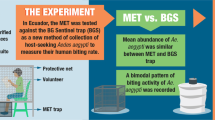
Abstract
Background
Mosquito-borne diseases, such as malaria, dengue, Zika and chikungunya, pose significant public health threats in tropical and subtropical regions worldwide. To mitigate the impact of these diseases on human health, effective vector surveillance and control strategies are necessary. Traditional vector control methods, which rely on chemical agents such as insecticides and larvicides, face challenges such as resistance and environmental concerns. Consequently, there has been a push to explore novel surveillance and control tools. Mass trapping interventions have emerged as a promising and environmentally friendly approach to reducing the burden of mosquito-borne diseases. This study assessed mass-trapping interventions using autocidal gravid ovitraps (AGOs) on Aedes aegypti populations in Reynosa, Tamaulipas, Mexico.
Methods
Four neighborhoods were selected to evaluate the effects of three treatments: AGO mass-trapping, integrated vector control (IVC), which included source reduction and the application of chemical larvicide and adulticide, and AGO + IVC on Ae. aegypti populations. A control area with no interventions was also included.
The effectiveness of the interventions was evaluated by comparing Ae. aegypti abundance between the pre-treatment period (9 weeks) and the post-treatment period (11 weeks) for each treatment.
Results
Only treatment using AGO mass trapping with an 84% coverage significantly reduced Ae. aegypti female populations by 47%, from 3.75 ± 0.32 to 1.96 ± 0.15 females/trap/week. As expected, the abundance of Ae. aegypti in the control area did not differ from the pre- and post-treatment period (range of 4.97 ± 0.59 to 5.78 ± 0.53); Ae. aegypti abundance in the IVC treatment was 3.47 ± 0.30 before and 4.13 ± 0.35 after, which was not significantly different. However, Ae. aegypti abundance in the AGO + IVC treatment increased from 1.43 ± 0.21 before to 2.11 ± 0.20 after interventions; this increase may be explained in part by the low AGO (56%) coverage.
Conclusions
This is the first report to our knowledge on the effectiveness of mass-trapping interventions with AGOs in Mexico, establishing AGOs as a potential tool for controlling Ae. aegypti in Northeastern Mexico when deployed with sufficient coverage.
Graphical Abstract

Similar content being viewed by others
Background
Aedes (Stegomyia) aegypti is among the most studied mosquitoes because of its role in the transmission of pathogens such as yellow fever, dengue, Zika and chikungunya viruses [1]. The risk of Ae. aegypti-borne arbovirus transmission is particularly high in the tropical and sub-tropical urban areas worldwide. This is attributed to abundant larval mosquito habitats in urban landscapes, especially in communities with low socioeconomic conditions which include elevated number of abandoned properties, urban decline and improper waste management practices [2, 3]. In Mexico, Ae. aegypti is considered the primary vector of dengue [4], Zika and chikungunya viruses [5, 6]. The Asian tiger mosquito, Aedes (Stegomyia) albopictus, is considered a potential vector of these arboviruses [7]. These two species co-exist in much of the tropical and subtropical regions of the world, with Ae. albopictus extending further into temperate regions [8, 9].
In Mexico, dengue, Zika and chikungunya have coexisted since 2015 [10], with dengue being the most prevalent and widespread, affecting 29 out of 32 States [11]. All four dengue virus serotypes circulate in Mexico, and over the last decade, the country has ranked as the second highest in Latin America in terms of dengue cases, second only to Brazil [12]. In the state of Tamaulipas, which borders the USA, an estimated 91,665 probable cases of dengue and 2966 cases of dengue hemorrhagic fever were reported between 2012 and 2023 [13]. Furthermore, there were a total of 823 reported cases of Zika in Tamaulipas between 2015 and 2023 [14].
Control of vector mosquitoes typically involves residual spraying of homes and surrounding areas with adulticides, treatment of peri-domestic water containers using larvicides or larval habitat source reduction [15]. However, these strategies have been insufficient for the sustained control of Aedes mosquitoes and reduction of human Aedes-borne diseases. A contributing factor to the challenge is the rapid development of insecticide-resistance populations of Ae. aegypti in diverse parts of Mexico [16,17,18]. The widespread rise of insecticide resistance has led to the search for new alternatives to control vector dengue mosquitoes to reduce of the reliance on chemical control [19].
