Abstract
Canine leishmaniasis is a widespread disease on the American continent, with cases reported from Uruguay to the USA and Canada. While numerous Leishmania spp. have been reported in dogs in this region, Leishmania infantum and Leishmania braziliensis are the most common etiological agents of canine leishmaniasis from a continental perspective. Nonetheless, other species may predominate locally in some countries. The participation of dogs in the transmission cycle of various Leishmania spp. has long been speculated, but evidence indicates that their role as reservoirs of species other than L. infantum is negligible. Various native wildlife (e.g., small rodents, marsupials, sloths, and monkeys) are, in fact, the primary hosts of Leishmania spp. in the Americas. In this review, an updated list of Leishmania spp. infecting dogs in the Americas is presented, along with their distribution and clinical and zoonotic importance
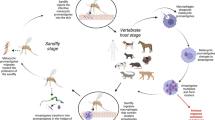
Graphical Abstract

Similar content being viewed by others
Background
Canine leishmaniasis is one of the most important vector-borne diseases affecting dogs worldwide [1]. This disease is caused by parasites of the genus Leishmania (Kinetoplastida: Trypanosomatidae), which are primarily transmitted by phlebotomine sand flies (Diptera: Psychodidae: Phlebotominae) [2]. Globally, Leishmania infantum is the most common species involved in the etiology of canine leishmaniasis [1]. Leishmania infantum infection in dogs may range from subclinical to life threatening, depending on the host’s ability to mount an effective immune response to the intracellular forms of the parasite [3]. In addition to the clinical importance, dogs are the most important reservoir hosts in the zoonotic transmission cycle of L. infantum [4, 5], which further highlights the importance of this disease from a public health perspective.
Although its clinical and zoonotic importance is beyond debate, L. infantum is not the only species involved. In fact, several other zoonotic Leishmania spp. have been detected in dogs worldwide [6,7,8], particularly in the Americas [9, 10]. In more recent years, the detection of distinct Leishmania spp. in dogs has been facilitated by the application of advanced DNA sequencing methodologies, including next-generation sequencing and nanopore sequencing [11, 12].
The objective of this review is to update and expand upon a previous review of canine leishmaniasis in South America [9]. The list of species infecting dogs is updated and extended to the whole American continent. An updated distribution map is provided, and the role of dogs as reservoirs of various Leishmania spp. on this continent is also discussed.
Etiology of canine leishmaniasis in the Americas
Numerous Leishmania spp. have been reported in dogs in the Americas (Table 1), including Leishmania amazonensis, Leishmania braziliensis, Leishmania colombiensis, Leishmania guyanensis, Leishmania infantum, Leishmania mexicana, Leishmania panamensis, Leishmania peruviana, Leishmania pifanoi, and Leishmania naiffi [9, 11,12,13,14,15,16,17,18]. Among these, L. colombiensis and L. pifanoi should be removed from the list of Leishmania spp. infecting dogs, as discussed below.
In 1990, Hashiguchi et al. [19] reported an isolate (MCAN/EC/88/INU2) identified as L. pifanoi obtained from a dog in Paute, Ecuador. In their original report, they provided no detailed information on the methods used for species identification. They said that the “INU 2 strain from Paute […] identified as Le. pifanoi (Tesh and Grimaldi, personal communication)” and “Detailed characterization of these Leishmania isolates from animals will be published elsewhere.” Previous review articles have cited this report of L. pifanoi in a dog from Ecuador [9, 10, 20]. Nonetheless, I recently dug a little deeper into the literature and found that the species reported by Hashiguchi et al. [19] as L. pifanoi was, in fact, L. mexicana, as they reported 1 year later, based on more comprehensive analyses, including restriction endonuclease analysis of Leishmania kinetoplast DNA [21]. Leishmania pifanoi is indeed very similar to L. mexicana (in fact, considered as synonyms by some authors), which probably resulted their initial misidentification by using monoclonal antibodies, isoenzyme electrophoresis, or both (unclear in Hashiguchi et al. [19]). Nonetheless, L. pifanoi is a species apparently restricted to Venezuela [22]. As a curiosity, my previous difficulty in finding this information in literature resulted from the fact that Hashiguchi et al. [21] slightly changed the strain name from MCAN/EC/88/INU2 (as reported originally in Hashiguchi et al. [19]) to MCAN/EC/88/PauteInu2.
Leishmania colombiensis is another species previously reported in dogs that should be excluded from the list of causative agents of canine leishmaniasis in the Americas. This species was reported in a dog from Venezuela that showed clinical signs of visceral leishmaniasis [23]. It is difficult to determine whether this parasite was the cause of the disease in the dog, and coinfection with L. infantum, which is also present in Venezuela, cannot be ruled out. More importantly, that species has recently been transferred to the genus Endotrypanum [24], so it should no longer be considered an agent of leishmaniasis in the narrow sense. Nonetheless, further investigations are needed to determine whether this parasite is pathogenic to dogs. If proven, the disease caused by E. colombiensis should be named canine endotrypanosomosis. Interestingly, this parasite is reputed to cause both cutaneous and visceral diseases in humans [25].
Other Leishmania spp. reported in humans but not in dogs in the Americas include L. lainsoni, L. lindenbergi, L. shawi, and L. venezuelensis [22, 26].
Geographical distribution
Available data indicate that canine leishmaniasis is widespread on the American continent, with cases described from Uruguay to the USA and Canada (Fig. 1) (Table 1). Across this wide distribution range, canine leishmaniasis is considered absent or rare in some countries, such as Belize, Chile, Guyana, and Suriname. However, there is enough evidence (e.g., the presence of sand fly vectors, human cases, and/or seropositive dogs) indicating the risk of canine leishmaniasis in these countries [27,28,29,30]. For instance, leishmaniasis is considered nonendemic in Chile, but supposedly, imported cases in humans [31] and seropositive dogs [29, 30] have been reported. Little is known about the sand fly fauna of Chile, where only a single sand fly species (Oligodontomyia isopsi) has been reported thus far [32]. Nonetheless, additional field studies are necessary to obtain a better picture of canine leishmaniasis in these countries, particularly to identify the causative agent and to confirm the presence of putative vectors.
