Abstract
Background
Anopheles sacharovi, a member of the Anopheles maculipennis complex, was a historical malaria vector in Italy, no longer found since the last report at the end of 1960s. In September 2022, within the Surveillance Project for the residual anophelism, a single specimen of An. maculipennis sensu lato collected in Lecce municipality (Apulia region) was molecularly identified as An. sacharovi. This record led to implement a targeted entomological survey in September 2023.
Methods
Investigation was conducted in the areas around the first discovery, focusing on animal farms, riding stables and potential breeding sites. Adult and immature mosquitoes were collected, using active search or traps, in several natural and rural sites. Mosquitoes belonging to An. maculipennis complex were identified morphologically and molecularly by a home-made routine quantitative polymerase chain reaction (qPCR) assay, developed specifically for the rapid identification of An. labranchiae, and, when necessary, by amplification and sequencing of the ITS-2 molecular marker.
Results
Out of the 11 sites investigated, 6 were positive for Anopheles presence. All 20 An. maculipennis s.l. (7 adults, 10 larvae and 3 pupae) collected in the areas were identified as An. sacharovi by ITS-2 sequencing.
Conclusions
The discovery of An. sacharovi, considered to have disappeared from Italy for over 50 years, has a strong health relevance and impact, highlighting an increase in the receptivity of the southern areas. As imported malaria cases in European countries are reported every year, the risk of Plasmodium introduction by gametocyte carriers among travellers from endemic countries should be taken into greater consideration. Our findings allow rethinking and building new models for the prediction and expansion of introduced malaria. Furthermore, to prevent the risk of reintroduction of the disease, the need to strengthen the surveillance of residual anophelism throughout the South should be considered.
Graphical Abstract
Similar content being viewed by others
Background
Malaria was endemic in Italy until the middle of the last century. Human cases, mainly due to Plasmodium falciparum and P. vivax, were widespread especially along the coastal plains, and before the decisive interventions of reclamation and the launch of the National Anti-malarial Campaign, the disease affected hundreds of people every year [1]. Although Italy was officially declared malaria free by the World Health Organization (WHO) in 1970, since then a few sporadic cases of non-imported malaria have been recorded. Among the most recent cases, four non-travel related malaria cases occurred in Ginosa (Taranto, Apulia region) in October 2017. Integrated entomological surveys were performed, and the presence of the potential malaria vector, Anopheles labranchiae, was recorded in the involved areas [2]. In Italy, there are currently six anopheline sibling species belonging to the Anopheles maculipennis Meigen complex that cannot be distinguished morphologically, including An. atroparvus Van Thiel, 1927, An. labranchiae Falleroni, 1926, An. maculipennis sensu stricto Meigen, 1818, An. melanoon Hackett, 1934, An. messeae Falleroni, 1926, and An. daciae species inquirenda Linton, Nicolescu & Harbach, 2004 [3, 4]. A seventh sibling taxon, Anopheles sacharovi Favre, 1903, once widely distributed throughout the country, progressively disappeared probably because of the progressive modification of its larval habitats [5,6,7,8,9,10]. Among the An. maculipennis complex, An. labranchiae and An. sacharovi were historically considered the two most competent malaria vectors in Italy, always associates with endemic malaria and involved in P. falciparum and P. vivax transmission [11,12,13]. These two species were common and sometimes found in sympatry in Central and South Italy, including the Apulia region [10,11,12,13,14]. Anopheles labranchiae is still abundant and widespread in the region, especially in the Gargano, as recently documented [12, 15]. Contrarily, despite two doubtful findings reported in Northern and Central Italy [16, 17], An. sacharovi has not been found since the last report in the late 1960s. This mosquito thrived in several rural areas, mostly along the north and southwestern coastal plains and in northern Sardinia [5]. Although exophagic activity has been described for this species, it commonly exhibited endophagic and endophilic behaviour, resting in human dwellings, flooded basements and animal shelters where it could bite both day and night [18, 19]. This paper describes the investigation that led to the rediscovery of An. sacharovi in Italy, specifically in the Apulia region.