The autocidal gravid ovitrap (AGO), developed by the United States Centers for Disease Control and Prevention (CDC), is an environmentally friendly, sticky, lethal ovitrap designed for surveillance and control of female gravid Aedes mosquitoes [20]. The implementation of AGOs in mosquito control interventions has been carried out mainly in Puerto Rico, showing positive outcomes by effectively decreasing mosquito densities in mass-trapping interventions in both small [21, 22] and large areas [23]. Furthermore, studies conducted in other countries such as Colombia and the US have demonstrated variable effectiveness of these traps for Ae. aegypti surveillance and control [24,25,26,27].
Here, we evaluated the effectiveness of AGO mass trapping on Ae. aegypti populations in an urban area of Northern Mexico. Our results confirm the utility of AGOs as an alternative control tool for Ae. aegypti populations in endemic neighborhoods of the US-Mexico border region.
Methods
Study areas
The city of Reynosa (704,767 inhabitants; 216, 207 houses; INEGI 2020) is located at 26°03′03.0ʺN, 98°17′52.4ʺW, 30 m above sea level, in the state of Tamaulipas, Mexico. This is a sister city to McAllen, Texas, in Hidalgo County, USA. Reynosa has a hot dry climate with an average temperature of 22 ºC; during the “canicula” event in the dry season, which lasts about 40 days, daily temperatures can reach 40 to 42 ºC during the months of July–August. During the winter, in the months of December–February, the minimum temperature ranged from 0 to 5 ºC [28]. The study was conducted from July to December 2022 in four urban areas of about 100 households/area. The choice of this period was based on the bimodal local seasonality of Ae. aegypti (March–June and September–November) in the region, where the highest abundance occurs in the autumn [29], concurrent with when human disease from Aedes-borne viruses are observed [30].
The four areas, Pedro José Mendez (26˚1′3.576ʺN, 98˚16′30.18ʺW), Balcones de Alcalá (26°00′08.1ʺN, 98°16′05.8" W), Independencia (26°00′41.3ʺN, 98°15′49.2ʺW) and Nuevo Amanecer (26°03′11.4ʺN, 98°14′33.3ʺW), were selected for this study (Fig. 1). Urban areas were chosen based on their history of recurrent dengue cases and their classification as low-income areas [29]. These areas are characterized by poor infrastructure, situated in semiurban areas with mostly one-story houses. Most streets are unpaved, with inadequate waste management practices and sewage systems. The streets frequently flood after rainfall, indicating poor storm water management with drains.
Study areas where SAGOs indicated by orange filled dots circles were deployed in Reynosa, Northern Mexico. The four areas are depicted individually and delineated by red lines: PJ: Pedro José Méndez, NA: Nuevo Amanecer, BA: Balcones de Alcalá, IN: Independencia; experimental households within each area were treated with Chlorpyrifos and cleaned; water-holding containers serving as breeding sites were removed. Map made with QGIS 3.16.6 (https://qgis.org/en/site/) and incorporated public domain map data from the Instituto Nacional de Estadística, Geografía e Informatica (National Institute of Statistics, Geography, and Computer Science [INEGI]; https://www.inegi.org.mx/app/mapas/), along with satellite images obtained from Google Maps (https://www.google.com/maps)
Experimental design
A repeated-measures design was used to assess the impact of three treatments, the placement of three AGOs, an IVC approach which included source reduction, larvicide (Spinosad) and adulticide (Chlorpyrifos) application, and a combination of both treatments (AGO + IVM), and a control area, on the reduction of Ae. aegypti female populations, comparing the temporal changes in female densities in the four selected areas (neighborhoods). These four areas were randomly assigned to each treatment. The experimental design included a 9-week pre-treatment period from July to September 2022 and an 11-week post-treatment period from September to December 2022 (Fig. 2).
In each area, approximately 100 houses per area were selected to assess the treatment effect. Balcones de Alcalá (103 houses) was randomly designated as the control area, Independencia (116 houses) received the AGO trap treatment, Pedro José Méndez (105 houses) received the IVC treatment, and Nuevo Amanecer (109 houses) received the AGO + IVC treatment.
Before commencing the study, the inhabitants of each area were informed about the scope and objectives of the study. The initial approach to the inhabitants for placing the AGO traps was attempted during the morning or afternoon. If this attempt was unsuccessful, a second attempt was made during the weekend. In cases where initial contact was not successful or the resident declined to participate in the study, we continued to approach the next household until we obtained verbal consent.
In addition, a series of actions were carried out aiming to reduce the presence of Ae. aegypti and achieve a low and similar population in the four evaluated areas. One week prior to the start of sentinel autocidal gravid ovitrap (SAGO) deployment, an IVC treatment was performed on all four sites. This IVC treatment was performed in a 2–3 street buffer around the core areas (Fig. 1).