Country-by-country distribution of Leishmania spp. in dogs in the Americas. This map was constructed with QGIS (https://qgis.org/en/site) and Natural Earth (https://www.naturalearthdata.com)
While canine leishmaniasis is not a notifiable disease in the Americas, it is reasonable to suppose that it is present in areas where human cases have been reported. As of 2012, human cases of visceral leishmaniasis have been reported in Argentina, Bolivia, Colombia, El Salvador, Honduras, Mexico, Nicaragua, Paraguay, and Venezuela [26]. Uruguay has now been added to this list, as both canine [33] and human cases [34] have been reported. French Guiana is still outside of this list, as no human cases of visceral leishmaniasis have been officially reported. Nonetheless, autochthonous cases of L. infantum infection in dogs have now been described in French Guiana [16], 13 years after a supposedly imported case [35]. This suggests that human cases of visceral leishmaniasis may be underdiagnosed in French Guiana. A similar situation has been observed in Panama, where the presence of Lutzomyia longipalpis (the main vector of L. infantum in the Americas) has long been known [26]. Supposedly imported cases of L. infantum infection have been reported in dogs from Panama [18]; therefore, the risk of visceral leishmaniasis establishment in this country, if not yet established, is high.
Human cases of cutaneous leishmaniasis caused by various Leishmania spp. have been reported in Argentina, Belize, Bolivia, Brazil, Colombia, Costa Rica, El Salvador, Ecuador, French Guiana, Guatemala, Guyana, Honduras, Mexico, Nicaragua, Panama, Paraguay, Peru, Suriname, and Venezuela [26]. Information on the presence of dogs infected by L. braziliensis or other Leishmania spp. causing cutaneous leishmaniasis in some of these countries (e.g., Belize, Costa Rica, El Salvador, Guatemala, Guyana, Honduras, and Nicaragua) is limited or virtually nonexistent. Nonetheless, further investigations in some of these countries will likely reveal that canine infections caused by species such as L. braziliensis and L. panamensis are common.
Leishmania infantum and L. braziliensis are the most widespread agents of canine leishmaniasis in the Americas. Canine infections caused by L. infantum have been reported in 17 countries, whereas those caused by L. braziliensis have been described in nine countries (Table 1). The apparent absence of L. braziliensis infections dogs in some countries (e.g., Belize, Costa Rica, Ecuador, French Guiana, Guatemala, Honduras, and Nicaragua) may be due to the lack of published reports, as L. braziliensis is known to occur in humans in these countries. Infections with other species in dogs are apparently more restricted geographically, but considering their distribution in humans, canine cases may currently be underestimated. This may well be the case for L. amazonensis, L. mexicana and L. panamensis.
Data on the prevalence of Leishmania spp. infection in dogs in the Americas have been reviewed elsewhere (e.g., [9, 10, 20, 36]). While discussing prevalence studies is outside of my objective here, I just want to emphasize that prevalence is an indicator that may vary widely in space and time. For instance, a study reported data from 73,964 dogs screened from 2008 to 2017 by state public health authorities in the Sobral municipality (Ceará state, northeastern Brazil), a traditional focus of human visceral leishmaniasis endemicity [37]. Considering the whole study period, the mean seroprevalence in the municipality was 3.8%, ranging from 1.6% to 13.1% according to district. However, the seroprevalence in each district varied widely annually, surpassing 50% on several occasions. In a similar fashion, the seroprevalence may vary widely according to the test used, as concluded from studies conducted in areas of active cutaneous leishmaniasis transmission. According to a comprehensive review article published in 1999 [20], the mean seroprevalence values were estimated to be 32.1% (range 14.7–58.9%) and 16.6% (range 0–63.2%) using the enzyme-linked immunosorbent assay (ELISA, eight studies) and indirect immunofluorescence (21 studies), respectively. The mean percentage of dogs that were positive according to the Montenegro skin test (13 studies) was 25.5% (range 0–66.7%). Another issue concerning serological studies is the possible cross-reactivity between different Leishmania spp. and Trypanosoma spp., which may be common in some foci [38, 39].
While cross-sectional studies are commonly conducted in the Americas, particularly in Latin America (reviewed in [10, 36], longitudinal studies are rare [40, 41], probably due to the inherent difficulties pertaining to this type of study. A study conducted in Goiana (Pernambuco, northeastern Brazil) and São Joaquim de Bicas (Minas Gerais, southeastern Brazil) reported yearly crude incidences of 19.6% and 43.8%, respectively, which were estimated by both serology and PCR [41]. This means that every year, a relatively high proportion of seronegative dogs living in these areas will seroconvert, become PCR positive, or both. This type of information is very important for understanding disease dynamics in endemic foci and may help to determine the magnitude of the disease control problem [40].
Clinical importance
From a clinical perspective, L. infantum is the agent of the most severe form of leishmaniasis in dogs [1, 42]. However, L. amazonensis has also been detected in dogs with clinical signs of visceral leishmaniasis [43, 44], which highlights the importance of using molecular approaches for a proper diagnosis and species identification. Excluding L. infantum and L. amazonensis, other Leishmania spp. have mostly been detected in dogs showing clinical signs of cutaneous leishmaniasis. While L. braziliensis is the most frequent agent of cutaneous leishmaniasis in dogs in the Americas, other species, such as L. panamensis, may also be common in some areas. For instance, during an outbreak of canine cutaneous leishmaniasis in Colombia, L. panamensis was isolated from 12 dogs, and L. braziliensis was isolated from eight dogs [45]. Regardless of the species involved, the dogs presented nodules or ulcers (0.4–10 cm in diameter), with evolution times ranging from 2 to 12 months [45]. Dogs presented single or multiple lesions but no systemic signs.
