Kaplan–Meier survival curve for maintaining bDMARD-free remission after discontinuation of different types of bDMARDs. X axis represents days after bDMARD discontinuation. Y axis represents rates of maintained BFR. BFR failure defined if DAS28-CRP exceeded 2.3 or if bDMARDs restarted. If disease activity not available for more than 6 months, patient was regarded as censored case at date of last disease activity record. Kaplan–Meier method used to estimate BFR maintenance time. bDMARD biological disease-modifying antirheumatic drug, BFR biological disease-modifying antirheumatic drug-free remission, TNFi(mAb) monoclonal antibodies against TNF (infliximab, adalimumab, and golimumab), TNFi(R/P) soluble TNF receptor or Fab fragments against TNF fused with polyethylene glycol (etanercept and certolizumab), CTLA4-Ig abatacept, IL-6Ri interleukin-6 receptor inhibitor (tocilizumab), CI confidence interval, TNF tumor necrosis factor
Because the patient backgrounds were different for those treated with different bDMARDs (Table 1), the BFR rate was compared only for the bDMARD-naïve patients (Additional file 2: Figure S2). Similar to the results in all patients, BFR was maintained the longest after withdrawal of TNFi(mAb), followed by CTLA4-Ig, TNFi(R/P), and IL-6Ri (Additional file 2: Figure S2). TNFi(mAb) use was consistently associated with a decreased risk of BFR failure compared with TNFi(R/P) use (Additional file 3: Table S1).
Clinical factors and achievement of BFR
We analyzed clinical factors that were associated with BFR maintenance (Table 2). Shorter disease duration (< 2 years) was associated with a decreased risk of BFR failure. Anti-CCP antibody and RF status or smoking status did not significantly affect BFR failure (Table 2). bDMARD-naïve patients were at a decreased risk for BFR failure compared to bDMARD-switched patients. bDMARD discontinuation due to remission was associated with a decreased risk of BFR failure.
If remission was continuously maintained for > 6 months before bDMARD discontinuation, patients achieved better BFR. Achievement of Boolean remission at the time of bDMARD discontinuation was also significantly associated with a decreased risk of BFR failure. The use and dosage of MTX at the time of bDMARD discontinuation were associated with a decreased risk of BFR failure, whereas glucocorticoid use at the time of discontinuation inversely increased the risk of BFR failure.
Multivariate analysis of factors associated with BFR maintenance
We performed a multivariate analysis of factors associated with BFR failure, including the types of bDMARDs and clinical factors selected based on the results of univariate analysis and clinical significance (Table 3). In addition, adjusted survival curves for different types of bDMARDs were constructed from the results of the multivariable Cox regression model (Fig. 2). After multivariate analysis, sustained remission (> 6 months) before bDMARD discontinuation, Boolean remission at the time of bDMARD discontinuation, and glucocorticoid-free medication at the time of bDMARD discontinuation remained as independent factors associated with a decreased risk of BFR failure. After adjustment, there was no significant difference in the BFR rate between pairs of bDMARDs, except for TNFi(mAb) and IL-6Ri. However, the adjusted survival curve revealed a clear difference between use of TNFi(mAb) or CTLA4-Ig and use of TNFi(R/P) or IL-6Ri (Fig. 2). Consistently, use of TNFi(mAb) or CTLA4-Ig was significantly associated with better survival for BFR compared with use of TNFi(R/P) or IL-6Ri (HR 0.64; 95% CI 0.42–0.96; p = 0.03), even after adjustment.
Adjusted survival curve based on Cox proportional hazard model. X axis represents days after bDMARD discontinuation. Y axis represents rates of maintained BFR. Survival curves adjusted for covariates based on Cox proportional hazard model. BFR biological disease-modifying antirheumatic drug-free remission, TNFi(mAb) monoclonal antibodies against TNF (infliximab, adalimumab, and golimumab), TNFi(R/P) soluble TNF receptor or Fab fragments against TNF fused with polyethylene glycol (etanercept and certolizumab), CTLA4-Ig abatacept, IL-6Ri interleukin-6 receptor inhibitor (tocilizumab), TNF tumor necrosis factor
Discussion
In this study, we analyzed favorable conditions for BFR achievement after bDMARD discontinuation in typical clinical practice using a multicenter RA registry in Japan (the ANSWER cohort). We found the following: BFR was achieved in 21.5% of patients at 1 year after bDMARD discontinuation in typical clinical practice; TNFi(mAb) or CTLA4-Ig was advantageous for achieving BFR compared with TNFi(R/P) or IL-6Ri; and sustained remission, Boolean remission, and glucocorticoid-free medication at the time of bDMARD discontinuation were important factors associated with a decreased risk of BFR failure. These findings will help decision-making in daily clinical practice when considering bDMARD discontinuation.