To monitor the abundance of Ae. aegypti females, 60 AGOs served as SAGOs with 15 deployed in each of the four study areas. The SAGOs were placed in the front yard of the randomly selected houses, ensuring a maximum distance of 100 m between each trap (Fig. 1). The monitoring of Ae. aegypti female populations during the pre-treatment period took place on a weekly basis from July 24 to September 18, 2022 (9 weeks).
At the end of the pre-intervention period, the treatments (AGO, IVC, AGO + IVC) were implemented (Fig. 2). The treatment with AGO traps consisted of placing three traps in the front and backyard of most of the selected houses in the area (56–84%), except for houses with a SAGO trap, where only two AGOs were deployed. The IVC treatment included source reduction and the application of chemical larvicide and adulticide, while in the control area, only the SAGOs were maintained.
The weekly post-treatment sampling period was from September 25 to December 4, 2022 (11 weeks). Preventive maintenance of AGOs was carried out every 2 months, involving removing external dirt and replacing grass infusions and sticky glue boards. All captured mosquitoes in SAGOs were visually identified to species [31], sexed and graded as fed and unfed based on the status of their abdomen.
Statistical analysis
To examine whether there was a significant interaction of captured Ae. aegypti females between traps and weeks, we conducted an analysis of covariance (ANCOVA) using the MIXED procedure with a first-order autoregressive structure variance [32]. ANCOVA allowed us to control for potential covariates that might influence mosquito abundance, which is a crucial step in repeated measures studies, enabling a more robust assessment of the effects of interest and a more solid interpretation of the results obtained [33]. In this repeated measures study, the experimental unit was the SAGOs, with the number of females captured as the dependent variable and the location and trap number as random factors.
Additionally, to evaluate the effect of the treatments (AGO, IVC, AGO + IVC) on Ae. aegypti abundance, we contrasted the mean of Ae. aegypti females per SAGO during the pre-intervention period (9 weeks) compared to the post-intervention period (11 weeks). A multivariate negative binomial regression model was used, where the four treatments and time (pre and post) were considered predictors as class variables. The goodness of fit of the statistical model was evaluated using Pearson’s chi-square test for degrees of freedom (χ2/df), where values < 2 indicated a good fit of the model.
Differences between treatments and time were examined using the GLIMMIX procedure [34], designed to evaluate the effect of categorical, discrete or continuous variables with any probability distribution upon a count as a response variable. Additionally, comparisons between treatment and the control areas were calculated as a relative reduction in female Ae. aegypti by a modification of Henderson’s formula [35]:
where subscripts denote the mean of females at time t and the pre-treatment mean and control area [36]. Also, a comparison of least square means was performed using Student’s t tests.
All statistical analyses were conducted using SAS OnDemand for Academics [37].
Results
Ninety-three households of 105 from Pedro José Mendez (IVC area) were treated (88% coverage); 98 of 116 households from Independencia (AGO area) were treated (84% coverage; 294 AGOs), while in Nuevo Amanecer (AGO + IVC), only 62 of 109 households were treated (56% coverage; 186 AGOs) because many houses are abandoned; thus, the access to properties for deploying AGOs was not possible. Balcones de Alcala served as a control area with no treatment.
The results of the covariance analysis (Cov = − 0.0559; χ2 = 1.53, df = 1, P = 0.2167) indicate no significant interaction in the number of captured Ae. aegypti females between traps or weeks, regardless of the treatments. Therefore, the female abundance was independent between treatment and weeks.
The average number of Ae. aegypti females/trap/week in Balcones de Alcalá ranged from 4.97 ± 0.59 before interventions to 5.78 ± 0.53 after interventions. In Pedro José Mendez (IVC area), it was from 3.47 ± 0.30 to 4.13 ± 0.35, respectively. In Independencia (AGO area) from 3.75 ± 0.32 to 1.96 ± 0.15, respectively; and in Nuevo Amanecer (AGO + IVC area) from 1.43 ± 0.21 to 2.11 ± 0.20, respectively.
Only AGO treatment (Independencia) resulted in a significant decrease in Ae. aegypti female population in the pre- and post-intervention periods (GLIMMIX: F(1, 298) = 29.10, P < 0.0001) indicating a reduction of 47% (Fig. 3). Contrarily, AGO + IVC treatment (Nuevo Amanecer) resulted in a higher number (2.11 ± 0.20 /trap/week) of females caught in SAGOs post-intervention (GLIMMIX: F(1, 298) = 5.20, P = 0.0233) than that (1.43 ± 0.21 /trap/week) of pre-intervention. However, for IVC treatment (Pedro José Méndez), the female population was slightly reduced in the second week after interventions, but during the following weeks, that increased (GLIMMIX: F(1, 298) = 1.70, P = 0.1935). As expected, the population did not vary (GLIMMIX: F(1, 298) = 0.91, P = 0.3404) in the control area (Balcones de Alcalá).