While canine cutaneous leishmaniasis is mostly a mild disease, some dogs may present disfiguring mucosal lesions and may sometimes die due to other health complications [46]. In Yucatan, Mexico, a 10-year-old intact female Chihuahua with cutaneous leishmaniasis attributed to L. mexicana died, probably due to renal failure (urea, 157 mg/dL; creatinine, 4 mg/dL) [46]. This dog was already receiving ramipril and furosemide due to congestive heart failure [46]. It is unlikely that L. mexicana infection itself caused this clinical condition, resulting in patient death, and information on the real-time PCR assay employed for species identification is incomplete. Nonetheless, although cutaneous leishmaniasis in dogs is usually a mild disease, this case highlights that a complete clinical evaluation of dogs with cutaneous leishmaniasis may be important, especially for geriatric dogs or dogs with other underlying medical conditions which could worsen the prognosis.
Zoonotic importance
Dogs are the primary reservoirs of L. infantum in the Americas [4, 5]. In a study conducted on Marajó Island, Pará state, northern Brazil, the basic case reproduction number (R0) was estimated to be 5.9 [40]. This means that, on average, each infected dog could generate approximately six new cases. Nonetheless, the infectiousness may vary widely from dog to dog, with dogs with high parasite numbers in their skin generally being more infectious to phlebotomine sand fly vectors than dogs with lower parasite numbers [47, 48]. Cats [49] and several other wildlife species (e.g., wild canids and nonhuman primates) [50, 51] may also serve as sources of infection for phlebotomine sand flies, although their actual epidemiological importance in the zoonotic transmission cycle of L. infantum needs further investigation. A contemporary example of the role played by other animals as reservoirs of L. infantum comes from Spain, where hares and rabbits were identified as the main sources of infection to phlebotomine sand fly vectors during an outbreak of human leishmaniasis in Madrid [52]. A series of studies clearly demonstrated that dogs played no role in this outbreak [53]. A study demonstrated that Lu. longipalpis can pick up L. infantum amastigotes while feeding on asymptomatic humans and that sick individuals coinfected with human immunodeficiency virus (HIV) are more infectious to this vector [54]. Again, the role of infected humans in endemic foci needs to be better understood considering the potential consequences for the control of visceral leishmaniasis.
Although the role of dogs as reservoirs of L. infantum is unequivocal, the indiscriminate elimination of seropositive dogs (i.e., dog culling strategy) has not been successful in controlling the incidence of human visceral leishmaniasis in Brazil [55]. The ineffectiveness of this strategy has been attributed to several factors, including the existence of other reservoirs [55].
The role of dogs as reservoirs of other Leishmania spp., particularly L. braziliensis, has been extensively investigated in the Americas. Although dogs are frequently exposed to L. braziliensis in endemic areas, their participation in the zoonotic transmission cycle of this parasite is likely negligible [4, 20, 56,57,58]. Indeed, excluding L. infantum, which was introduced in the Americas [59], all other Leishmania spp. detected in dogs in the Americas are native to the Neotropical region and are primarily maintained by wildlife reservoirs [4, 60, 61]. For instance, small rodents are exceptional hosts for L. braziliensis [62,63,64].
Dogs have been repeatedly suggested as reservoirs of L. peruviana and L. mexicana in Peru and Ecuador, respectively [65]. However, these conclusions are based on weak circumstantial evidence, as reviewed elsewhere [4, 20]. Indeed, there are apparently no studies demonstrating the infectiousness of dogs infected by these parasites to their respective phlebotomine sand fly vectors. Moreover, both L. peruviana and L. mexicana have also been detected in a wide range of small mammal species, some of which are considered potential reservoirs [60, 65].
Similarly, there is no evidence suggesting that dogs are potential reservoirs of L. panamensis in Colombia, as discussed appropriately by Vélez et al. [45]. Reports of infection by L. guyanensis and L. naiffi in dogs are very rare and clearly incidental. For example, L. naiffi has only recently been found in humans and dogs [11, 66] in Colombia, where the wild animal reservoir is, in fact, unknown.
Outstanding questions
Since 2009, an extraordinary number of field and laboratory studies on canine leishmaniasis in the Americas have been published in international literature. These include epidemiological studies focused on prevalence and risk factors (e.g., [67, 68]), studies validating new diagnostic tools (e.g., [69]), and clinical trials assessing the efficacy or effectiveness of therapeutic protocols (e.g., [70]) and prevention and control strategies (e.g., [71, 72]). The unified efforts of scientists, nongovernmental organizations [e.g., the Brasileish group (https://www.brasileish.com.br)], and public health authorities effectively contributed to positively changing our practices in terms of the diagnosis, treatment, prevention, and control of canine leishmaniasis, particularly in Brazil, where mass culling of seropositive dogs is no longer a common practice, 4% deltamethrin-impregnated dog collars are often applied to dogs in high-risk areas, and miltefosine is now licensed for use in dogs [55, 73, 74].
While most studies continue to be conducted by Brazilian leishmaniacs, the number of studies from other countries is also rapidly increasing (e.g., [18, 27,28,29,30, 33, 45]). It also amazes me the extraordinary number of studies on feline leishmaniasis, a neglected disease that is finally receiving the attention it deserves. These studies have, for instance, unequivocally demonstrated that cats are also infectious to sand fly vectors [49]. One of the outstanding research questions is the possible role of cats as reservoirs in the zoonotic transmission cycle of L. infantum [75]. The answer to this question may have practical implications for the control of leishmaniasis in areas where dogs, humans, and cats are at risk of infection.
Another important aspect to be understood is the unstoppable spread of canine leishmaniasis caused by L. infantum to new areas in the southern cone of South America [33] and to urbanized areas in already endemic regions [76]. The disease is also apparently expanding in the Caribbean region [77,78,79,80]. Drivers of this spreading process may include the movements of infected dogs and people and the expanding distribution range of sand fly vectors. For instance, Lu. longipalpis, which is present in Argentina, Bolivia, Brazil, Colombia, Costa Rica, El Salvador, Guatemala, Honduras, Mexico, Nicaragua, Panama, Paraguay, Uruguay, and Venezuela [81], is the main vector of L. infantum in most of the Americas. This vector has adapted to new areas in the southern cone of South America may be related to climate change [82]. In areas where Lu. longipalpis is absent, other species may be acting as local vectors of L. infantum. For instance, Lutzomyia cruzi is considered a vector of L. infantum in Corumbá city (Mato Grosso do Sul state, central-western Brazil) [83] and Bolivia [84]. Similarly, Migonemyia migonei is also a permissive vector of L. infantum and suspected to be involved in its transmission in some areas of Brazil [85] and Bolivia [84]. In the USA, Psathyromyia shannoni is a putative vector of L. infantum in areas where cases of canine leishmaniasis have been reported [86]. These examples suggest the need for more studies on the vectors involved in the Leishmania spp. transmission to dogs in the Americas, including in the USA [86].