Methods
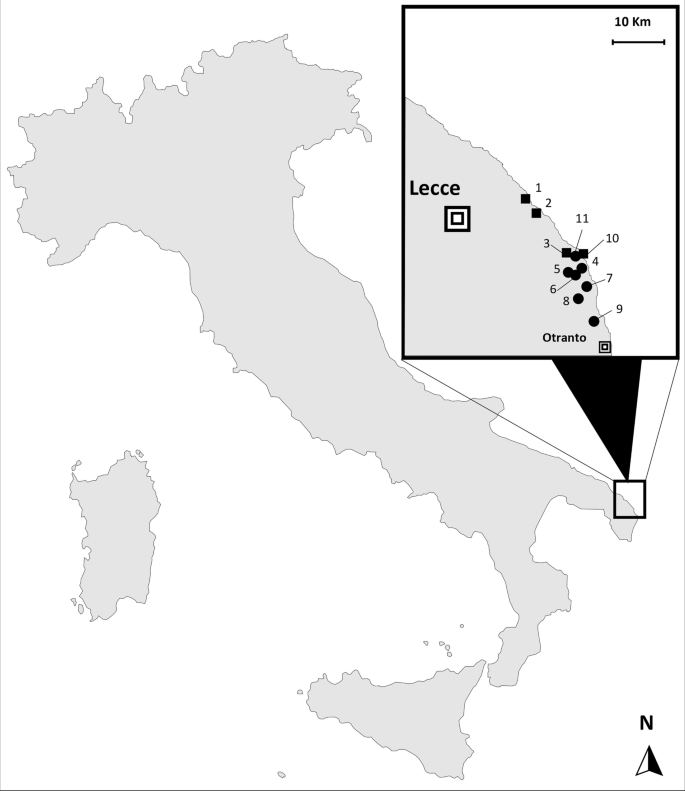
In 2022, the Italian Ministry of Health funded a research project (RC IZSPB 06/2022) to monitor the presence and the extent of An. maculipennis complex mosquitoes in Apulia and Basilicata territories. The Centers for Disease Control light trap (CDC-LT) network was extended to the area of Lecce Province in southeastern Italy. During the year, only one Anopheles specimen was collected in early September, in a horse-riding stable, and it was morphologically identified as An. maculipennis s.l. according to Severini et al. [20]. The mosquito was subsequently analysed by a home-made qPCR, an assay developed for routine rapid screening of An. labranchiae identification, the most widespread species of the complex in the region. A primer set was designed to amplify a 95-bp fragment of nuclear ribosomal internal transcribed spacer 2 (ITS-2) of An. labranchiae. The reaction was carried out in a 20-µl reaction using the SsoAdvanced Universal ProbeMix (BIORAD) with primers L1: 5′-GGTCATCGTGAGGCGTTATC-3′ and R1: 5′-GCTAGGAGCCGGTCTTGTAT-3′ at concentrations of 0.5 µM each and the probe P1: FAM-5′-AAGCACTCGCTGCTGCGCGT-BHQ1′ at a concentration of 0.2 µM under the following conditions: 95 ℃ for 3 min, 30 cycles at 95 ℃ for 10 s and 60 ℃ for 20 s. The negative qPCR sample was tested by conventional PCR. ITS-2 amplicon was sequenced at Eurofins Genomics (Ebersberg, Germany) and analysed by National Center for Biotechnology Information’s (NCBI) Basic Local Alignment Search (BLAST) for the identification of mosquito species [21]. The resulting sequence was deposited in GenBank (accession no. OQ748043). In September 2023, the entomological investigation was extended from the positive horse-riding stable (site 6) to neighbouring coastal areas of Lecce Province, including ten other different sites. In Fig. 1, the study area with the geographical distribution of the visited sites, for both adult and larval collections, is shown. The collection sites were selected in areas characterized by the presence of several marshes, brackish water basins and natural lakes and farms with animals (cattle, horses, sheep and poultry). The adult collections were carried out in animal shelters and human dwellings, using manual or battery-powered aspirators (modified Katcha® Bug Buster Spider Vacuum Blue) or CDC light traps. In potential breeding sites (i.e. irrigation and drainage canals, streams, marshes, large ponds and other permanent water collections), larvae and pupae were collected using a 500-ml standard dipper. All mosquito specimens were morphologically identified [20] and, when necessary, screened by molecular tools for species detection. Rapid DNA extraction for a single specimen was performed using a microwave method [22]. Culex and Aedes species and all specimens belonging to the An. maculipennis complex caught in 2023 were directly tested by conventional PCR, according to Marinucci et al. [21]. ITS-2 amplicons were sequenced at Eurofins Genomics (Ebersberg, Germany) and analysed using NCBI’s Basic Local Alignment Search (BLAST) for the identification of mosquito species.