In this study, BFR was achieved in 21.5% of patients at 1 year after bDMARD discontinuation; the rate of BFR was lower than the rates reported in previous clinical trials [1, 3,4,5,6, 14]. The low BFR achievability in this study may be because of the stricter BFR protocol used (maintaining remission at every visit) or the diverse patient backgrounds encountered in daily clinical practice (longer disease duration, fewer bDMARD-naive patients, more patients with comorbidities, etc.). The results of this study suggest that maintaining BFR after bDMARD discontinuation is more difficult in typical clinical practice than has been reported by clinical trials.
The present results showed that TNFi(mAb) is more advantageous for achieving BFR than TNFi(R/P). This is the first study to show a substantial difference between TNFi(mAb) and TNFi(R/P) with respect to the achievability of BFR in the same observational cohort. TNFi(mAb) not only binds to soluble TNF-α but also to transmembrane TNF-α, the binding of which induces outside-to-inside signaling, leading to apoptosis of the pathogenic cells bearing transmembrane TNF-α [19]. Therefore, TNFi(mAb) but not TNFi(R/P) may not only neutralize soluble TNF but also inhibit the granuloma formation of TNF-expressing cells. The latter might create favorable conditions for successful BFR achievement after TNFi(mAb) discontinuation [19]. In addition, TNF-α inhibition might expand or restore the suppressive function of regulatory T (Treg) cells that are important for the maintenance of immunological tolerance [25,26,27]. Transmembrane TNF-α might be involved in this process because ADA but not ETN drives regulatory T-cell expansion via TNF-receptor 2 expressed by Treg cells [28].
CTLA4-Ig provided better survival for BFR, followed by TNFi(mAb) in the unadjusted model (Fig. 1), whereas it was almost equal to TNFi(mAb) in the adjusted model (Fig. 2). Since CTLA4-Ig targets CD4 T cells upstream of the pathological condition of RA, it might be easier to maintain a good condition even after discontinuation of bDMARDs. In fact, CTLA4-Ig reduces the number of follicular helper T cells and consequently reduces the number of switched memory B cells and autoantibodies, which may favor BFR achievement [29, 30].
It is known that IL-6 inhibition increases Treg and reduces effector T cells, which can create favorable conditions for immunological tolerance [30]. However, the BFR rate after IL-6Ri use was not as high as that associated with TNFi(mAb) or CTLA4-Ig use. Since the IL-6 signal is restored after discontinuation of TCZ, it is possible that a Treg-dominant condition might be reversed after withdrawal of IL-6Ri. Alternatively, DAS28-CRP remission might not be suited as the cutoff value considering BFR after TCZ therapy because TCZ masks CRP production and DAS28-CRP remission by TCZ can be overestimated.
Even using any bDMARDs, BFR can be achieved only in 21.5% of patients at 1 year after bDMARD discontinuation. This result suggests that immunological tolerance that could lead to long-lasting BFR has not yet been established after current bDMARD therapies, and there are still unmet needs for an RA “cure.”
This study demonstrated that BFR can be successfully achieved after achieving sustained and strict remission at the time of bDMARD discontinuation (Table 3). This result is mostly consistent with previous recommendations and the consensus for bDMARD discontinuation [1]. The importance of minimal disease activity for > 6 months has been indicated in clinical trials [1]. The importance of achieving more stringent remission than DAS28 remission before withdrawing bDMARDs has been indicated by previous studies; for example, using a lower DAS28-CRP cutoff value or the absence of Doppler signals on an ultrasonogram [5, 8, 31]. This study showed that sustained and stringent remission at the time of bDMARD discontinuation is important for successfully achieving BFR, not only in clinical trials but also in real-world clinical practice.
