Abstract
Background
Microsporidia MB (MB) is a naturally occurring symbiont of Anopheles and has recently been identified as having a potential to inhibit the transmission of Plasmodium in mosquitoes. MB intensity is high in mosquito gonads, with no fitness consequences for the mosquito, and is linked to horizontal (sexual) and vertical (transovarial) transmission from one mosquito to another. Maximising MB intensity and transmission is important for maintaining heavily infected mosquito colonies for experiments and ultimately for mosquito releases. We have investigated how diet affects the MB-Anopheles arabiensis symbiosis phenotypes, such as larval development and mortality, adult size and survival, as well as MB intensity in both larvae and adults.
Methods
F1 larvae of G0 females confirmed to be An. arabiensis and infected with MB were either combined (group lines [GLs]) or reared separately (isofemale lines [IMLs]) depending on the specific experiment. Four diet regimes (all mg/larva/day) were tested on F1 GLs: Tetramin 0.07, Tetramin 0.3, Gocat 0.3 and Cerelac 0.3. GLs reared on Tetramin 0.3 mg/larva/day were then fed either a 1% or 6% glucose diet to determine adult survival. Larvae of IMLs were fed Tetramin 0.07 mg and Tetramin 0.3 mg for larval experiments. The mosquitoes in the adult experiments with IMLs were reared on 1% or 6% glucose.
Results
Amongst the four larval diet regimes tested on An. arabiensis development in the presence of MB, the fastest larval development highest adult emergence, largest body size of mosquitoes, highest prevalence and highest density of MB occurred in those fed Tetramin 0.3 mg/larva/day. Although adult MB-positive mosquitoes fed on 6% glucose survived longer than MB-negative mosquitoes, there was no such effect for those fed on the 1% glucose diet. Development time, wing length and adult survival were not significantly different between MB-infected and uninfected An. arabiensis fed on the Tetramin 0.07 mg/larva/day diet, demonstrating that the MB-conferred fitness advantage was diet-dependent.
Conclusions
Microsporidia MB does not adversely impact the development and fitness of An. arabiensis, even under limited dietary conditions. The diet regime of Tetramin 0.3 mg/larva/day + 6% glucose for adults is the superior diet for the mass rearing of MB-infected An. arabiensis mosquitoes. These results are important for rearing MB-infected An. arabiensis in the laboratory for experiments and the mass rearing required for field releases.
Graphical Abstract

Similar content being viewed by others
Background
Despite the massive effort and investment directed towards malaria control through the improvement of healthcare systems, vector control and development of drugs for treatment of the disease, malaria remains an important public health problem in the world, especially in Africa [1, 2]. In 2023, there were 608,000 deaths due to malaria worldwide, with children being the most affected group [3]. Malaria deaths increased by 12% in 2020 compared to 2019, likely due to the challenges imposed on the operation of malaria control programmes by the COVID-19 pandemic [4]. Malaria transmission in the past three decades has drastically declined due to the adoption of long-lasting insecticide-treated bed nets (LLINs) and indoor residual spraying (IRS) [2]. However, the combined impact of climate change on mosquito vectors [5], insecticide resistance [6, 7] behavioural changes, such as early biting, and other factors have started to reverse the gains that had been made and are a threat to case burden reduction targets [1, 8, 9]. Considering these developments, there is an urgent need for alternative vector control approaches.
Symbiosis is a term that defines a close physical or ecological interaction between two different species. This association could be beneficial to one (commensal) or both (mutual) of the organisms involved, detrimental to one of the organisms or at times have a neutral effect on the organisms. Endosymbionts are symbiotic organisms that reside within a host organism, either inside the host’s cells (intracellular) or outside the cells (extracellular) in multicellular hosts. Endosymbionts play a very crucial role in insect biology by enhancing both nutritional provision [10] and defense [11], affecting fitness either positively or negatively (e.g. [12], [13]). However, environmental factors such as temperature and nutrition determine the stability and intensity of these symbionts in their host insects and their ability to provide protective effects or cause harmful effects on the insects (e.g. [13,14,15,]–16])
The nutritional adaptations and requirements of the aquatic larvae stage of Anopheles mosquitoes differ significantly from those of terrestrial adult-stage mosquitoes [16, 17]. The mouth brushes of the omnivorous scavenger larvae are used to collect suspended food particles from submerged surfaces [18]. Adult mosquitoes, on the other hand, gain energy mainly from plant sources, such as the nectar of flowers [19]. Several studies have investigated the effect of diet on Anopheles mosquitoes [19,20,21]. One notable finding of such studies is that the content of larval diet has a significant effect on both larval development and adult body size [22]. Moreover, restricting the food of mosquito larvae can result in negative effects on survival and development [23]. A study on Anopheles arabiensis found that fatty acid profiles in mosquitos were modified by the larval diet, which controlled mosquito size, phosphorus nutrition and population size [24].
Studies have shown that the reproductive capacity of mosquitoes is also affected by the type and quantity of diet they feed on [20, 25]. Variations in the concentrations of sugar derived from plants have been demonstrated to impact the lifespan and reproductive capabilities of adult female Anopheles gambiae. Longevity, body size and biting frequency are determined by the quality and quantity of food consumed by the mosquito in its lifetime [21]. This, in turn, affects the vectorial capacity or disease transmission potency of the mosquito [21, 26]. Adult Anopheles stephensi mosquitoes fed a lower abundance of larval diet and infected with Plasmodium falciparum exhibited a delay in parasite development and an increase in adult mortality, resulting in the mosquito requiring a longer period to become infectious [27]. The prevalence and intensity of adult infection in Anopheles coluzzii were significantly impacted by the larval diet they received during their larval stage when infected with Plasmodium berghei [28].
Nutrition is well recognised as a crucial factor in influencing the interactions between a host and its symbiotic organisms [29, 30]. Diet composition significantly influences the interaction between Wolbachia, which are maternally inherited bacterial endosymbionts in insects, and the host's diet selection [29, 31, 32]. This, in turn, affects the intensity of Wolbachia and its impact on the host's longevity and fertility [33]. There are three processes through which the host regulates endosymbionts [33]. The first mechanism suggests that the intensity of the symbionts is frequently controlled by the dietary requirements of the host; for example, hosts may have a varying intensity of symbionts depending on the quality of their food [34].