Compared to the pre-treatment period, the overall reduction percentage in Pedro José Mendez (IVC area) was –2.3%, in Independencia (AGO area) it was 54.9%, and in Nuevo Amanecer (AGO + IVC area) it was –26.5% compared to the control area over the 11-week post-treatment period. Multiple comparisons of least square means revealed significant differences among all treatments (Additional file 1: Table S1).
Discussion
Among the three treatments we evaluated, only the AGO intervention (deploying three AGOs/household with 84% coverage of area) reduced the female Ae. aegypti by 47% when comparing the mean number of females/trap/week. Additionally, it achieved a reduction of 54.9% when compared against control area using this repeated measures study design. Previous studies [38, 39] have assessed mass trapping interventions as an alternative strategy for controlling dengue vector mosquito populations. These studies have shown the efficacy of various types of traps, including AGOs [21,22,23, 40], standard lethal ovitraps [41, 42] and BG-GATs, to suppress Ae. aegypti populations [43]. However, some interventions, like those assessing three MosquiTRAPs (a sticky ovitrap) [44] or three BG-sentinel traps per household, failed to reduce adult Ae. aegypti populations [45].
Similar interventions with AGOs (placing three AGO traps per home) have proven effective at reducing Ae. aegypti female populations in Puerto Rico by 70% [21] and 79% [22] compared to the control areas. Furthermore, larger scale interventions in a medium-sized city from Puerto Rico demonstrated that a decrease in female population could be achieved when trap coverage exceeded 60% [23]. Our study reinforces the utility of AGOs for the control of Ae. aegypti populations.
The IVC intervention initially showed a slight reduction in the number of Ae. aegypti females after the second week, but it was not steady. Overall, it did not effectively reduce the female population. In contrast, the AGO intervention (Independencia) decreased the Ae. aegypti population, and this decline was steady for the rest of the study. The most recent reports on resistance to insecticides or larvicides in the Tamaulipas state have indicated susceptibility to those used in our study, namely Spinosad and Chlorpyrifos [46, 47]. Hence, insecticide resistance may not have influenced the results of our study. Surprisingly, we were expecting the intervention treatment in Nuevo Amanecer (AGO + IVC) to reduce the Ae. aegypti abundance. Contrarily, the female population was even higher post-AGO + IVC intervention than that of pre-intervention. This outcome can only be attributed to the lower AGO coverage (56%) in the selected area, primarily because of the many abandoned houses where it was impossible to deploy AGOs. As reported in Juarez et al. [27], this inadequate coverage may result in insufficient adult control by the traps, limiting the effect of these interventions. Furthermore, a recent study in Puerto Rico demonstrated the influence of vacant, abandoned or uninhabited buildings on the productivity of Ae. aegypti, where mosquito pupae were abundant in abandoned and inhabited houses. This highlights their significant contribution to increasing mosquito densities, resulting in ineffective control of vector mosquitoes [48].
The influence of non-residential areas on mosquito densities during field evaluations is a critical factor to consider. Diverse breeding sites within these areas provide suitable habitats for mosquito larvae, contributing to their proliferation [49,50,51]. This could play a significant role in the maintenance of Ae. aegypti populations after treatments or interventions. Mosquitoes from these non-residential areas could then migrate (i.e. ‘spillover’) to study areas, potentially confounding the outcomes of the treatments.
In Puerto Rico, a similar intervention (source reduction, larviciding and placing 3 AGOs per house) [40] with a coverage of at least 80% of the area resulted in 92% and 84% reduction of the Ae. aegypti female population in the two intervened sites, respectively.
The level of coverage thus plays a crucial role in decreasing mosquito populations using mass trapping interventions as shown here. Evaluations conducted in Puerto Rico have shown that a coverage of ≥ 60% is necessary to effectively decrease Ae. aegypti populations in interventions using AGOs [21,22,23]. Similar effects have also been observed in interventions with BG-GATs, where areas with high coverage (≥ 80%) showed a significantly lower abundance of Ae. albopictus compared to areas with lower coverage (< 80) [43]. In contrast, interventions with low coverage (< 60%) using MosquiTRAPs [44] or BG-sentinel traps [45] did not result in a reduction of mosquito populations. In the present study, AGO coverage was only 56% in Nuevo Amanecer (AGO + IVC), which did not reduce the Ae. aegypti population; however, the AGO coverage in Independencia was 84% and thus significantly decreased the mean number of Ae. aegypti females.