This study also showed that no glucocorticoid use at the time of bDMARD discontinuation is important for achieving BFR. The importance of tapering the glucocorticoid dose before bDMARD discontinuation has been suggested in the EULAR recommendations, which state the following: “If a patient is in persistent remission after having tapered glucocorticoids, one can consider tapering bDMARDs” [32]. However, the clinical evidence supporting this recommendation is insufficient. The present study strongly suggests that the glucocorticoid dose should first be tapered when considering bDMARD discontinuation because the use of glucocorticoids at the time of bDMARD discontinuation was associated with failure of BFR in the real-world observational cohort (Table 3).
The present study has several limitations. First, the number of patients was small. Even including a multicenter cohort, serial disease activity at every visit was available only in limited cases. Therefore, the present results need to be confirmed by future studies including a larger number of participants. Second, due to the small number of study participants, all patients who met the inclusion and exclusion criteria were included regardless of the reasons for drug discontinuation. The reason for drug discontinuation may have affected the BFR survival time, although we adjusted for discontinuation due to remission in multivariate analysis. Third, since this study had a retrospective design and used an observational cohort from daily clinical practice, the unknown background factors (e.g., the use of csDMARDs other than MTX or disease status at the initiation of bDMARDs) may have affected the results. Finally, the radiographic progression of joint destruction was not evaluated in this study. Notably, bDMARDs have strong protective activity against bone destruction; therefore, radiographic destruction can be inhibited by bDMARD use, even though disease activity cannot be fully controlled [33, 34]. Future studies should address whether radiographic remission can be maintained if strict BFR is maintained after bDMARD discontinuation.
Conclusions
This study investigated the real-world conditions affecting BFR achievement in patients with RA. Although BFR is difficult to achieve in typical clinical practice, after strained and strict remission without glucocorticoid use, bDMARDs can be successfully withdrawn while retaining remission after discontinuation. Furthermore, TNFi(mAb) or CTLA4-Ig may be more advantageous for achieving BFR than TNFi(R/P) or IL-6Ri.
Abbreviations
- ABT:
-
Abatacept
- ADA:
-
Adalimumab
- ANSWER:
-
Kansai Consortium for Well-being of Rheumatic Disease Patients
- bDMARD:
-
Biological disease-modifying antirheumatic drug
- BFR:
-
bDMARD-free remission
- CCP:
-
Cyclic citrullinated peptide
- CI:
-
Confidence interval
- csDMARD:
-
Conventional synthetic DMARD
- CZP:
-
Certolizumab pegol
- DAS28-CRP:
-
Disease Activity Score 28—C-reactive protein
- ETN:
-
Etanercept
- EULAR:
-
European League Against Rheumatism
- GLM:
-
Golimumab
- HR:
-
Hazard ratio
- IFX:
-
Infliximab
- IL:
-
Interleukin
- MTX:
-
Methotrexate
- RA:
-
Rheumatoid arthritis
- RCT:
-
Randomized controlled trial
- RF:
-
Rheumatoid factor
- TCZ:
-
Tocilizumab
- TNFi:
-
TNF inhibitor
- TNFi(mAb):
-
Monoclonal antibodies against TNF
- TNFi(R/P):
-
Soluble TNF receptor or Fab fragments against TNF fused with polyethylene glycol
References
Schett G, Emery P, Tanaka Y, et al. Tapering biologic and conventional DMARD therapy in rheumatoid arthritis: current evidence and future directions. Ann Rheum Dis. 2016;75(8):1428–37.
Quinn MA, Conaghan PG, O'Connor PJ, et al. Very early treatment with infliximab in addition to methotrexate in early, poor-prognosis rheumatoid arthritis reduces magnetic resonance imaging evidence of synovitis and damage, with sustained benefit after infliximab withdrawal: results from a twelve-month randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2005;52(1):27–35.
van den Broek M, Klarenbeek NB, Dirven L, et al. Discontinuation of infliximab and potential predictors of persistent low disease activity in patients with early rheumatoid arthritis and disease activity score-steered therapy: subanalysis of the BeSt study. Ann Rheum Dis. 2011;70(8):1389–94.