The endosymbiotic microsporidium Microsporidia MB has been found to prevent An. arabiensis in Kenya from transmitting the Plasmodium parasite [35]. Microsporidia are obligate intracellular eukaryotes that are related to fungi [36]. Microsporidia MB are localised in the gonads of An. arabiensis and are transmitted between mosquitoes by two routes: horizontal transmission, which occurs through mating, and vertical transmission from mother to offspring [35, 37]. The intensity of Microsporidia MB infection is important for vertical transmission, and possibly for horizontal transmission [35]. However, how environmental factors such as diet affect Microsporidia MB transmission and aspects of the host-symbiont interaction are unknown.
A high intensity of Microsporidia MB infection in Anopheles mosquitoes is linked to higher vertical transmission [35]. Hence, to advance the development of Microsporidia MB as a malaria control tool, it is important to understand the factors that contribute to the maintenance of a high prevalence and intensity of Microsporidia MB in mosquito colonies. In addition, the spread of Microsporidia MB will be affected by impacts on host fitness under a variety of natural conditions. We have investigated the effects of different diet regimes on Microsporidia MB infection parameters in An. arabiensis and determined how different diet regimes affect An. arabiensis host fitness in the presence of Microsporidia MB.
Methods
Mosquitoes, species identification and Microsporidia MB screening
The progeny of field-collected mosquitoes was used for this study. Blood-fed and indoor resting Anopheles mosquitoes were aspirated from houses around the Ahero irrigation scheme, Kisumu County, Kenya. The mosquitoes were transferred to the laboratory (International Centre Of Insect Physiology And Ecology [Icipe], Thomas Odhiambo Campus, Mbita, Kenya) in cages (30 × 30 × 30 cm) covered with a moist towel with mosquitoes provided access to 6% glucose solution. In the laboratory, oviposition was induced by transferring an individual female mosquito (G0) to a 1.5-ml Eppendorf tube that had been lined with filter paper and filled with 100 µl of water [38]. The eggs were dispensed into the water in larval trays/tubs (21 × 15 × 8.5 cm) kept in semi-field conditions in screened houses. The G0 females were screened by PCR to determine the species and presence of Microsporidia MB following established protocols, briefly described in the following text. The F1 larvae of G0 females were confirmed to be An. arabiensis and infected with Microsporidia MB were either combined (group lines [GLs]) or reared separately (isofemale lines [IMLs]) depending on the experiments. Similarly, the larvae of females confirmed to be An. arabiensis but not infected with Microsporidia MB were combined or reared separately to serve as controls for the experiments. All experiments were carried out on either F1 larvae or adults.
After oviposition, wild-caught females were screened using morphological characteristics [39] and PCR methods [40]. Quantitative PCR (qPCR) was performed by first isolating DNA using the ammonium acetate protein precipitation technique [35]. The DNA samples thus obtained were analysed to determine whether they were infected with Microsporidia MB using the specific primers MB18SF (CGCCGG CCGTGAAAAATTTA) and MB18SR (CCTTGGACGTG GGAGCTATC) that target the Microsporidia MB 18S ribosomal RNA (rRNA) region [35]. The PCR analyses were carried out in a reaction volume of 10 µl containing 2 µl of HOT FIREPol Blend Master Mix Ready-To-Load (Solis Biodyne, Tartu, Estonia), 0.5 µl of forward and reverse primers at a concentration of 5 pmol/µl, 2 µl of the DNA template and 5 µl of nuclease-free water. The thermocycling protocol consisted of an initial denaturation step at 95 °C for 15 min, followed by 35 cycles of denaturation at 95 °C for 1 min, annealing at 62 °C for 90 s and extension at 72 °C for 60 s, with a final extension at 72 °C for 5 min. Microsporidia MB-positive samples underwent relative qPCR analysis to measure infection levels [35]. The qPCR utilised the MB18SF/MB18SR primers, with normalisation performed using the Anopheles ribosomal S7 gene (primers: S7F [TCCTGGAGCTGGAGATGAAC] and S7R [GACGGGTCTGTACCTTCTGG]) as the reference gene. The PCR analyses were carried out in a reaction volume of 10 µl containing 2 µl of HOT FIREPol EvaGreen HRM no ROX Mix Solis qPCR Master Mix (Solis Biodyne), 0.5 µl of forward and reverse primers at a concentration of 5 pmol/µl, 2 µl of DNA template from Microsporidia MB-positive samples and 5 µl of nuclease-free PCR. The thermocycling protocol consisted of an initial denaturation step at 95 °C for 15 min, followed by 35 cycles of denaturation at 95 °C for 1 min, annealing at 62 °C for 90 s and extension at 72 °C for 60 s. Ultimately, the melting curves were produced by a melting step, utilising temperatures ranging from 65 °C to 95 °C. The PCR and qPCR were performed using a MIC qPCR cycler (Bio Molecular Systems, Upper Coomera, Australia). Confirmation was obtained for each sample, indicating the presence of the distinctive melt curve that is related to the Microsporidia MB MB18SF/MB18SR primers.
Effect of Microsporidia MB on developmental fitness under different larval diet regimes
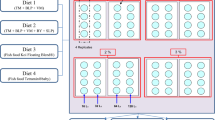
The selection of diet regimes was based on previous literature [20, 41, 42]. In total, there were eight treatments/diet regimes (Additional file 1, Figure 2), and each treatment was replicated 3 times. Each replicate consisted of 60 unfed 24 h-old An. arabiensis larvae placed in a larval tray (21 × 15 × 8.5 cm) filled with 1 l of distilled water. The larvae came from either Microsporidia MB-infected or uninfected GL lines. Three types of diets were tested [41]: (i) TetraMin® baby fish diet (TD; Tetra GmbH, Melle, Germany), Cerelac® baby diet (CD) and diary powdered milk (Nestle S.A., Vevey, Switzerland) and Gocat® diet (GD; Purina®, St. Louis, MO, USA). The nutritional content of each diet is given in Additional file 2, Table 1. Two doses of TD were tested, namely 0.3 mg/larva/day and 0.07 mg/larva/day, and one dose of CD and GD was tested, namely 0.3 mg/larva/day. The size of the flakes or granules was reduced by grinding, a routine larval-rearing practice in the laboratory [28]. The TD 0.3 mg/larva/day diet was used as the reference diet and served as a positive control diet regime, while the TD 0.07 mg/larva/day diet was tested to determine how low diet availability during larval development influences the effect of Microsporidia MB on mosquito fitness [43]. The number of dead larvae was recorded and removed daily. The number of pupae was also recorded daily, and these were transferred to emergence cages. The diet added to the larval trays was adjusted to the number of larvae that remained in the larval trays. The emerging adults from each treatment group were fed on 6% glucose solution ad libitum. Twenty adults (10 males and 10 females) were harvested on day 3 from each group and used to determine wing length as a proxy for body size [44, 45]; the remaining mosquitoes were screened for Microsporidia MB presence and intensity. The wing length was measured in millimetres using a Dino-Lite® Premier handheld microscope at a magnification of 32.1× (Huatang Optical Industry Co., Ltd, Taiwan). The regime that resulted in the highest survival and Microsporidia MB intensity was used for all the other experiments unless otherwise stated.