Further research is needed to obtain a more reliable picture of the epidemiological situation of canine leishmaniasis in different American countries, where information is currently limited or virtually inexistent. Researchers should focus on the capture and identification of phlebotomine sand flies, detection of anti-Leishmania spp. antibodies, and molecular characterization of Leishmania spp. circulating in dogs. For instance, these studies could reveal the circulation of L. braziliensis among dogs in countries like Belize, Costa Rica, Ecuador, Guatemala, French Guiana, Honduras, and Nicaragua, where this parasite causes cutaneous and mucocutaneous leishmaniasis in humans [26].
Conclusions
Canine leishmaniasis is a widespread disease in the Americas, with a seroprevalence exceeding 50% in highly endemic foci. The disease may be caused by different Leishmania spp., but L. infantum and L. braziliensis are the most widespread and prevalent from a continental perspective. Leishmania infantum clearly expanded southwards in recent decades and is now endemic to parts of the southern cone of South America, including Uruguay. Considering the clinical importance of canine leishmaniasis and the limited treatment availability in the Americas, the use of preventive measures [55, 72,73,74, 87,88,89] is key to mitigating the risk of infection in uninfected dogs. This may also minimize the role of infected dogs as reservoirs, which is pivotal to reduce the risk of infection in humans and other susceptible animals, including cats.
Availability of data and materials
All the data supporting the conclusions of this review are cited in the references.
References
Dantas-Torres F, Solano-Gallego L, Baneth G, Ribeiro VM, de Paiva-Cavalcanti M, Otranto D. Canine leishmaniosis in the Old and New Worlds: unveiled similarities and differences. Trends Parasitol. 2012;28:531–8.
Maroli M, Feliciangeli MD, Bichaud L, Charrel RN, Gradoni L. Phlebotomine sandflies and the spreading of leishmaniases and other diseases of public health concern. Med Vet Entomol. 2013;27:123–47.
Giunchetti RC, Silveira P, Resende LA, Leite JC, Melo-Júnior OAO, Rodrigues-Alves ML, et al. Canine visceral leishmaniasis biomarkers and their employment in vaccines. Vet Parasitol. 2019;271:87–97.
Dantas-Torres F. The role of dogs as reservoirs of Leishmania parasites, with emphasis on Leishmania (Leishmania) infantum and Leishmania (Viannia) braziliensis. Vet Parasitol. 2007;149:139–46.
Quinnell RJ, Courtenay O. Transmission, reservoir hosts and control of zoonotic visceral leishmaniasis. Parasitology. 2009;136:1915–34.
Baneth G, Yasur-Landau D, Gilad M, Nachum-Biala Y. Canine leishmaniosis caused by Leishmania major and Leishmania tropica: comparative findings and serology. Parasit Vectors. 2017;10:113.
Mendoza-Roldan JA, Latrofa MS, Iatta R, Manoj RRS, Panarese R, Annoscia G, et al. Detection of Leishmania tarentolae in lizards, sand flies and dogs in southern Italy, where Leishmania infantum is endemic: hindrances and opportunities. Parasit Vectors. 2021;14:461.
Baneth G, Nachum-Biala Y, Adamsky O, Gunther I. Leishmania tropica and Leishmania infantum infection in dogs and cats in central Israel. Parasit Vectors. 2022;15:147.
Dantas-Torres F. Canine leishmaniosis in South America. Parasit Vectors. 2009;2:S1.
Marcondes M, Day MJ. Current status and management of canine leishmaniasis in Latin America. Res Vet Sci. 2019;123:261–72.
Patiño LH, Castillo-Castañeda AC, Muñoz M, Jaimes JE, Luna-Niño N, Hernández C, et al. Development of an amplicon-based next-generation sequencing protocol to identify Leishmania species and other trypanosomatids in leishmaniasis endemic areas. Microbiol Spectr. 2021;9:e0065221.
Patiño LH, Ballesteros N, Muñoz M, Jaimes J, Castillo-Castañeda AC, Madigan R, et al. Validation of Oxford nanopore sequencing for improved New World Leishmania species identification via analysis of 70-kDA heat shock protein. Parasit Vectors. 2023;16:458.
Herrer A, Christensen HA. Natural cutaneous leishmaniasis among dogs in Panama. Am J Trop Med Hyg. 1976;25:59–63.
Santaella J, Ocampo CB, Saravia NG, Méndez F, Góngora R, Gomez MA, et al. Leishmania (Viannia) infection in the domestic dog in Chaparral. Colombia Am J Trop Med Hyg. 2011;84:674–80.
Ramírez JD, Hernández C, León CM, Ayala MS, Flórez C, González C. Taxonomy, diversity, temporal and geographical distribution of cutaneous leishmaniasis in Colombia: A retrospective study. Sci Rep. 2016;6:28266.
Medkour H, Davoust B, Dulieu F, Maurizi L, Lamour T, Marié JL, et al. Potential animal reservoirs (dogs and bats) of human visceral leishmaniasis due to Leishmania infantum in French Guiana. PLoS Negl Trop Dis. 2019;13:e0007456.
Santos FJA, Nascimento LCS, Silva WB, Oliveira LP, Santos WS, Aguiar DCF, et al. First report of canine infection by Leishmania (Viannia) guyanensis in the Brazilian Amazon. Int J Environ Res Public Health. 2020;17:8488.
Terrero I, Pineda V, Vásquez V, Miranda A, Saldaña A, Calzada JE, et al. First report of imported canine visceral leishmaniasis cases in Panama, Central America: public health implications. Vet Parasitol Reg Stud Reports. 2022;32:100745.