Allaart CF, Lems WF, Huizinga TW. The BeSt way of withdrawing biologic agents. Clin Exp Rheumatol. 2013;31(4 Suppl 78):S14–8.
Tanaka Y, Takeuchi T, Mimori T, et al. Discontinuation of infliximab after attaining low disease activity in patients with rheumatoid arthritis: RRR (remission induction by Remicade in RA) study. Ann Rheum Dis. 2010;69(7):1286–91.
Ghiti Moghadam M, Vonkeman HE, Ten Klooster PM, et al. Stopping tumor necrosis factor inhibitor treatment in patients with established rheumatoid arthritis in remission or with stable low disease activity: a pragmatic multicenter, open-label randomized controlled trial. Arthritis Rheumatol. 2016;68(8):1810–7.
Harigai M, Takeuchi T, Tanaka Y, et al. Discontinuation of adalimumab treatment in rheumatoid arthritis patients after achieving low disease activity. Mod Rheumatol. 2012;22(6):814–22.
Tanaka Y, Hirata S, Kubo S, et al. Discontinuation of adalimumab after achieving remission in patients with established rheumatoid arthritis: 1-year outcome of the HONOR study. Ann Rheum Dis. 2015;74(2):389–95.
Tanaka Y, Yamanaka H, Ishiguro N, et al. Adalimumab discontinuation in patients with early rheumatoid arthritis who were initially treated with methotrexate alone or in combination with adalimumab: 1 year outcomes of the HOPEFUL-2 study. RMD Open. 2016;2(1):e000189.
Tanaka Y, Yamanaka H, Ishiguro N, et al. Low disease activity for up to 3 years after adalimumab discontinuation in patients with early rheumatoid arthritis: 2-year results of the HOPEFUL-3 study. Arthritis Res Ther. 2017;19(1):56.
Smolen JS, Nash P, Durez P, et al. Maintenance, reduction, or withdrawal of etanercept after treatment with etanercept and methotrexate in patients with moderate rheumatoid arthritis (PRESERVE): a randomised controlled trial. Lancet. 2013;381(9870):918–29.
Smolen JS, Szumski A, Koenig AS, et al. Predictors of remission with etanercept-methotrexate induction therapy and loss of remission with etanercept maintenance, reduction, or withdrawal in moderately active rheumatoid arthritis: results of the PRESERVE trial. Arthritis Res Ther. 2018;20(1):8.
Yamanaka H, Nagaoka S, Lee SK, et al. Discontinuation of etanercept after achievement of sustained remission in patients with rheumatoid arthritis who initially had moderate disease activity-results from the ENCOURAGE study, a prospective, international, multicenter randomized study. Mod Rheumatol. 2016;26(5):651–61.
Atsumi T, Tanaka Y, Yamamoto K, et al. Clinical benefit of 1-year certolizumab pegol (CZP) add-on therapy to methotrexate treatment in patients with early rheumatoid arthritis was observed following CZP discontinuation: 2-year results of the C-OPERA study, a phase III randomised trial. Ann Rheum Dis. 2017;76(8):1348–56.
Emery P, Burmester GR, Bykerk VP, et al. Evaluating drug-free remission with abatacept in early rheumatoid arthritis: results from the phase 3b, multicentre, randomised, active-controlled AVERT study of 24 months, with a 12-month, double-blind treatment period. Ann Rheum Dis. 2015;74(1):19–26.
Nishimoto N, Amano K, Hirabayashi Y, et al. Drug free REmission/low disease activity after cessation of tocilizumab (Actemra) monotherapy (DREAM) study. Mod Rheumatol. 2014;24(1):17–25.
Huizinga TW, Conaghan PG, Martin-Mola E, et al. Clinical and radiographic outcomes at 2 years and the effect of tocilizumab discontinuation following sustained remission in the second and third year of the ACT-RAY study. Ann Rheum Dis. 2015;74(1):35–43.
Yoshida K, Sung YK, Kavanaugh A, et al. Biologic discontinuation studies: a systematic review of methods. Ann Rheum Dis. 2014;73(3):595–9.
Horiuchi T, Mitoma H, Harashima S, et al. Transmembrane TNF-alpha: structure, function and interaction with anti-TNF agents. Rheumatology (Oxford). 2010;49(7):1215–28.