Effect of Microsporidia MB on adult mosquito survival under different adult diet regimes
Larvae of Microsporidia MB-infected and -uninfected GLs were reared on Tetramin 0.3 mg/larva/day; the diet regime was selected based on results from previous experiments (Additional file Figure 3). At least 80 adults from either Microsporidia MB-infected or -uninfected GLs were used in the experiment. The adult mosquitoes (< 1 day old) were divided into two treatment groups (n = 40) and placed in separate cages (15 × 15 × 15 cm). In one cage, mosquitoes had ad libitum access to 1% glucose solution and in the second cage mosquitoes had ad libitum access to 6% glucose solution (Additional file Figure 3). The glucose solution was provided in a vial and mosquitoes could feed through a paper towel wick. The vial, glucose solution and wick were replaced every 2 days. The above procedure was conducted concurrently for both Microsporidia MB-positive and -negative GLs. Daily adult mortality was recorded. The dead mosquitoes were screened for Microsporidia MB. The prevalence and densities of Microsporidia MB infection in mosquitoes from each diet regime were recorded. This experiment was conducted in two rounds, with three replicates for each diet treatment group in each round.
Effect of larval and adult diet quantity on the intensity of Microsporidia MB infection in An. arabiensis adult with a similar genetic background.
To determine if the effect of different quantities of larval and adult diet on Microsporidia MB prevalence and intensity is influenced by the genetic background of the mosquitoes, experiments were conducted with IMLs (Additional file Figure 4). This study was implemented to enhance detection of variations in intensity among mothers as data suggest that the intensity of endosymbionts can be inherited. For this experiment (Additional file Figure 4A), the two larval diet regimes from TD, 0.07 mg and 0.3 mg per larva/day, were referred to as the low and high nutritional diet, respectively [43]. Larvae from Microsporidia MB-positive IMLs were divided into two trays and reared on either the low or high nutritional diet.
Dead larvae were removed daily and recorded. Pupae were also collected daily and placed in a 15 × 15 × 15-cm cage. The adults that emerged were fed on 6% glucose solution ad libitum and harvested to quantify Microsporidia MB intensity when 3 days old. This experiment was carried out in nine replicates, with each replicate consisting of at least 20 unfed 24-h-old An. arabiensis larvae from the same mother placed in a larval tray (21 × 15 × 8.5 cm) with 1 l of distilled water.
To determine if the effect of adult diet on Microsporidia MB intensity was influenced by genetic background, larvae from IMLs were reared on 0.3 mg TD/larva. The IMLs that produced at least 40 adults were used for this experiment (Additional file Figure 4B). The adults were split in two groups and placed in separate cages (15 × 15 × 15 cm). Mosquitoes in one cage were fed on 1% glucose solution and those in the second cage were fed on 6% glucose solution ad libitum. The sugar solution was replaced with a fresh one every 2 days. On day 14, the adult mosquitoes were harvested to quantify Microsporidia MB using qPCR. A period of 14 days was chosen to ensure the accurate measurement of the impact of the adult diet.
Data analysis
Kaplan–Meier survival analysis and Cox regression were used to determine the effect of different diet regimes on larval development and adult survival. Prevalence and adult emergence data was arcsine transformed and analysed using the Tukey’s honestly significant difference (HSD) test of analysis of variance (ANOVA) to determine the best-fit diet regime. Non-parametric Mann–Whitney U and Kruskal–Wallis tests were used to compare the Microsporidia MB intensities of the adult and larval diet treatment groups. Phenotypic characteristics, such as length of wing, were tested for significant variation within and across treatment groups using the generalised linear model (GLM) following gamma distribution. R software version 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria) was used for analysis with a P-value < 0.05 considered to be significant at a 95% confidence interval.
Results
Prevalence and Intensity of Microsporidia MB under different larval diet regimes
There was no significant difference in Microsporidia MB prevalence across the four diet regimes (ANOVA after arcsine transformation of the data, F = 2.655, df = 3, P = 0.185) (Fig. 1a). This result showed that larval diet regimes did not affect the prevalence of Microsporidia MB in emerging adults. The intensity of Microsporidia MB was affected by the different larval diet regimes (Kruskal–Wallis test, X2 = 22.85, df = 3, P-value < 0.0001). Multiple pairwise comparisons using Wilcoxon rank sum test with continuity correction showed that 3-day-old adults emerging from the larvae feeding on TetraMin (TD) 0.3 mg/larva/day had a higher intensity of Microsporidia MB than those feeding on TD 0.07 mg/larva/day (P = 0.007) and Cerelac (CD) 0.3 mg/larva/day (P < 0.001) (Fig. 1b). There was no significant difference between Microsporidia MB intensity between larvae fed TD 0.3 and Gocat (GD) 0.3 mg/larva/day diets. Furthermore, there was a significant difference in Microsporidia MB intensity between larvae fed on GD 0.3 mg/larva/day and those fed on CD 0.3 mg/larva/day (P < 0.006); however, there was no difference in Microsporidia MB intensity between larvae fed TD 0.07 mg/larva/day and those fed CD 0.3 mg/larva/day (P > 0.254).