Results
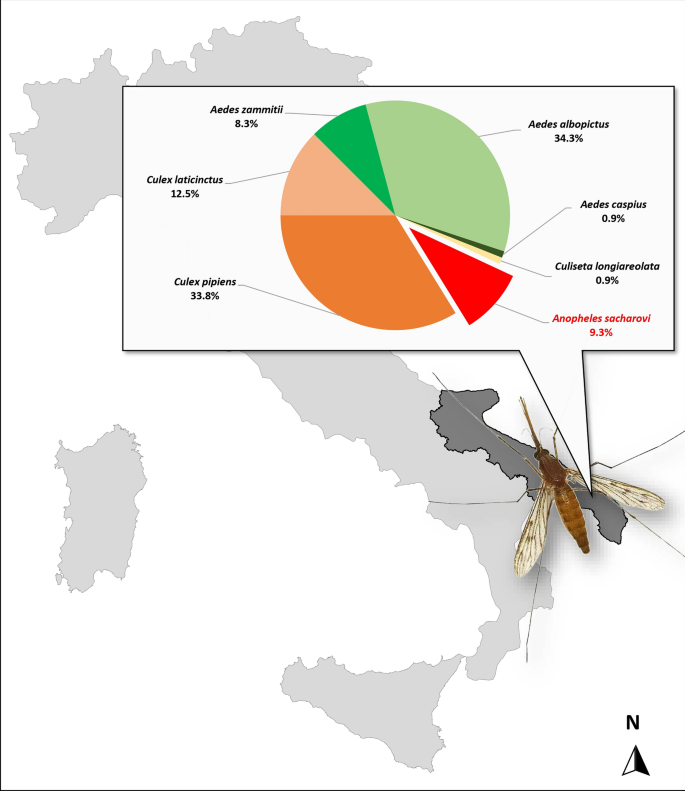
The single An. maculipennis s.l. collected in September 2022, at site 6 (Lecce Province), was molecularly analysed and identified as An. sacharovi (GenBank accession no. OQ748043). During the subsequent 2023 entomological survey, a total of 216 mosquitoes (125 immatures and 91 adults) were captured and identified morphologically, when possible. For Culex pipiens, Cx. laticinctus, Aedes mariae complex and An. maculipennis complex, targeted molecular analyses allowed the exact identification of the different taxa. Seven An. maculipennis s.l. females were collected on four farms (sites 4, 5, 8 and 9) and in a human dwelling (site 11); only one larval breeding site was positive for Anopheles presence, with 10 larvae and 3 pupae detected (site 2). All An. maculipennis s.l. were molecularly analysed and identified as An. sacharovi. ITS2 sequences obtained shared 100% nucleotide sequence identity with the sequence from a single specimen collected in 2022 (GenBank accession no. OQ748043). Table 1 summarizes the result obtained in this study, showing all sites visited in 2023, the number of specimens collected and the mosquito species detected. Overall, seven species were identified: An. sacharovi (9.3%), Cx. pipiens (33.8%), Cx. laticinctus (12.5%), Aedes zammitii (8.3%), Ae. albopictus (34.3%), Ae. caspius (0.9%), Culiseta longiareolata (0.9%).
Discussion
Even after the anti-malarial campaign following the Second World War, An. labranchiae and An. sacharovi were recorded in Apulia region, often found in sympatry [23]. However due to the diking and draining of marshes and retrodunal ponds, the use of insecticides, urbanization and pollution, many larval habitats, typical of An. sacharovi, have been progressively reduced, and the species was reported in the region until the late 1960s [14]. This heavy anthropic impact has contributed to the rarefaction of An. sacharovi in its distribution, perhaps confining small and limited mosquito populations in restricted natural sites. More recently, a reduction in anthropic pressure, the expansion of natural areas and the creation of new ones, together with other favourable climatic and environmental factors, could have contributed to the slow reconquest of territories and reappearance of the species in Apulia region. The record of Anopheles algeriensis Theobald, 1903, a potential secondary malaria vector, recently reported in Apulia, confirms how these changes can positively allow the Anopheles species to spread and/or recolonize some areas [14, 24]. The recolonization of areas with characteristics favourable to the larval development of An. algeriensis could be a positive signal of environmental requalification, which could also be beneficial for the spread of An. sacharovi. As supported by the literature, An. sacharovi can colonise different types of water collections, such as swamps, marshes, river margins, streams, pools and ditches, and can develop in both freshwater and brackish-salt water collections [18].