The cost of mosquito traps, especially in mass trapping interventions, is an important factor to consider. According to Barrera et al. [21], the material cost for an AGO is USD$ 12.5. The SpringStar Biocare® Autocidal Gravid Ovitrap is sold commercially for USD$ 60 per two pack [52] but this price can be reduced for research and public health applications. This cost could be a limiting factor, especially in countries with a limited budget for mosquito control, where this may restrict the widespread adoption and long-term sustainability of mass trapping interventions.
Moreover, understanding the fundamental ecology and biology of Ae. aegypti, from larvae to adults, is crucial for effective vector control. The knowledge encompassing breeding site preferences, feeding behavior and flight range across various environments allows us to predict mosquito populations and target interventions. Additionally, insights into adult mosquito behavior, such as host-seeking and resting habits, are vital for implementing strategies like insecticide application and trap placement, maximizing effectiveness and minimizing cost [53, 54]. Since AGOs are designed to capture and eliminate the adult stage, the larval life stage could persist. Therefore, it would be advisable to combine AGOs with a larval control method to achieve better results.
This is the first report on the effectiveness of mass trapping interventions with AGOs in Mexico. One limitation of the interventions was the inability to completely isolate the evaluated areas because this was conducted in an urban setting. Thus, the possibility of mosquito migration from non-intervention areas could not be ruled out, which could have influenced results. In addition, the interventions included only a single replicate of each treatment, and future studies should scale up the evaluation to include multiple replicates.
Conclusions
This study highlights the potential of AGO mass trapping interventions as a valuable component of integrated vector management strategy for controlling Ae. aegypti populations. We found evidence of Ae. aegypti population suppression when AGO coverage was > 80%, confirming observations of prior work. However, a more comprehensive assessment of its effectiveness is necessary. Replication across diverse environmental conditions at multiple sites throughout different seasons is crucial. Furthermore, incorporating data on arboviral disease incidence and assessing the impact of mosquito populations from non-residential areas would provide more robust evidence regarding AGOs true potential, particularly as an alternative in areas where conventional adulticides are ineffective, such as those with insecticide resistance.
Availability of data and materials
The datasets employed and/or analyzed in the present study are available in the online supplementary material: Additional file 2: Aedes aegypti database from SAGOs.
Abbreviations
- AGO:
-
Autocidal gravid traps
- SAGO:
-
Sentinel autocidal gravid traps
- ICV:
-
Integrated vector control
References
Kraemer MUG, Reiner RC Jr, Brady OJ, Messina JP, Gilbert M, Pigott DM, et al. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat Microbiol. 2019;4:854–63.
de Jesús CR, Rogers RE. Habitat segregation patterns of container breeding mosquitos: the role of urban heat islands, vegetation cover, and income disparity in cemeteries of New Orleans. Int J Environ Res Public Health. 2022;19:245.
Damtew YT, Tong M, Varghese BM, Anikeeva O, Hansen A, Dear K, et al. Effects of high temperatures and heatwaves on dengue fever: a systematic review and meta-analysis. EBioMedicine. 2023;91:104582.
Fernández-Salas I, Danis-Lozano R, Casas-Martínez M, Ulloa A, Bond JG, Marina CF, et al. Historical inability to control Aedes aegypti as a main contributor of fast dispersal of chikungunya outbreaks in Latin America. Antiviral Res. 2015;124:30–42.
Izquierdo-Suzán M, Zárate S, Torres-Flores J, Correa-Morales F, González-Acosta C, Sevilla-Reyes EE, et al. Natural vertical transmission of Zika virus in larval Aedes aegypti populations, Morelos, Mexico. Emerg Infect Dis. 2019;25:1477–84.
Dzul-Manzanilla F, Martínez NE, Cruz-Nolasco M, Gutiérrez-Castro C, López-Damián L, Ibarra-López J, et al. Arbovirus surveillance and first report of Chikungunya virus in wild populations of Aedes aegypti from Guerrero, Mexico. J Am Mosq Control Assoc. 2015;31:275–7.
Pech-May A, Moo-Llanes DA, Puerto-Avila MB, Casas M, Danis-Lozano R, Ponce G, et al. Population genetics and ecological niche of invasive Aedes albopictus in Mexico. Acta Trop. 2016;157:30–41.
Lubinda J, Treviño CJA, Walsh MR, Moore AJ, Hanafi-Bojd AA, Akgun S, et al. Environmental suitability for Aedes aegypti and Aedes albopictus and the spatial distribution of major arboviral infections in Mexico. Parasite Epidemiol Control. 2019;12:e00116.