Microsporidia MB prevalence and intensity under different larval diet regimes. a Prevalence (%) of Microsporidia MB in 3-day-old F1 adults reared on different diet regimes. Error bars represent the standard deviation. b Relative Microsporidia MB intensity in 3-day-old F1 adults reared on different diet regimes using qPCR. Bar represents a significant difference between the diet regimes. There were 3 independent biological replicates, with each replicate comprising 60 larvae. The experimental design is shown in Additional file 2. Asterisks indicate the level of significance (*P < 0.05, **P < 0 .001, ***P < 0.0001); ns indicates no significance. qPCR Quantitative PCR
Effect of Microsporidia MB on larval mortality and pupation under different larval diet regimes
The Kaplan–Meier survival analysis showed that larval mortality was highest under the TD 0.07 mg/larva/day regime, being 26% in Microsporidia MB-infected and 28.33% in Microsporidia MB-uninfected larvae, out of the total 180 larvae in three biological replicates (n = 60 larvae) in each replicate. Mortality in Microsporidia MB-infected and -uninfected was 4.45% and 6.67%, respectively, under the GD 0.3 mg/larva/day diet and 1.70% and 2.78%, respectively, under the CD 0.3 mg/larva/day diet (Fig. 2a). There was a significantly higher survival under the TD 0.3 mg/larva/day diet, with a mortality of 1.67% in Microsporidia MB-infected larvae and 15% in Microsporidia MB-uninfected larvae (X2 = 15, df = 1, P < 0.001). Under the TD 0.3 mg/larva/day diet regime and GD diet regime, Microsporidia MB significantly reduced larva mortality (X2 = 15, df = 1, P < 0.001 and X2 = 5.43, df = 1, P = 0.012, respectively).
Effect of Microsporidia MB on mortality and larval development under different larval diet conditions. a Effect of Microsporidia MB on larval mortality under different diet regimes, b effect of Microsporidia MB on larval developmental time under different diet regimes. Error bars represent standard deviations
As previously observed [35], we noted that Microsporidia MB-infected larvae developed faster than their uninfected counterparts. The enhancement of Microsporidia MB growth rate was diet dependent. Microsporidia MB-infected larvae developed significantly faster than their uninfected counterparts only when fed on the TD 0.3 and GD 0.3 mg/larva/day diet regimes (Hazard ratio (HR) = 1.7, 95% CI = 1.4–2.2, P < 0.001 and HR = 1.4, 95% CI = 1.1–1.7, P < 0.01, respectively). Under the TD 0.3 mg/larva/day diet regime, the median (± standard deviation) developmental time was shorter for Microsporidia MB-infected larvae than for their uninfected controls (9 ± 0.06 vs 10 ± 0.15 days, respectively) (Fig. 2b). The hazard ratio of 1.7 (95% CI, 1.4–2.2; P<0.001) showed that Microsporidia MB-infected larvae developed 1.7 days faster than the uninfected controls. Under the GD 0.3 mg/larva/day diet regime, the median development time for Microsporidia MB-infected larvae was also shorter than that of the uninfected larvae (10 ± 0.12 vs 11 ± 0.14 days, respectively). The median developmental time for Microsporidia MB-infected and -uninfected larvae fed CD 0.3 mg/larva/day was 12 ± 0.12 days and 11 ± 0.21 days, respectively. However, in larvae fed the TD 0.07 mg/larva/day diet there was little difference in the developmental period between the Microsporidia MB-infected larvae and the control larvae (15 ± 0.52 days and 15 ± 0.72 days, respectively).
The different diets affected the emergence of pupated larvae into adults (F = 4.66, df = 3, P = 0.005) (Fig. 3). It must be noted that not all pupae emerged into adult mosquitoes, and this was especially noted for those fed the TD 0.07 mg/larva/day diet; this accounted for the difference in pupae and emerged adult mosquito numbers. However, for each treatment, there was no significant difference between Microsporidia MB-infected GLs and control GLs (F = 4.66 , df = 3, P = 0.99).
Microsporidia MB's on adult emergence under the different larval diet regimes. The data shown are the percentage of larvae that developed into adult mosquitoes from larvae fed the various diet regimes and represent the total number of adults that emerged from the collected pupae from each diet. Error bars represent standard deviation
Effect of Microsporidia MB on adult mosquito survival under different adult diet regimes
The median survival time for Microsporidia MB-infected and uninfected adult mosquitoes from GLs reared on the same larval diet regime (TD 0.3 mg/larva/day), when fed a 1% glucose diet (Fig. 4a), was 4 ± 0.17 days and 4 ± 0.18 days, respectively. There was no significant difference in survival between Microsporidia MB-infected and -uninfected adults (X2 = 2.95, df = 1, P = 0.073).
On the other hand, among those adult mosquitoes fed on the 6% glucose diet (Fig. 4b), Microsporidia MB-infected mosquitoes survived significantly longer than their uninfected counterparts (X2 = 5.84, df = 1, P = 0.007). The median survival time for the Microsporidia MB-infected adults was 12 ± 0.49 days, compared to 10 ± 0.47 days for their uninfected counterparts.
Effect of larval and adult diet quantity on Microsporidia MB intensity in IMLs
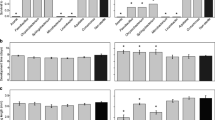
We compared Microsporidia MB intensity in adult mosquitoes emerged from IML larvae which had been fed on the TD 0.07 mg/larva/day and TD 0.3 mg/larva/day diets, respectively (Fig. 5b). The intensity of female mosquitoes was significantly affected by the two larval diets, with the latter mosquitoes having the higher Microsporidia MB intensity than those on the low nutritional diet ( X2 = 6.38, df = 1, P-value = 0.011). Meanwhile, the adult male mosquitoes (Fig. 5a) did not show any significant difference in intensity between both diets ( X2= 0.30, df = 1, P-value = 0.584).
IMLs which were fed on the TD 0.07 mg/larva/day diet were shown to result in significantly smaller sized adult mosquitoes when compared to those fed the TD 0.3 mg/larva/day regime (t = 4.23, df = 1, P < 0.0001). Males fed on both diets had smaller body sizes than their female counterparts (t = 5.93, df = 1, P < 0.0001). Microsporidia MB-positive IML female mosquitoes had a significantly longer wing length under both the TD 0.07 mg/larva/day diet (X2 = 4.18, df = 1, P= 0.040) and the TD 0.3 mg/larva/day diet ( X2= 23.21, df = 1, P < 0.0001) than their male counterparts.