Conclusions
Although research projects have often been short and discontinuous, in recent years the collaborative activities conducted by the Experimental Zooprophylactic Institute of Puglia and Basilicata and the Istituto Superiore di Sanità have been able to clearly document the presence of potential malaria vectors, such as An. labranchiae, Anopheles superpictus Grassi, 1899 and An. algeriensis in the Apulia and Basilicata regions [14, 24]. Therefore, after decades of entomological investigations, this notable rediscovery in the coastal areas of southeastern Italy allows us to reintegrate An. sacharovi in the Italian Culicidae fauna. Adults of An. sacharovi were found indoors, either resting in animal shelters or landing on a human during dusk. This highlights not only an endophagic and endophilic behaviour but also a certain degree of anthropophilia of the species. Furthermore, a typical natural breeding site was identified, completing the entomological investigation. Beyond the exceptionality of such findings, it is necessary to underline the importance of strengthening and maintaining constant surveillance of residual anophelism, especially in vulnerable areas where the occurrence of sporadic malaria transmission could be possible. Further investigations will need to assess the distribution and seasonal abundance of An. sacharovi along the southeastern coasts of Italy. However, our findings, confirmed by two point-in-time investigations in 2 consecutive years, represent a valid basis for rethinking and building new models for the prediction and expansion of introduced malaria and for reconsidering the receptivity of the studied areas to malaria to prevent the risk of reintroduction of the disease. Although vector densities do not currently appear to be epidemiologically relevant enough to pose a health threat, the conditions for a renewal of transmission in several Mediterranean countries still exist, as reported in Greece [25], and the occurrence of foci of introduced malaria (in particular by P. vivax) in some regions of our country should not to be underestimated [13].
Data availability
The datasets generated and analysed during the current study are available in the NCBI (GenBank) repository by accession no. OQ748043. https://www.ncbi.nlm.nih.gov/nuccore/OQ748043.1
References
Majori G. Short history of malaria and its eradication in Italy with short notes on the fight against the infection in the Mediterranean basin. Mediterr J Hematol Infect Dis. 2012;4:e2012016. https://doi.org/10.4084/MJHID.2012.016.
Boccolini D, Menegon M, Di Luca M, Toma L, Severini F, Marucci G, et al. Non-imported malaria in Italy: paradigmatic approaches and public health implications following an unusual cluster of cases in 2017. BMC Public Health. 2020;20:857. https://doi.org/10.1186/s12889-020-08748-9.
Toma L, Severini F, Di Luca M, Menegon M. Insecta Diptera Culicidae. In: Bologna MA, Zapparoli M, Oliverio M, Minelli A, Bonato L, Cianferoni F, Stoch F, editors. Checklist of the Italian Fauna. Version 1.0. 2021. https://www.lifewatchitaly.eu/en/initiatives/checklist-fauna-italia-en/checklist/.
Calzolari M, Desiato R, Albieri A, Bellavia V, Bertola M, Bonilauri P, et al. Mosquitoes of the Maculipennis complex in Northern Italy. Sci Rep. 2021;11:6421. https://doi.org/10.1101/2020.11.02.365262.
Romi R, Pierdominici G, Severini C, Tamburro A, Cocchi M, Menichetti D, et al. Status of malaria vectors in Italy. J Med Entomol. 1997;34:263–71. https://doi.org/10.1093/jmedent/34.3.263.
Zamburlini R, Cagnus E. Residual mosquitoes in the northern Adriatic seacoast 50 years after the disappearance of malaria. Parassitologia. 1998;40:431–7.
Romi R, Sabatinelli G, Majori G. Could malaria reappear in Italy? Emerg Infect Dis. 2001;7:915–9. https://doi.org/10.3201/eid0706.010601.
Romi R, Sabatinelli G, Majori G. Malaria epidemiological situation in Italy and evaluation of malaria incidence in Italian travelers. J Travel Med. 2001;8:6–11. https://doi.org/10.2310/7060.2001.5140.
Bietolini S, Zamburlini R, Candura F, Musella V, Rinaldi L, Cringoli G, et al. Is Anopheles sacharovi disappeared from Italy? Retrospective analysis and new field data. Parassitologia. 2004;46:81.
Bietolini S, Candura F, Coluzzi M. Spatial and long term temporal distribution of the Anopheles maculipennis complex species in Italy. Parassitologia. 2006;48:581–608.
Hackett LW, Missiroli A. The varieties of Anopheles maculipennis and their relation to the distribution of malaria in. Europe Riv Malariol. 1935;14:45–109.
Raffaele G. Note sull’eradicazione della malaria in Italia. Riv Malariol. 1964;43:1–27.
Jetten TH, Takken W. Anophelism without malaria in Europe: a review of the ecology and distribution of the genus Anopheles in Europe. Wageningen Agricultural University Papers. 1994. p. 69.