Hashiguchi Y, Gomez EA, Mimori T, Furuya M, Flor T. A further trial of Leishmania isolation from wild and domestic animals in Ecuador. In: Hashiguchi Y, editor. Studies on the New World leishmaniasis and its transmission, with particular reference to Ecuador. Kochi: Kyowa Printing; 1990. p. 27–30.
Reithinger R, Davies CR. Is the domestic dog (Canis familiaris) a reservoir host of American cutaneous leishmaniasis? A critical review of the current evidence. Am J Trop Med Hyg. 1999;61:530–41.
Hashiguchi Y, Gomez EA, de Coronel VV, Mimori T, Kawabata M, Furuya M, et al. Andean leishmaniasis in Ecuador caused by infection with Leishmania mexicana and L. major-like parasites. Am J Trop Med Hyg. 1991;44:205–17.
Lainson R. Espécies neotropicais de Leishmania: uma breve revisão histórica sobre sua descoberta, ecologia e taxonomia. Rev Pan-Amaz Saude. 2010;1:13–32.
Delgado O, Castes M, White AC Jr, Kreutzer RD. Leishmania colombiensis in Venezuela. Am J Trop Med Hyg. 1993;48:145–7.
Espinosa OA, Serrano MG, Camargo EP, Teixeira MMG, Shaw JJ. An appraisal of the taxonomy and nomenclature of trypanosomatids presently classified as Leishmania and Endotrypanum. Parasitology. 2018;145:430–42.
Rodriguez-Bonfante C, Bonfante-Garrido R, Grimaldi G Jr, Momen H, Cupolillo E. Genotypically distinct Leishmania colombiensis isolates from Venezuela cause both cutaneous and visceral leishmaniasis in humans. Infect Genet Evol. 2003;3:119–24.
Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE. 2012;7:e35671.
Sophia DC, Aitor C, Claudia UC, Javier C, Delia G, Valeria G, et al. Large-scale survey for canine vector-borne parasites in free-ranging dogs and foxes from six diverse bioclimatic regions of Chile. Vet Parasitol Reg Stud Reports. 2022;30:100721.
Cevidanes A, Di Cataldo S, Muñoz-San Martín C, Latrofa MS, Hernández C, Cattan PE, et al. Co-infection patterns of vector-borne zoonotic pathogens in owned free-ranging dogs in central Chile. Vet Res Commun. 2023;47:575–85.
Milstein MS, Shaffer CA, Suse P, Marawanaru A, Heinrich DA, Larsen PA, et al. A mixed-methods approach to understanding domestic dog health and disease transmission risk in an indigenous reserve in Guyana South America. PLoS Negl Trop Dis. 2022;16:e0010469.
Kent A, Ramkalup P, Mans D, Schallig H. Is the dog a possible reservoir for cutaneous leishmaniasis in Suriname? J Trop Med. 2013;2013:324140.
Navarrete-Dechent C, Cevallos C, Jercic MI, Saldias-Fuentes C, González S, Labarca J. Liposomal amphotericin B treatment of cutaneous leishmaniasis caused by L. braziliensis: an imported case report. Rev Chilena Infectol. 2018;35:612–66.
Leger N, Ferte H. Première mention de phlébotomes au Chili et description de Lutzomyia isopsi n. sp. (Diptera-Psychodidae). Parasite. 1996;3:193–5.
Satragno D, Faral-Tello P, Canneva B, Verger L, Lozano A, Vitale E, et al. Autochthonous outbreak and expansion of canine visceral leishmaniasis. Uruguay Emerg Infect Dis. 2017;23:536–8.
Cabrera A, Pita S, González T, Viera A, Verger L, Piegas S, et al. Genetic variability highlights the invasion route of the Lutzomyia longipalpis complex, the main vector of Visceral Leishmaniasis in Uruguay. Zoonoses Public Health. 2023;70:383–92.
Rotureau B, Ravel C, Aznar C, Carme B, Dedet JP. First report of Leishmania infantum in French Guiana: canine visceral leishmaniasis imported from the Old World. J Clin Microbiol. 2006;44:1120–2.
Maggi RG, Krämer F. A review on the occurrence of companion vector-borne diseases in pet animals in Latin America. Parasit Vectors. 2019;12:145.
Sousa-Paula LC, Silva LGD, Sales KGS, Dantas-Torres F. Failure of the dog culling strategy in controlling human visceral leishmaniasis in Brazil: a screening coverage issue? PLoS Negl Trop Dis. 2019;13:e0007553.
Silva DA, Madeira MF, Teixeira AC, de Souza CM, Figueiredo FB. Laboratory tests performed on Leishmania seroreactive dogs euthanized by the Leishmaniasis control program. Vet Parasitol. 2011;179:257–61.
Alves AS, Mouta-Confort E, Figueiredo FB, Oliveira RV, Schubach AO, Madeira MF. Evaluation of serological cross-reactivity between canine visceral Leishmaniasis and natural infection by Trypanosoma caninum. Res Vet Sci. 2012;93:1329–33.
Quinnell RJ, Courtenay O, Garcez L, Dye C. The epidemiology of canine leishmaniasis: transmission rates estimated from a cohort study in Amazonian Brazil. Parasitology. 1997;115:143–56.
Dantas-Torres F, Figueredo LA, Sales KGS, Miranda DEO, Alexandre JLA, da Silva YY, et al. Prevalence and incidence of vector-borne pathogens in unprotected dogs in two Brazilian regions. Parasit Vectors. 2020;13:195.
Baneth G, Solano-Gallego L. Leishmaniasis. Vet Clin North Am Small Anim Pract. 2022;52:1359–75.
Tolezano JE, Uliana SR, Taniguchi HH, Araújo MF, Barbosa JA, Barbosa JE, et al. The first records of Leishmania (Leishmania) amazonensis in dogs (Canis familiaris) diagnosed clinically as having canine visceral Leishmaniasis from Araçatuba County, São Paulo State Brazil. Vet Parasitol. 2007;149:280–4.