Furthermore, the sizes of the female Microsporidia MB IML mosquitoes were significantly larger compared to the control IMLs (uninfected IMLs from the same infected mother) when fed the TD 0.3 mg/larva/day diet (X2= 23, df = 1, P< 0.0001; Fig. 6); male mosquitoes on the same diet did not show any difference in size (U(64) = 3.58, P = 0.115).
Effects of Microsporidia MB on wing length of IMLs under different larval diet quantity regimes. Microsporidia MB significantly increased the length of female wings both under the Tetramin 0.07 and 0.3 mg/larva/day diet, respectively. The line and the bars in the middle of each treatment indicate the mean with 95% CI, respectively.
There was no significant difference in Microsporidia MB intensity between adult mosquitoes fed on the 1% and 6% glucose diet regimes U(76)= 8.08, P = 0.445; Fig. 7c). Under the low nutritional diet (1%) glucose, there was no significant difference in Microsporidia MB intensity between male and female mosquitoes (U(39)= 7.4, P = 0.430). In contrast, in adult mosquitoes fed the the 6% diet, Microsporidia MB intensity was significantly higher in the female IMLs than in the male IMLs (Fig. 7b; U(46)= 12.57, P < 0.009).
Microsporidia MB intensity under different adult diet quantities. a Effect of low adult diet (1% glucose) on Microsporidia MB intensity in male and female IMLs mosquitoes. b Effect of high adult diet (6% glucose) on Microsporidia MB intensity in male and female IMLs mosquitoes. c Effect of low (1% glucose) and high (6% glucose) diet on the intensity of Microsporidia MB. Bars represent the mean with a 95% confidence interval of the Microsporidia MB intensities of each diet regime. IMLs, Isofemale lines
Discussion
Despite being harboured inside the cells of their hosts, insect endosymbionts are affected by the environmental conditions experienced by their hosts. In this study we investigated how host nutrient availability affects the interaction between An. arabiensis mosquitoes and Microsporidia MB. Understanding the dynamics of this interaction is important for predicting the ability of Microsporidia MB to spread in natural An. arabiensis populations and for establishing optimal methods to rear Microsporidia MB-infected An. arabiensis mosquitoes [21, 46].
Some life history traits were not affected by either Microsporidia MB or diet. The prevalence of Microsporidia MB was not affected by diet although diet conditions could play an essential role in ensuring successful transmission with a significant increase in symbiont intensity. The results of the intensity experiments showed that in the absence of the TD 0.3 mg/larva/day diet regime, larvae raised on the GD 0.3 mg/larva/day diet can be used to achieve a comparable high Microsporidia MB intensity in the mosquito host. The low-intensity results seen under the TD 0.07 mg/larva/day and CD diets could be attributed to the low protein content by quantity and composition, respectively.
Firstly, Microsporidia MB reduced larval mortality across the four larval diet regimes, although the impact was more prominent under the TD 0.3 and GD 0.3 mg/larva/d diets. A similar trend was seen for larval development where larvae unde the TD 0.3 g/larva/day diet regime showed the fastest larval development, followed (in order of increased developmental time) by those on the GD 0.3 mg/larva/day diet, the CD diet and finally the TD 0.07 mg/larva/day diet. Microsporidia MB-colonised An. arabiensis larvae pupated 1.75 days faster than the control larvae without the symbiont under the TD 0.3 mg/larva/day regime; however under the TD 0.07 mg/larva/day regime the development time was delayed and there was no significant difference between Microsporidia MB-positive and -negative groups, indicating the impact of diet quality and quantity on development [47, 48]. Larval diet conditions impacted the adult emergence of mosquitoes, although Microsporidia MB did not affect the emergence of adults from pupae under the four diet regimes. These findings are similar to those reported in Herren et al. [37].
Under the different larval diets, larvae fed on the TD 0.3 mg/larva/day diet had the lowest larval mortality, fastest larval development and highest adult emergence. These results confirm those of earlier research showing that the diet of a mosquito influences the life history characteristics of that mosquito [21]. In terms of diet composition, only the protein content differed significantly among the various diets, with TD having the highest protein content, followed by the BD and CD diets. Hence, the difference seen in mosquito development must be due to the protein content in the TD diet, as supported by earlier observations that medium levels of protein are essential for a bigger size and weight [21]. The results clearly showed that not only the mosquito (host) is affected by diet but the symbiont as well. The Microsporidia MB-conferred fitness advantage on mosquitoes is therefore diet-dependent. These results indicate that the TD 0.3 mg/larva/day diet regime is the most suitable nutritional regime for mass rearing of Microsporidia MB mosquitoes among the options tested.
The survival of Microsporidia MB adult mosquitoes was affected by the quantity of the adult mosquito’s diet quantity. The longer survival of the Microsporidia MB adult An. arabiensis iso-groups compared to the negative groups reaffirms that the Microsporidia MB fitness advantage is diet-dependent. This finding is similar an earlier report of the higher survival of Rickettsia-infected whiteflies compared to uninfected ones [49]. It is also worth noting that the quantity of sugar in the adult mosquito’s diet did not affect the intensity of Microsporidia MB in the mosquitoes; rather, it affected the presence of Microsporidia MB, with the diet condition determining the fitness benefit to the host.
In the IMLs, larval diet quantity significantly affected the intensity of Microsporidia MB in adult female mosquitoes but not in adult males. One explanation could be the difference in protein profiles between male and female mosquitoes, with 682 and 422 exclusively different proteins in females and males, respectively [50]. Vertically transmitted symbionts strategically manipulate the female host to ensure their effective transmission to the next generation by various mechanisms [51, 52]. Hence, the higher intensity of Microsporidia MB in the females both under the TD 0.3 mg/larva/day diet and compared to males may be ensuring that the symbiont is successfully transmitted to the offspring. The size of mosquitoes was affected by both diet quantity and sex of the mosquito. Research has proven that female mosquitoes with larger body sizes are more likely to become gravid than those with smaller body size [53]. Furthermore, Larger females lay more eggs and undergo shorter gonotrophic cycles than small ones [43, 54, 55]. The quantity of the larval diet affects the size of the mosquito [43], as also noted in the present study. The corresponding higher Microsporidia MB intensity with larger body size of Microsporidia MB-positive females in the IMLs confirms the reproductive advantage conferred to the host by the symbiont. A maternally transmitted Rickettsia symbiont in whitefly causes similar biases in the female as a reproductive surety [49].