Mitoma H, Horiuchi T, Tsukamoto H, Ueda N. Molecular mechanisms of action of anti-TNF-alpha agents—comparison among therapeutic TNF-alpha antagonists. Cytokine. 2018;101:56–63.
Nikiphorou E, Buch MH, Hyrich KL. Biologics registers in RA: methodological aspects, current role and future applications. Nat Rev Rheumatol. 2017;13(8):503–10.
Ebina K, Hirao M, Takagi K, et al. Comparison of the effects of forefoot joint-preserving arthroplasty and resection-replacement arthroplasty on walking plantar pressure distribution and patient-based outcomes in patients with rheumatoid arthritis. PLoS One. 2017;12(8):e0183805.
Inoue E, Yamanaka H, Hara M, et al. Comparison of disease activity score (DAS)28-erythrocyte sedimentation rate and DAS28-C-reactive protein threshold values. Ann Rheum Dis. 2007;66(3):407–9.
Makuch RW. Adjusted survival curve estimation using covariates. J Chronic Dis. 1982;35(6):437–43.
Ehrenstein MR, Evans JG, Singh A, et al. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J Exp Med. 2004;200(3):277–85.
Nadkarni S, Mauri C, Ehrenstein MR. Anti-TNF-alpha therapy induces a distinct regulatory T cell population in patients with rheumatoid arthritis via TGF-beta. J Exp Med. 2007;204(1):33–9.
Nie H, Zheng Y, Li R, et al. Phosphorylation of FOXP3 controls regulatory T cell function and is inhibited by TNF-alpha in rheumatoid arthritis. Nat Med. 2013;19(3):322–8.
Nguyen DX, Ehrenstein MR. Anti-TNF drives regulatory T cell expansion by paradoxically promoting membrane TNF-TNF-RII binding in rheumatoid arthritis. J Exp Med. 2016;213(7):1241–53.
Scarsi M, Paolini L, Ricotta D, et al. Abatacept reduces levels of switched memory B cells, autoantibodies, and immunoglobulins in patients with rheumatoid arthritis. J Rheumatol. 2014;41(4):666–72.
Nakayamada S, Kubo S, Yoshikawa M, Miyazaki Y, Yunoue N, Iwata S, Miyagawa I, Hirata S, Nakano K, Saito K, Tanaka Y. Differential effects of biological DMARDs on peripheral immune cell phenotypes in patients with rheumatoid arthritis. Rheumatology (Oxford). 2018;57(1):164–74.
Naredo E, Valor L, De la Torre I, et al. Predictive value of Doppler ultrasound-detected synovitis in relation to failed tapering of biologic therapy in patients with rheumatoid arthritis. Rheumatology (Oxford). 2015;54(8):1408–14.
Smolen JS, Landewe R, Bijlsma J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76(6):960–77.
Smolen JS, Han C, Bala M, et al. Evidence of radiographic benefit of treatment with infliximab plus methotrexate in rheumatoid arthritis patients who had no clinical improvement: a detailed subanalysis of data from the anti-tumor necrosis factor trial in rheumatoid arthritis with concomitant therapy study. Arthritis Rheum. 2005;52(4):1020–30.
Smolen JS, Avila JC, Aletaha D. Tocilizumab inhibits progression of joint damage in rheumatoid arthritis irrespective of its anti-inflammatory effects: disassociation of the link between inflammation and destruction. Ann Rheum Dis. 2012;71(5):687–93.
Acknowledgements
The authors thank all medical staff at all institutions participating in the ANSWER cohort study for providing data.