Laporta GZ, Potter AM, Oliveira JFA, Bourke BP, Pecor DB, Linton YM. Global distribution of Aedes aegypti and Aedes albopictus in a climate change scenario of regional rivalry. Insects. 2023;4:49.
Grijalva I, Grajales-Muñiz C, González-Bonilla C, Borja-Aburto VH, Paredes-Cruz M, Guerrero-Cantera J, et al. Zika and dengue but not chikungunya are associated with Guillain-Barré syndrome in Mexico: a case-control study. PLoS Negl Trop Dis. 2020;14:e0008032.
Secretaría de Salud. Programa de Acción Específico. Prevención y Control de Dengue 2013–2018. 2014. http://www.cenaprece.salud.gob.mx/descargas/pdf/PAE_PrevencionControlDengue2013_2018.pdf. Accessed 17 June 2024.
Pan American Health Organization (PAHO). Dengue fever in the Americas. 2023. https://www3.paho.org/data/index.php/en/mnu-topics/indicadores-dengue-en/dengue-nacional-en/252-dengue-pais-ano-en.html. Accessed 17 June 2024.
Secretaría de Salud. Histórico Boletín Epidemiológico. 2023. https://www.gob.mx/salud/acciones-y-programas/historico-boletin-epidemiologico. Accessed 17 June 2024.
Secretaría de Salud. Casos Confirmados de Infección por Virus Zika 2023. 2023. https://www.gob.mx/salud/documentos/casos-confirmados-de-infeccion-por-virus-zika-2023. Accessed 17 June 2024.
Flores-Suarez AE, Ponce-Garcia G, Lopez- Monroy B, Villanueva-Segura OK, Rodriguez-Sanchez IP, Arredondo-Jimenez JI, et al. Current status of the insecticide resistance in Aedes aegypti (Diptera: Culicidae) from Mexico. In: Trdan S, editor., et al., Insecticides resistance. London: InTech; 2016. p. 99–109.
Solis-Santoyo F, Rodriguez AD, Penilla-Navarro RP, Sanchez D, Castillo-Vera A, Lopez-Solis AD, et al. Insecticide resistance in Aedes aegypti from Tapachula, Mexico: spatial variation and response to historical insecticide use. PLoS Negl Trop Dis. 2021;15:e0009746.
Vazquez-Prokopec GM, Medina-Barreiro A, Che-Mendoza A, Dzul-Manzanilla F, Correa-Morales F, Guillermo-May G, et al. Deltamethrin resistance in Aedes aegypti results in treatment failure in Merida. Mexico PLoS Negl Trop Dis. 2017;11:e0005656.
Kuri-Morales PA, Correa-Morales F, González-Acosta C, Moreno-Garcia M, Santos-Luna R, Román-Pérez S, et al. Insecticide susceptibility status in Mexican populations of Stegomyia aegypti (= Aedes aegypti): a nationwide assessment. Med Vet Entomol. 2018;32:162–74.
Achee NL, Grieco JP, Vatandoost H, Seixas G, Pinto J, Ching-Ng L, et al. Alternative strategies for mosquito-borne arbovirus control. PLoS Negl Trop Dis. 2019;13:e0006822.
Mackay AJ, Amador M, Barrera R. An improved autocidal gravid ovitrap for the control and surveillance of Aedes aegypti. Parasit Vectors. 2013;6:225.
Barrera R, Amador M, Acevedo V, Caban B, Felix G, Mackay AJ. Use of the CDC autocidal gravid ovitrap to control and prevent outbreaks of Aedes aegypti (Diptera: Culicidae). J Med Entomol. 2014;51:145–54.
Barrera R, Amador M, Acevedo V, Hemme RR, Félix G. Sustained, area-wide control of Aedes aegypti using CDC autocidal gravid ovitraps. Am J Trop Med Hyg. 2014;91:1269–76.
Barrera R, Harris A, Hemme RR, Felix G, Nazario N, Muñoz-Jordan JL, et al. Citywide Control of Aedes aegypti (Diptera: Culicidae) during the 2016 Zika epidemic by integrating community awareness, education, source reduction, larvicides, and mass mosquito trapping. J Med Entomol. 2019;56:1033–46.
Montenegro D, Martinez L, Tay K, Hernandez T, Noriega D, Barbosa L, et al. Usefulness of autocidal gravid ovitraps for the surveillance and control of Aedes (Stegomyia) aegypti (Diptera: Culicidae) in eastern Colombia. Med Vet Entomol. 2020;34:379–84.