Interestingly, adult diet quantity did not affect Microsporidia MB intensity. The significantly higher female Microsporidia MB intensity compared to that in the male reflects the synergistic impact of both a high larval diet (TD 0.3 mg/larva/day) and a high adult diet (6% glucose).
Insect hosts regulate the intensity of obligate intracellular nutritional symbionts depending on the nutritional status of the host [56]. Under limited thiamine conditions, obligate mutualist Wigglesworthia intensity in Glossina morsitans increased while an enriched thiamine diet significantly decreased the intensity of the symbiont [34]. As a nutritional symbiont, Wigglesworthia synthesises thiamine, hence under an enriched diet, the intensity of the symbiont is reduced by the host since there is no need for nutrient provision [57]. Another experimental manipulation of nutrition in Acyrthosiphon pisum (pea aphid) showed an increased obligate Buchnera aphidicola population intensity with a corresponding increase in the size of the host when fed on a rich nitrogen diet [58]. The proportional increase in Buchnera was to increase nutrient provision by the symbiont to compensate for the growth of the insect. Wolbachia pipientis, an intracellular proteobacterium that infects about 60% of insects, is both maternally and horizontally transmitted [56]. It has been demonstrated that the ability of Wolbachia symbiont to induce recombination in Drosophila melanogaster is titer-dependent [56].
The results from this study demonstrate that Microsporidia MB is not a nutritional symbiont. Consequently, there was a resultant lack of directly proportional changes in intensity according to diet quantity [33]. Insect host regulation of symbionts could be responsible for the restriction of the Microsporidia MB fitness benefit to An. arabiensis under very limited diet conditions, as all resources are channelled to host-symbiont survival [33]. However, under normal diet conditions, the metabolism of the symbiont is not restricted, leading to a full expression of fitness advantage seen in both larval and adult diets. Our results also showed clearly that there was no significant fitness cost to the host even under very limited diet conditions.
Conclusions
Microsporidia MB has no negative effect on the development of An. arabiensis even under low diet conditions, which are often experienced in nature, and hence can be used as an effective malaria control tool. The Tetramin 0.3 mg/larva/day diet regime results in a high Microsporidia MB intensity in the mosquito. The longer survival of the adult Microsporidia MB mosquito fed on 6% glucose is an added advantage for the proliferation of Microsporidia MB in the uninfected mosquito population. The diet regimes can, therefore, be utilised for mass rearing of Microsporidia MB-infected An. arabiensis.
Availability of data and materials
All data supporting the findings of this study are available as Additional file Data 5.
Abbreviations
- MB :
-
Microsporidia MB
- GLs:
-
Group lines
- IMLs:
-
Isofemale lines
References
Wilson AL, Courtenay O, Kelly-Hope LA, Scott TW, Takken W, Torr SJ, et al. The importance of vector control for the control and elimination of vector-borne diseases. PLoS Negl Trop Dis. 2020;14:e0007831.
WHO. World Malaria Report: 20 years of global progress and challenges. 2020. https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2020#:~:text=The%202020%20edition%20of%20the,and%207.6%20million%20deaths%20averted. Accessed 14 Jul 2023.
WHO. World malaria report 2023. 2023. https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2023#:~:text=Latest%20malaria%20report%20spotlights%20the,the%20malaria%2Dcarrying%20Anopheles%20mosquito. Accessed 10 Jan 2024.
WHO. Word Malaria Report 2021. 2021.https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2021. Accessed 14 Jul 2023.
Giesen C, Roche J, Redondo-Bravo L, Ruiz-Huerta C, Gomez-Barroso D, Benito A, et al. The impact of climate change on mosquito-borne diseases in Africa. Pathog Glob Health. 2020;114:287–301.
Hemingway J, Ranson H, Magill A, Kolaczinski J, Fornadel C, Gimnig J, et al. Averting a malaria disaster: Will insecticide resistance derail malaria control? Lancet. 2016;387:1785–8.
Lindsay SW, Thomas MB, Kleinschmidt I. Threats to the effectiveness of insecticide-treated bednets for malaria control: thinking beyond insecticide resistance. Lancet Glob Health. 2021;9:e1325–31.
Sougoufara S, Ottih EC, Tripet F. The need for new vector control approaches targeting outdoor biting anopheline malaria vector communities. Parasit Vectors. 2020;13:1–15.
The malERA Consultative Group on Vector Control. A research agenda for malaria eradication: Vector control. PLoS Med. 2011;8:e1000401.
Hansen AK, Pers D, Russell JA. Chapter 5—Symbiotic solutions to nitrogen limitation and amino acid imbalance in insect diets. Adv Insect Physiol. 2020;58:161–205.
Waterworth SC, Flórez LV, Rees ER, Hertweck C, Kaltenpoth M, Kwan JC. Horizontal gene transfer to a defensive symbiont with a reduced genome in a multipartite beetle microbiome. MBio. 2020. 11:10–128. https://doi.org/10.1128/mBio.02430-19.
Anbutsu H, Fukatsu T. Spiroplasma as a model insect endosymbiont. Environ Microbiol Rep. 2011;3:144–53.
Doremus MR, Kelly SE, Hunter MS. Exposure to opposing temperature extremes causes comparable effects on Cardinium density but contrasting effects on Cardinium induced cytoplasmic incompatibility. PLoS Pathog. 2019;15:e1008022.
Mouton L, Henri H, Bouletreau M, Vavre F. Effect of temperature on Wolbachia density and impact on cytoplasmic incompatibility. Parasitology. 2006;132:49–56.
Zhang B, Leonard SP, Li Y, Moran NA. Obligate bacterial endosymbionts limit thermal tolerance of insect host species. Proc Natl Acad Sci USA. 2019;116:24712–8.
Clements AN. Larval nutrition, excretion and respiration. Biol Mosq Dev Nutr Reprod. 1992;1:100–23.