Valdivia HO, Almeida LV, Roatt BM, Reis-Cunha JL, Pereira AA, Gontijo C, et al. Comparative genomics of canine-isolated Leishmania (Leishmania) amazonensis from an endemic focus of visceral leishmaniasis in Governador Valadares, southeastern Brazil. Sci Rep. 2017;7:40804.
Vélez ID, Carrillo LM, López L, Rodríguez E, Robledo SM. An epidemic outbreak of canine cutaneous leishmaniasis in Colombia caused by Leishmania braziliensis and Leishmania panamensis. Am J Trop Med Hyg. 2012;86:807–11.
Ortega-Pacheco A, Gutierrez-Blanco E, Escamilla- Flores W, Cordero-Guillermo L, Jiménez-Coello M, Loria-Cervera N. A fatal case of canine cutaneous leishmaniosis in a dog. Ann Parasitol. 2019;65:183–6.
Courtenay O, Carson C, Calvo-Bado L, Garcez LM, Quinnell RJ. Heterogeneities in Leishmania infantum infection: using skin parasite burdens to identify highly infectious dogs. PLoS Negl Trop Dis. 2014;8:e2583.
Courtenay O, Peters NC, Rogers ME, Bern C. Combining epidemiology with basic biology of sand flies, parasites, and hosts to inform Leishmaniasis transmission dynamics and control. PLoS Pathog. 2017;13:e1006571.
Batista JF, Magalhães Neto FDCR, Lopes KSPDP, Sato MO, Costa CHN, Mendonça IL. Transmission of Leishmania infantum from cats to dogs. Rev Bras Parasitol Vet. 2020;29:e017820.
Mol JP, Soave SA, Turchetti AP, Pinheiro GR, Pessanha AT, Malta MC, et al. Transmissibility of Leishmania infantum from maned wolves (Chrysocyon brachyurus) and bush dogs (Speothos venaticus) to Lutzomyia longipalpis. Vet Parasitol. 2015;212:86–91.
Rodrigues de Oliveira A, Pinheiro GRG, Tinoco HP, Loyola ME, Coelho CM, Dias ES, et al. Competence of non-human primates to transmit Leishmania infantum to the invertebrate vector Lutzomyia longipalpis. PLoS Negl Trop Dis. 2019;13:e0007313.
Fernández-Arévalo A, González E, Ballart C, Martín-Martín I, Tebar S, Muñoz C, et al. Typing of Leishmania isolates from vectors and leporids of the Madrid (Spain) outbreak. Parasitology. 2024;151:213–9.
Miró G, Müller A, Montoya A, Checa R, Marino V, Marino E, et al. Epidemiological role of dogs since the human leishmaniosis outbreak in Madrid. Parasit Vectors. 2017;10:209.
Ferreira GR, Castelo Branco Ribeiro JC, Meneses Filho A, de Farias Pereira TJC, Parente DM, Pereira HF, et al. Human competence to transmit Leishmania infantum to Lutzomyia longipalpis and the influence of human immunodeficiency virus infection. Am J Trop Med Hyg. 2018;98:126–33.
Dantas-Torres F, Miró G, Bowman DD, Gradoni L, Otranto D. Culling dogs for zoonotic visceral leishmaniasis control: the wind of change. Trends Parasitol. 2019;35:97–101.
Vexenat JA, Barretto AC, Rosa AC. Infecção experimental de Lutzomyia whitmani em cães infectados com Leishmania braziliensis braziliensis. Mem Inst Oswaldo Cruz. 1986;81:125–6.
Travi BL, Tabares CJ, Cadena H. Leishmania (Viannia) braziliensis infection in two Colombian dogs: a note on infectivity for sand flies and response to treatment. Biomedica. 2006;26:249–53.
Calzada JE, Saldaña A, González K, Rigg C, Pineda V, Santamaría AM, et al. Cutaneous leishmaniasis in dogs: is high seroprevalence indicative of a reservoir role? Parasitology. 2015;142:1202–14.
Leblois R, Kuhls K, François O, Schönian G, Wirth T. Guns, germs and dogs: on the origin of Leishmania chagasi. Infect Genet Evol. 2011;11:1091–5.
Roque AL, Jansen AM. Wild and synanthropic reservoirs of Leishmania species in the Americas. Int J Parasitol Parasites Wildl. 2014;3:251–62.
Maia C, Dantas-Torres F, Campino L. Parasite biology: the reservoir hosts. In: Bruschi F, Gradoni L, editors. The Leishmaniases: old neglected tropical diseases. Berlin: Springer; 2018. p. 79–106.
Roque AL, Cupolillo E, Marchevsky RS, Jansen AM. Thrichomys laurentius (Rodentia; Echimyidae) as a putative reservoir of Leishmania infantum and L. braziliensis: patterns of experimental infection. PLoS Negl Trop Dis. 2010;4:e589.
Andrade MS, Courtenay O, Brito ME, Carvalho FG, Carvalho AW, Soares F, et al. Infectiousness of sylvatic and synanthropic small rodents implicates a multi-host reservoir of Leishmania (Viannia) braziliensis. PLoS Negl Trop Dis. 2015;9:e0004137.
Marinho-Júnior JF, Monteiro JFCLS, Sales de Carvalho AW, de Carvalho FG, de Paiva CM, Shaw J, et al. High levels of infectiousness of asymptomatic Leishmania (Viannia) braziliensis infections in wild rodents highlights their importance in the epidemiology of American tegumentary leishmaniasis in Brazil. PLoS Negl Trop Dis. 2023;17:e0010996.
Hashiguchi Y, Gomez LEA, Cáceres AG, Velez LN, Villegas NV, et al. Andean cutaneous Leishmaniasis (Andean-CL, uta) in Peru and Ecuador: the causative Leishmania parasites and clinico-epidemiological features. Acta Trop. 2018;177:135–45.
Correa-Cárdenas CA, Pérez J, Patiño LH, Ramírez JD, Duque MC, Romero Y, et al. Distribution, treatment outcome and genetic diversity of Leishmania species in military personnel from Colombia with cutaneous Leishmaniasis. BMC Infect Dis. 2020;20:938.