Availability of data and materials
The datasets used and/or analyzed in the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
MH was responsible for conception and design. MF, WY, TF, RH, MK, AO, KA, SY, KN, YS, HA, TH, KE, HI, MT, KO, TF, and TM contributed to execution or analysis and interpretation of the data. MH and RU contributed to statistical analysis. MH prepared the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the ethics committee of Kyoto University (approval number R0357) as well as the ethics committees of all six institutions (Osaka University, Osaka Medical College, Kansai Medical University, Kobe University, Nara Medical University, and Osaka Red Cross Hospital). The study was conducted in accordance with the Declaration of Helsinki and written informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
MH and MT are affiliated with a department that is financially supported by four pharmaceutical companies (Mitsubishi-Tanabe, Chugai, Ayumi, and UCB Japan) and the city government (Nagahama City). MH received a research grant from Astellas. AO received a research grant from Eisai, Asahi-Kasei Pharma, and Mitsubishi-Tanabe. TH received a research grant and/or speaker fee from Astellas, Chugai, Eisai, Mitsubishi Tanabe, Abbvie, and Asahi-Kasei. MT received research grants from Astellas, Abbvie, Pfizer, and Taishotoyama. HI received a research grant and/or speaker fee from Bristol-Myers, Astellas, Asahi-Kasei, and Eli Lily. TF received a research grant and/or speaker fee from Pfizer Japan Inc., Ono Pharmaceutical Co., Ltd, Daiichi Sankyo Co., Ltd, Mitsubishi-Tanabe Pharma Corporation, Eisai Co., Ltd, AbbVie GK, and Astellas Pharma Inc. KO received a research grant and/or speaker fee from Abbvie, Astellas, Bristol-Myers Squibb, Eli Lily, Mitsubishi-Tanabe, Pfizer, and Takeda. TM received research grants from Acterion, Astellas, Asahi Kasei Pharma, Ayumi, Chugai, Daiichi Sankyo, Eisai, JB, Mitsubishi Tanabe, MSD, Nippon Shinyaku, Pfizer, Sanofi, and Takeda, and has participated in speakers’ bureaus for Astellas, Bristol-Myers Squibb, Chugai, and Mitsubishi-Tanabe. The remaining authors have no financial conflicts of interest to disclose concerning this manuscript. The pharmaceutical companies had no role in the design of the study, the collection or analysis of the data, the writing of the manuscript, or the decision to submit the manuscript for publication.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Figure S1. Kaplan–Meier survival curve for maintaining bDMARD-free remission after discontinuation of each bDMARD. X axis represents days after bDMARD discontinuation. Y axis represents rates of maintained BFR. bDMARD biological disease-modifying anti-rheumatic drug, BFR biological disease-modifying anti-rheumatic drug-free remission, ABT abatacept, ADA adalimumab, CZP certolizumab, ETN etanercept, GLM golimumab, IFX infliximab, TCZ tocilizumab (PDF 80 kb) (PDF 79 kb)
Additional file 2:
Figure S2. Kaplan–Meier survival curve for maintaining bDMARD-free remission after discontinuation of different types of bDMARDs in bDMARD-naïve patients. bDMARD-naïve patients classified into four groups based on types of bDMARDs. Kaplan–Meier method used to estimate BFR maintenance time. bDMARD biological disease-modifying anti-rheumatic drug, BFR biological disease-modifying anti-rheumatic drug-free remission, TNFi(mAb) monoclonal antibodies against TNF (infliximab, adalimumab, and golimumab), TNFi(R/P) soluble TNF receptor or Fab fragments against TNF fused with polyethylene glycol (etanercept and certolizumab), CTLA4-Ig abatacept, IL-6Ri interleukin-6 receptor inhibitor (tocilizumab), CI confidence interval (PDF 80 kb) (PDF 79 kb)
Additional file 3:
Table S1. Types of bDMARDs and hazard ratios for bDMARD-free remission failure in bDMARD-naïve patients (univariate analysis). bDMARD-naïve patients classified into four groups based on types of bDMARDs. Hazard ratios with 95% CIs obtained using Cox’s proportional hazard model. CI confidence interval, bDMARD biological disease-modifying antirheumatic drug, TNFi(mAb) monoclonal antibodies against TNF (infliximab, adalimumab, and golimumab), TNFi(R/P) soluble TNF receptor or Fab fragments against TNF fused with polyethylene glycol (etanercept and certolizumab), CTLA4-Ig abatacept, IL-6Ri interleukin-6 receptor inhibitor (tocilizumab) (DOCX 15 kb) (DOCX 15 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Hashimoto, M., Furu, M., Yamamoto, W. et al. Factors associated with the achievement of biological disease-modifying antirheumatic drug-free remission in rheumatoid arthritis: the ANSWER cohort study. Arthritis Res Ther 20, 165 (2018). https://doi.org/10.1186/s13075-018-1673-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13075-018-1673-1