Clements AN. Growth and development. In Biology of Mosquitoes, Volume 1: development, nutrition and reproduction. CABI, Wallingford, United Kingdom. 1992, p. 150–170. https://doi.org/10.1079/97808519937440007.
Clements AN. Larval feeding. In Biology of Mosquitoes, Volume 1: development, nutrition and reproduction. CABI, Wallingford, United Kingdom. 1992, p.74–99. https://doi.org/10.1079/9780851993744.0004.
Müller G, Schlein Y. Sugar questing mosquitoes in arid areas gather on scarce blossoms that can be used for control. Int J Parasitol. 2006;36:1077–80.
Zeller M, Koella JC. Effects of food variability on growth and reproduction of Aedes aegypti. Ecol Evol. 2016;6:552–9.
Carvajal-Lago L, Ruiz-López MJ, Figuerola J, Martínez-de la Puente J. Implications of diet on mosquito life history traits and pathogen transmission. Environ Res. 2021;195:110893.
Damiens D, Benedict MQ, Wille M, Gilles JRL. An inexpensive and effective larval diet for Anopheles arabiensis (Diptera: Culicidae): Eat like a horse, a bird, or a fish? J Med Entomol. 2012;49:1001–11.
Gilles JRL, Lees RS, Soliban SM, Benedict MQ. Density-dependent effects in experimental larval populations of Anopheles arabiensis (Diptera: Culicidae) can be negative, neutral, or overcompensatory depending on density and diet levels. J Med Entomol. 2011;48:296–304.
Hood-Nowotny R, Schwarzinger B, Schwarzinger C, Soliban S, Madakacherry O, Aigner M, et al. An analysis of diet quality, how it controls fatty acid profiles, isotope signatures and stoichiometry in the malaria mosquito Anopheles arabiensis. PLoS ONE. 2012;7:e45222.
Nayar JK, Sauermann DM. The effects of nutrition on survival and fecundity in Florida mosquitoes. Part 3. Utilization of blood and sugar for fecundity. J Med Entomol. 1975;12:220–5.
Zirbel KE, Alto BW. Maternal and paternal nutrition in a mosquito influences offspring life histories but not infection with an arbovirus. Ecosphere. 2018;9:e02469.
Shapiro LLM, Murdock CC, Jacobs GR, Thomas RJ, Thomas MB. Larval food quantity affects the capacity of adult mosquitoes to transmit human malaria. Proc R Soc B Biol Sci. 2016; 283:20160298. https://doi.org/10.1098/rspb.2016.0298.
Linenberg I, Christophides GK, Gendrin M. Larval diet affects mosquito development and permissiveness to Plasmodium infection. Sci Rep. 2016;6:38230.
Ponton F, Wilson K, Holmes A, Raubenheimer D, Robinson KL, Simpson SJ. Macronutrients mediate the functional relationship between Drosophila and Wolbachia. Proc R Soc B Biol Sci. 2014; 282:20142029. https://doi.org/10.1098/rspb.2014.2029.
Herren JK, Paredes JC, Schüpfer F, Arafah K, Bulet P, Lemaitre B. Insect endosymbiont proliferation is limited by lipid availability. Elife. 2014;3:e02964.
Caragata EP, Rezende FO, Simões TC, Moreira LA. Diet-induced nutritional stress and pathogen interference in Wolbachia-infected Aedes aegypti. PLoS Negl Trop Dis. 2016;10:e0005158.
Camacho M, Oliva M, Serbus LR. Dietary saccharides and sweet tastants have differential effects on colonization of Drosophila oocytes by Wolbachia endosymbionts. Biol Open. 2017;6:1074–83.
Whittle M, Barreaux AMG, Bonsall MB, Ponton F, English S. Insect-host control of obligate, intracellular symbiont density. Proc Biol Sci. 2021;288:20211993.
Snyder AK, Mclain C, Rio RVM. The tsetse fly obligate mutualist Wigglesworthia morsitans alters gene expression and population density via exogenous nutrient provisioning. Appl Environ Microbiol. 2012;78:7792–7.
Herren JK, Mbaisi L, Mararo E, Makhulu EE, Mobegi VA, Butungi H, et al. A microsporidian impairs Plasmodium falciparum transmission in Anopheles arabiensis mosquitoes. Nat Commun. 2020;11:2187.
Han B, Weiss LM. Microsporidia: Obligate intracellular pathogens within the fungal kingdom. Microbiol Spectr. 2017;5:97–113. https://doi.org/10.1128/9781555819583.ch5.
Nattoh G, Maina T, Makhulu EE, Mbaisi L, Mararo E, Otieno FG, et al. Horizontal transmission of the symbiont Microsporidia MB in Anopheles arabiensis. Front Microbiol. 2021; 12:647183. https://doi.org/10.3389/fmicb.2021.647183.
Nepomichene TN, Andrianaivolambo L, Boyer S, Bourgouin C. Efficient method for establishing F1 progeny from wild populations of Anopheles mosquitoes. Malar J. 2017;16:21.
Coetzee M. Key to the females of Afrotropical Anopheles mosquitoes (Diptera: Culicidae). Malar J. 2020;19:70.
Santolamazza F, Mancini E, Simard F, Qi Y, Tu Z, Della TA. Insertion polymorphisms of SINE200 retrotransposons within speciation islands of Anopheles gambiae molecular forms. Malar J. 2008;7:1–10.
Takken W, Smallegange RC, Vigneau AJ, Johnston V, Brown M, Mordue-Luntz AJ, et al. Larval nutrition differentially affects adult fitness and Plasmodium development in the malaria vectors Anopheles gambiae and Anopheles stephensi. Parasites and Vectors. 2013; 6:1. https://doi.org/10.1186/1756-3305-6-345.
Van Schoor T, Kelly ET, Tam N, Attardo GM. Impacts of dietary nutritional composition on larval development and adult body composition in the yellow fever mosquito (Aedes aegypti). Insects. 2020;11:535.
Yan J, Kibech R, Stone CM. Differential effects of larval and adult nutrition on female survival, fecundity, and size of the yellow fever mosquito, Aedes aegypti. Front Zool. 2021;18:10.