Martin E, Medeiros MCI, Carbajal E, Valdez E, Juarez JG, Garcia-Luna S, et al. Surveillance of Aedes aegypti indoors and outdoors using Autocidal Gravid Ovitraps in South Texas during local transmission of Zika virus, 2016 to 2018. Acta Trop. 2019;192:129–37.
Obregón JA, Ximenez MA, Villalobos EE, de Valdez MRW. Vector mosquito surveillance using centers for disease control and prevention autocidal gravid ovitraps in San Antonio, Texas. J Am Mosq Control Assoc. 2019;35:178–85.
Juarez JG, Chaves LF, Garcia-Luna SM, Martin E, Badillo-Vargas I, Medeiros MCI, et al. Variable coverage in an Autocidal Gravid Ovitrap intervention impacts efficacy of Aedes aegypti control. J Appl Ecol. 2021;58:2075–86.
Instituto Nacional de Estadística y Geografía (INEGI). Anuario estadístico y geográfico de Tamaulipas 2017. 2017. https://www.inegi.org.mx/contenidos/productos/prod_serv/contenidos/espanol/bvinegi/productos/nueva_estruc/anuarios_2017/702825094928.pdf. Accessed 10 Jan 2023.
Rodriguez-Perez MA, Russell TL, Olguin-Rodriguez O, Laredo-Tiscareño SV, Garza-Hernández JA, Reyes-Villanueva F. Dengue serotypes circulating in Aedes aegypti and humans in a poor or peripheral neighborhood at Reynosa, Mexico. Southwest Entomol. 2021;45:1025–38.
Kolimenakis A, Heinz S, Wilson ML, Winkler V, Yakob L, Michaelakis A, et al. The role of urbanisation in the spread of Aedes mosquitoes and the diseases they transmit—a systematic review. PLoS Negl Trop Dis. 2021;15:e0009631.
Carpenter S, LaCasse W. Mosquitoes of North America (North of Mexico). Berkeley: University of California Press; 1955.
Stevens JP. Analysis of covariance. In: Stevens JP, editor. Applied multivariate statistics for the social sciences. 5th ed. New York: Routledge; 2009. p. 287–314.
Littell RC, Pendergast J, Natarajan R. Modelling covariance structure in the analysis of repeated measures data. Stat Med. 2000;19:1793–819.
Jallow MFA, Dahab AA, Albaho MS, Devi VY, Jacob J, Al-Saeed O. Efficacy of mating disruption compared with chemical insecticides for controlling Tuta absoluta (Lepidoptera: Gelechiidae) in Kuwait. Appl Entomol Zool. 2020;55:213–21.
Henderson CF, Tilton EW. Tests with acaricides against the brown wheat mite. J Econ Entomol. 1955;48:157–61.
Figurskey AC, Hollingsworth B, Doyle MS, Reiskind MH. Effectiveness of autocidal gravid trapping and chemical control in altering abundance and age structure of Aedes albopictus. Pest Manag Sci. 2022;78:2931–9.
SAS OnDemand for Academics. 2021. https://www.sas.com/en_us/software/on-demand-for-academics.html. Accessed 20 Feb 2023.
Johnson BJ, Ritchie SA, Fonseca DM. The state of the art of lethal oviposition trap-based mass interventions for arboviral control. Insects. 2017;8:5.
Jaffal A, Fite J, Baldet T, Delaunay P, Jourdain F, Mora-Castillo R, et al. Current evidences of the efficacy of mosquito mass-trapping interventions to reduce Aedes aegypti and Aedes albopictus populations and Aedes-borne virus transmission. PLoS Negl Trop Dis. 2023;17:e0011153.
Barrera R, Amador M, Munoz J, Acevedo V. Integrated vector control of Aedes aegypti mosquitoes around target houses. Parasit Vectors. 2018;11:88.
Perich MJ, Kardec A, Braga IA, Portal IF, Burge R, Zeichner BC, et al. Field evaluation of a lethal ovitrap against dengue vectors in Brazil. Med Vet Entomol. 2003;17:205–10.
Kittayapong P, Chansang U, Chansang C, Bhumiratana A. Community participation and appropriate technologies for dengue vector control at transmission foci in Thailand. J Am Mosq Control Assoc. 2006;22:538–46.
Johnson BJ, Brosch D, Christiansen A, Wells E, Wells M, Bhandoola AF, et al. Neighbors help neighbors control urban mosquitoes. Sci Rep. 2018;8:15797.
Degener CM, de Ázara TM, Roque RA, Rösner S, Rocha ES, Kroon EG, et al. Mass trapping with MosquiTRAPs does not reduce Aedes aegypti abundance. Mem Inst Oswaldo Cruz. 2015;110:517–27.