Silva DM, Passarella Teixeira AI, Sierra Romero GA. Socioeconomic status of guardians as a risk factor for canine visceral leishmaniasis: a cohort study in an endemic area of the Federal District. Brazil Am J Trop Med Hyg. 2022;108:328–34.
Chiyo L, Dos Santos AG, de Souza AB, Rivas AV, Valle SB, Sevá ADP, et al. Cross-sectional spatial and epidemiological analysis of canine visceral leishmaniasis cases in the triple border region, Brazil, Argentina and Paraguay, between 2015 and 2020. Acta Trop. 2023;239:106811.
Figueiredo FB, Vasconcelos TCB, Madeira MF, Menezes RC, Maia-Elkhoury ANS, Marcelino AP, et al. Validation of the Dual-path Platform chromatographic immunoassay (DPP® CVL rapid test) for the serodiagnosis of canine visceral leishmaniasis. Mem Inst Oswaldo Cruz. 2018;113:e180260.
Nogueira FS, Avino VC, Galvis-Ovallos F, Pereira-Chioccola VL, Moreira MAB, Romariz APPL, et al. Use of miltefosine to treat canine visceral leishmaniasis caused by Leishmania infantum in Brazil. Parasit Vectors. 2019;12:79.
Alves EB, Figueiredo FB, Rocha MF, Castro MC, Werneck GL. Effectiveness of insecticide-impregnated collars for the control of canine visceral leishmaniasis. Prev Vet Med. 2020;182:105104.
Werneck GL, Figueiredo FB, Cruz MDSPE. Impact of 4% deltamethrin-impregnated dog collars on the incidence of human visceral Leishmaniasis: a community intervention trial in Brazil. Pathogens. 2024;13:135.
Dantas-Torres F, Miró G, Baneth G, Bourdeau P, Breitschwerdt E, Capelli G, et al. Canine Leishmaniasis control in the context of one health. Emerg Infect Dis. 2019;25:1–4.
Dantas-Torres F, Nogueira FDS, Menz I, Tabanez P, da Silva SM, Ribeiro VM, et al. Vaccination against canine Leishmaniasis in Brazil. Int J Parasitol. 2020;50:171–6.
Dalvi APR, Carvalho TDG, Werneck GL. Is there an association between exposure to cats and occurrence of visceral Leishmaniasis in humans and dogs? Vector Borne Zoonotic Dis. 2018;18:335–42.
Arbeláez N, Moreno J, Murillo J, Montoya A, Robledo SM, Vélez A, et al. First report of an urban case of canine visceral Leishmaniasis in the municipality of Cali. Colombia Am J Trop Med Hyg. 2020;102:289–93.
Rosypal AC, Tripp S, Kinlaw C, Sharma RN, Stone D, Dubey JP. Seroprevalence of canine Leishmaniasis and American trypanosomiasis in dogs from Grenada. West Indies J Parasitol. 2010;96:228–9.
Kumthekar S, Chikweto A, Chawla P, Tiwari KP, Gozlan J, Langeois Q, et al. Seroprevalence of canine Leishmaniasis in owned and stray dogs from Grenada. West Indies J Anim Res. 2014;4:131–9.
Castillo-Alcala F, Marshall S, Beeler-Marfisi J, Beierschmitt A, Scorpio D, Yao C. First case of canine Leishmaniasis on the island of St Kitts, West Indies. Vet Parasitol Reg Stud Reports. 2016;6:39–41.
Yao C. Leishmania spp. and Leishmaniasis on the Caribbean islands. Trans R Soc Trop Med Hyg. 2020;114:73–8.
Shimabukuro PHF, de Andrade AJ, Galati EAB. Checklist of American sand flies (Diptera, Psychodidae, Phlebotominae): genera, species, and their distribution. Zookeys. 2017;660:67–106.
Peterson AT, Campbell LP, Moo-Llanes DA, Travi B, González C, Ferro MC, et al. Influences of climate change on the potential distribution of Lutzomyia longipalpis sensu lato (Psychodidae: Phlebotominae). Int J Parasitol. 2017;47:667–74.
Falcão de Oliveira E, Oshiro ET, Fernandes WS, Murat PG, de Medeiros MJ, Souza AI, et al. Experimental infection and transmission of Leishmania by Lutzomyia cruzi (Diptera: Psychodidae): aspects of the ecology of parasite-vector interactions. PLoS Negl Trop Dis. 2017;11:e0005401.
Mollinedo JS, Mollinedo ZA, Gironda WJ, Mollinedo RE, Mollinedo P, Salomón OD. Visceral Leishmaniasis in Bolivia: current status. Rev Soc Bras Med Trop. 2020;53:e20190421.
Alexandre J, Sadlova J, Lestinova T, Vojtkova B, Jancarova M, Podesvova L, et al. Experimental infections and co-infections with Leishmania braziliensis and Leishmania infantum in two sand fly species, Lutzomyia migonei and Lutzomyia longipalpis. Sci Rep. 2020;10:3566.
Beasley EA, Mahachi KG, Petersen CA. Possibility of Leishmania transmission via Lutzomyia spp. sand flies within the USA and implications for human and canine autochthonous infection. Curr Trop Med Rep. 2022;9:160–8.
Miró G, Petersen C, Cardoso L, Bourdeau P, Baneth G, Solano-Gallego L, et al. Novel areas for prevention and control of canine leishmaniosis. Trends Parasitol. 2017;33:718–30.
Travi BL, Cordeiro-da-Silva A, Dantas-Torres F, Miró G. Canine visceral leishmaniasis: Diagnosis and management of the reservoir living among us. PLoS Negl Trop Dis. 2018;12:e0006082.
Ibiapina AB, Batista FMA, Aguiar BGA, Mendonça VJ, Costa DL, Costa CHN, et al. Evidence map of diagnosis, treatment, prognosis, prevention, and control in visceral leishmaniasis. Rev Panam Salud Publica. 2022;46:e89.