Huestis DL, Yaro AS, Traoré AI, Adamou A, Kassogué Y, Diallo M, et al. Variation in metabolic rate of Anopheles gambiae and A. arabiensis in a Sahelian village. J Exp Biol. 2011;214:2345–53.
Maïga H, Dabiré RK, Lehmann T, Tripet F, Diabaté A. Variation in energy reserves and role of body size in the mating system of Anopheles gambiae. J Vector Ecol. 2012;37:289–97.
Bourtzis K, Dobson SL, Xi Z, Rasgon JL, Calvitti M, Moreira LA, et al. Harnessing mosquito-Wolbachia symbiosis for vector and disease control. Acta Trop. 2014;132:S150–63.
Couret J, Dotson E, Benedict MQ. Temperature, larval diet, and density effects on development rate and survival of Aedes aegypti (Diptera: Culicidae). PLoS ONE. 2014;9:e87468.
Araújo MDS, Gil LHS, E-Silva ADA. Larval food quantity affects development time, survival and adult biological traits that influence the vectorial capacity of Anopheles darlingi under laboratory conditions. Malar J. 2012;11:1–9.
Himler AG, Adachi-Hagimori T, Bergen JE, Kozuch A, Kelly SE, Tabashnik BE, et al. Rapid spread of a bacterial symbiont in an invasive whitefly is driven by fitness benefits and female bias. Science. 2011;332:254–6.
Shettima A, Joseph S, Ishak IH, Raiz SHA, Hasan HA, Othman N. Evaluation of total female and male Aedes aegypti proteomes reveals significant predictive protein–protein interactions, functional ontologies, and differentially abundant proteins. Insects. 2021;12:752.
Koga R, Tanahashi M, Nikoh N, Hosokawa T, Meng XY, Moriyama M, et al. Host’s guardian protein counters degenerative symbiont evolution. Proc Natl Acad Sci USA. 2021;118: e2103957118. https://doi.org/10.1073/pnas.2103957118.
Hosokawa T, Kikuchi Y, Fukatsu T. How many symbionts are provided by mothers, acquired by offspring, and needed for successful vertical transmission in an obligate insect-bacterium mutualism? Mol Ecol. 2007;16:5316–25.
Lyimo EO, Takken W. Effects of adult body size on fecundity and the pre-gravid rate of Anopheles gambiae females in Tanzania. Med Vet Entomol. 1993;7:328–32.
Briegel H. Metabolic relationship between female body size, reserves, and fecundity of Aedes aegypti. J Insect Physiol. 1990;36:165–72.
Huck DT, Klein MS, Meuti ME. Determining the effects of nutrition on the reproductive physiology of male mosquitoes. J Insect Physiol. 2021;129:104191.
Bryant KN, Newton ILG. The intracellular symbiont Wolbachia pipientis enhances recombination in a dose-dependent manner. Insects. 2020;11:284.
Snyder AK, Deberry JW, Runyen-Janecky L, Rio RVM. Nutrient provisioning facilitates homeostasis between tsetse fly (Diptera: Glossinidae) symbionts. Biol Sci. 2010;277:2389–97.
Wilkinson TL, Koga R, Fukatsu T. Role of host nutrition in symbiont regulation: Impact of dietary nitrogen on proliferation of obligate and facultative bacterial endosymbionts of the pea aphid Acyrthosiphon pisum. Appl Environ Microbiol. 2007;73:1362–6.
Acknowledgements
We are grateful for the assistance of David Alila, Elisha Obudho, and Charles Amara from ICIPE Mbita insectary team and Ian Okello and Sampson Okello. We also acknowledge the support provided by project administrators, Faith Kyengo and Ibrahim Kiche.
Funding
The authors gratefully acknowledge the financial support for this research by the following organisations and agencies: Open Philanthropy (SYMBIOVECTOR Track A); the Bill and Melinda Gates Foundation (INV0225840); the Children’s Investment Fund Foundation (SMBV-FFT); National Research Foundation of South Africa (Ref Numbers SRUG2203311457); the Swedish International Development Cooperation Agency (Sida); the Swiss Agency for Development and Cooperation (SDC); the Australian Centre for International Agricultural Research (ACIAR); the Norwegian Agency for Development Cooperation (Norad); the German Federal Ministry for Economic Cooperation and Development (BMZ); and the Government of the Republic of Kenya. The views expressed herein do not necessarily reflect the official opinion of the donors.”
Author information
Authors and Affiliations
Contributions
GYB.: Conceptualisation, data curation, validation, visualisation, formal analysis, investigation, methodology, writing—original draft and writing—review & editing. LLK: Conceptualisation, supervision, writing—review & editing. TB: Conceptualisation, data curation, formal analysis, methodology, supervision, validation, visualisation, writing—original draft, writing—review & editing. JKH: Conceptualisation, data curation, formal analysis, funding acquisition, methodology, supervision, validation, visualisation, writing—review & editing.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
A research permit was sought from the National Commission for Science, Technology and Innovation, Kenya before conducting the research (Reference No. 643670). Ethical approval for the collection of mosquitoes from households was obtained from The Scientific and Ethics Review Unit-Kenya Medical Research Institute (Non-KEMRI protocol number 4520). Ethics certificate number M220622 from the University of the Witwatersrand HREC, South Africa was also obtained.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
The manuscript does not report any detail on individuals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Experimental design to determine the effect of different larval diet regimes on Microsporidia MB prevalence and intensity and influence on Microsporidia MB on >An. arabiensis development fitness.
Additional file 2.
Main nutritional components of the diets.
Additional file 3.
Experimental design to determine the effect of Microsporidia MB on adult mosquito survival under different adult diet regimes.
Additional file 4.
(A) Experimental design to determine the effect of different larval diet quantity on Microsporidia MB intensity in the isofemale line of An. arabiensis after vertical transmission. (B) Experimental design to determine the effect of adult diet quantity on Microsporidia MB intensity in isofemale line of An. arabiensis after vertical transmission.
Additional file 5.
Data generated from the study.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Boanyah, G.Y., Koekemoer, L.L., Herren, J.K. et al. Effect of Microsporidia MB infection on the development and fitness of Anopheles arabiensis under different diet regimes. Parasites Vectors 17, 294 (2024). https://doi.org/10.1186/s13071-024-06365-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-024-06365-8