Degener CM, Eiras AE, Azara TM, Roque RA, Rösner S, Codeço CT, et al. Evaluation of the effectiveness of mass trapping with BG-sentinel traps for dengue vector control: a cluster randomized controlled trial in Manaus, Brazil. J Med Entomol. 2014;51:408–20.
CENAPRECE. Resultados Finales Estudio de Larvicidas y Reguladores 2019. Mexico. 2020. https://www.gob.mx/cms/uploads/attachment/file/545444/Resultados_Finales_Larvicidas_2019.pdf. Accessed 27 Apr 2024.
CENAPRECE. Resultados Finales Estudio de Adulticidas 2018. Mexico. 2020. https://www.gob.mx/cms/uploads/attachment/file/545443/Resultados_Finales_Adulticidas_2018.pdf. Accessed 27 Apr 2024.
Barrera R, Acevedo V, Amador M. Role of abandoned and vacant houses on Aedes aegypti productivity. Am J Trop Med Hyg. 2021;104:145–50.
Dharmamuthuraja D, Rohini PD, Iswarya Lakshmi M, Isvaran K, Ghosh SK, Ishtiaq F. Determinants of Aedes mosquito larval ecology in a heterogeneous urban environment—a longitudinal study in Bengaluru, India. PLoS Negl Trop Dis. 2023;17:1–22.
Dos Reis IC, Honório NA, Codeço CT, de Magalhães MAFM, Lourenço-de-Oliveira R, Barcellos C. Relevance of differentiating between residential and non-residential premises for surveillance and control of Aedes aegypti in Rio de Janeiro, Brazil. Acta Trop. 2010;114:37–43.
Morrison AC, Sihuincha M, Stancil JD, Zamora E, Astete H, Olson JG, et al. Aedes aegypti (Diptera: Culicidae) production from nonresidential sites in the Amazonian city of Iquitos, Peru. Ann Trop Med Parasitol. 2006;100:73–86.
BFG Supply Co. SpringStar Biocare® Autocidal Gravid Ovitrap. 2024. https://www.bfgsupply.com/order-now/product/0/527362/springstar-ago-mosquito-traps-2ct-retail. Accessed 17 June 2024.
Benelli G, Jeffries CL, Walker T. Biological control of mosquito vectors: past, present, and future. Insects. 2016;3:52.
Mwingira V, Mboera LEG, Dicke M, Takken W. Exploiting the chemical ecology of mosquito oviposition behavior in mosquito surveillance and control: a review. J Vector Ecol. 2020;45:155–79.
Acknowledgements
The authors thank the residents of Pedro José Méndez, Independencia, Balcones de Alcalá and Nuevo Amanecer for their participation and cooperation in the study. We acknowledge Dr. Lihua Wei, Ricardo Palacios Santana, Abner Alejandro Soto Hernández, Miriam Anahi Andrade Reyes, Julissa Patricia Domínguez Osorio and the personnel from Jurisdicción Sanitaria 4: Reynosa (Iliana Villarreal Cantu and the vector control program staff) for their valuable assistance in the field work. We thank Dr. Olga Real-Najarro from Consejería de Educación, Madrid, Spain, for her assistance in reviewing the language. Last but not least, we thank Mr. Joshua Ramirez from the City Health Department in Harlingen, Texas, for supporting the field work approach.
Funding
The study was supported by Instituto Politécnico Nacional; México (SIP-20201972) and the National Institutes of Health R21AI128953. JGEF was supported by Grants SIP-IPN-20222157, 20221576, 20226932, 20230712 and 20231063. Texas A&M AgriLife Research supported the APC.
Author information
Authors and Affiliations
Contributions
JAAD drafted the initial version, collected data and conducted trap monitoring. GLH supervised, revised the draft and composed the final version of the manuscript. FRV performed statistical analysis on the data. NAFS and SUC supervised the field work and contributed to the early draft. LMRP, JGEF and MARP took part in study design and contributed to the final version of the draft.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
13071_2024_6361_MOESM1_ESM.docx
Additional file 1: Table S1. Student’s t-test multiple comparisons of least squares means among the four treatments evaluated in the average number of female Aedes aegypti caught in SAGOs.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Aguilar-Durán, J.A., Hamer, G.L., Reyes-Villanueva, F. et al. Effectiveness of mass trapping interventions using autocidal gravid ovitraps (AGO) for the control of the dengue vector, Aedes (Stegomyia) aegypti, in Northern Mexico. Parasites Vectors 17, 344 (2024). https://doi.org/10.1186/s13071-024-06361-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-024-06361-y