Brazil RP, Rodrigues AAF, Andrade Filho JD. Sand fly vectors of Leishmania in the Americas—a mini review. Entomol Ornithol Herpetol. 2015;4:144.
Cantanhêde LM, Cupolillo E. Leishmania (Viannia) naiffi Lainson & Shaw 1989. Parasit Vectors. 2023;16:194.
Herrera G, Higuera A, Patiño LH, Ayala MS, Ramírez JD. Description of Leishmania species among dogs and humans in Colombian visceral Leishmaniasis outbreaks. Infect Genet Evol. 2018;64:135–8.
Marco JD, Barroso PA, Calvopiña M, Kumazawa H, Furuya M, Korenaga M, et al. Species assignation of Leishmania from human and canine American tegumentary leishmaniasis cases by multilocus enzyme electrophoresis in North Argentina. Am J Trop Med Hyg. 2005;72:606–11.
Le Pont F, Mollinedo S, Mouchet J, Desjeux P. Leishmaniasis in Bolivia. IV. The dog in the cycles of Leishmaniasis in Bolivia. Mem Inst Oswaldo Cruz. 1989;1989:417–21.
Pirmez C, Coutinho SG, Marzochi MC, Nunes MP, Grimaldi G Jr. Canine American cutaneous leishmaniasis: a clinical and immunological study in dogs naturally infected with Leishmania braziliensis braziliensis in an endemic area of Rio de Janeiro. Brazil Am J Trop Med Hyg. 1988;38:52–8.
Arjona-Jiménez G, Villegas N, López-Céspedes A, Marín C, Longoni SS, Bolio-González ME, et al. Prevalence of antibodies against three species of Leishmania (L. mexicana, L. braziliensis, L. infantum) and possible associated factors in dogs from Mérida, Yucatán, Mexico. Trans R Soc Trop Med Hyg. 2012;106:252–8.
Oddone R, Canese A, Jamjoon M, Nolder D, Miles MA, Noyes H. Genetic diversity among Paraguayan isolates of Leishmania braziliensis. Trans R Soc Trop Med Hyg. 2002;96:364.
Aguilar CM, Fernández E, de Fernández R, Deane LM. Study of an outbreak of cutaneous Leishmaniasis in Venezuela. The role of domestic animals. Mem Inst Oswaldo Cruz. 1984;79:181–95.
Torrellas A, Ferrer E, Cruz I, Lima H, Delgado O, Rangel JC, et al. Molecular typing reveals the co-existence of two transmission cycles of American cutaneous Leishmaniasis in the Andean Region of Venezuela with Lutzomyia migonei as the vector. Mem Inst Oswaldo Cruz. 2018;113:e180323.
Marco JD, Barroso PA, Locatelli FM, Cajal SP, Hoyos CL, Nevot MC, et al. Multilocus sequence typing approach for a broader range of species of Leishmania genus: describing parasite diversity in Argentina. Infect Genet Evol. 2015;30:308–17.
Braga RR, Lainson R, Shaw JJ, Ryan L, Silveira FT. Leishmaniasis in Brazil. XXII: Characterization of Leishmania from man, dogs and the sandfly Lutzomyia longipalpis (Lutz & Neiva, 1912) isolated during an outbreak of visceral leishmaniasis in Santarém, Pará State. Trans R Soc Trop Med Hyg. 1986;80:143–5.
Gin TE, Lashnits E, Wilson JM, Breitschwerdt EB, Qurollo B. Demographics and travel history of imported and autochthonous cases of leishmaniosis in dogs in the United States and Canada, 2006 to 2019. J Vet Intern Med. 2021;35:954–64.
Gaskin AA, Schantz P, Jackson J, Birkenheuer A, Tomlinson L, Gramiccia M, et al. Visceral leishmaniasis in a New York foxhound kennel. J Vet Intern Med. 2002;16:34–44.
Zerpa O, Pratlong F, Ulrich M, Convit J. Isolation of Leishmania infantum, zymodeme MON-1 from canine and human visceral leishmaniasis on Margarita Island. Venezuela Mem Inst Oswaldo Cruz. 2001;96:901–2.
Velasco-Castrejón O, Rivas-Sanchez B, Munguia-Saldaña A, Hobart O. Leishmaniasis cutánea de perros en México. Enf Inf Microbiol. 2009;29:135–40.
Castillo-Ureta H, Zazueta-Moreno JM, Rendón-Maldonado JG, Torres-Avendaño JI, López-Moreno HS, Olimón-Andalón V, et al. First report of autochthonous canine leishmaniasis caused by Leishmania (L.) mexicana in Sinaloa, Mexico. Acta Trop. 2019;190:253–6.
Kipp EJ, Mariscal J, Armijos RX, Weigel M, Waldrup K. Genetic evidence of enzootic Leishmaniasis in a stray canine and Texas mouse from sites in west and central Texas. Mem Inst Oswaldo Cruz. 2016;111:652–4.
Dereure J, Espinel I, Barrera C, Guerrini F, Martini A, Echeverria R, et al. Leishmaniasis in Ecuador. 4. Natural infestation of the dog by Leishmania panamensis. Ann Soc Belg Med Trop. 1994;74:29–33.
Christensen HA, Fairchild GB, Herrer A, Johnson CM, Young DG, de Vásquez AM. The ecology of cutaneous leishmaniasis in the Republic of Panama. J Med Entomol. 1983;20:463–84.
Acknowledgements
I thank all the students and colleagues who have collaborated with me during the past 20 years in laboratory and field studies on canine leishmaniasis in Brazil. I am also grateful to Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco (FACEPE), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and Elanco Animal Health (formerly Bayer Animal Health) for supporting these studies.
Funding
No funding was received to assist in the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Filipe Dantas-Torres is the sole author of this manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Filipe Dantas-Torres is the Editor-in-Chief of Parasites and Vectors. This review was independently edited by Adnan Hodžić (Subject Editor).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Dantas-Torres, F. Canine leishmaniasis in the Americas: etiology, distribution, and clinical and zoonotic importance. Parasites Vectors 17, 198 (2024). https://doi.org/10.1186/s13071-024-06282-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-024-06282-w