Rivosecchi L, Stella E. Artropodi ematofagi delle aree naturali da proteggere. Nota II. La zona del Massiccio Garganico. In Proceedings of the Atti del IV Simposio Nazionale sulla Conservazione della Natura, Bari, Italy, 23–28 April 1974; Volume I, Cacucci Editore. pp. 149–170.
Raele DA, Severini F, Boccolini D, Menegon M, Toma L, Vasco I, et al. Entomological surveillance in former malaria-endemic areas of southern Italy. Pathogens. 2021;10:1521. https://doi.org/10.3390/pathogens10111521.
Talbalaghi A, Shaikevich E. Molecular approach for identification of mosquito species (Diptera: Culicidae) in Province of Alessandria, Piedmont, Italy. Eur J Entomol. 2011;108:35–40.
Möhlmann TWR, Wennergren U, Tälle M, Favia G, Damiani C, Bracchetti L, et al. Community analysis of the abundance and diversity of mosquito species (Diptera: Culicidae) in three European countries at different latitudes. Parasit Vectors. 2017;10:510. https://doi.org/10.1186/s13071-017-2481-1.
Bertola M, Mazzucato M, Pombi M, Montarsi F. Updated occurrence and bionomics of potential malaria vectors in Europe: a systematic review (2000–2021). Parasit Vectors. 2022;15:88. https://doi.org/10.1186/s13071-022-05204-y.
Boreham PF, Garrett-Jones C. Prevalence of mixed blood meals and double feeding in a malaria vector (Anopheles sacharovi Favre). Bull World Health Organ. 1973;48:605–14.
Severini F, Toma L, Di Luca M. Zanzare in Italia: raccolta, identificazione e conservazione delle specie più comuni. Roma: Istituto Superiore di Sanità; 2022. (Rapporti ISTISAN 22/3).
Marinucci M, Romi R, Mancini P, Di Luca M, Severini C. Phylogenetic relationships of seven palearctic members of the maculipennis complex inferred from ITS2 sequence analysis. Insect Mol Biol. 1999;8:469–80. https://doi.org/10.1046/j.1365-2583.1999.00140.x.
Dashti AA, Jadaon MM, Abdulsamad AM, Dashti HM. Heat treatment of bacteria: a simple method of DNA extraction for molecular techniques. Kuwait Med J. 2009;41:117–22.
Romi R, Severini C, Pierdominici G, Marchi A, Erbi G, Mantega V, et al. Anofelismo residuo in Italia: distribuzione in quattro regioni meridionali. Ann Ist Super Sanità. 1994;30:237–42.
Menegon M, Tomazatos A, Severini F, Raele DA, Lilja T, Werner D, et al. Molecular characterization of Anopheles algeriensis theobald, 1903 (Diptera: Culicidae) populations from Europe. Pathogens. 2022;30:990. https://doi.org/10.3390/pathogens11090990.
Tseroni M, Georgitsou M, Baka A, Pinaka O, Pervanidou D, Tsironi M, et al. The importance of an active case detection (ACD) programme for malaria among migrants from malaria endemic countries: the Greek experience in a receptive and vulnerable area. Int J Environ Res Public Health. 2020;17:4080.
Acknowledgements
We are grateful to Inspectors of ASL of Lecce for their help with field surveys and farm contacts and all the breeders, who have always cordially made their stables accessible for the inspections. We also acknowledge the assistance and cooperation provided by the personnel of the Carabinieri Biodiversity Protection Unit of San Cataldo, on duty at the San Cataldo State Nature Reserve, Lecce. Finally, special thanks to Roberto Romi, whose teachings guided and inspired us.
Funding
The surveillance study was supported by funding obtained through IZS PB 06/2022 RC by Ministry of Health, Italy, and partially by EU funding within the NextGeneration EU-MUR PNRR Extended Partnership Initiative on Emerging Infectious Diseases (project no. PE00000007, INF-ACT).
Author information
Authors and Affiliations
Contributions
Marco Di Luca (MD) and Maria Assunta Cafiero (MAC) contributed equally to this article and share last authorship. Conceptualization, DAR, MD and MAC; methodology, DAR, FS, LT, GT, MM, DB and MAC; formal analysis, DAR, FS, LT, DB, MM and MD; writing—original draft preparation, DAR.; writing—review and editing, MDL, FS, LT, DAR, DB, MM and MAC; project administration, MAC and DAR; funding acquisition, DAR, MAC and MDL. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Raele, D.A., Severini, F., Toma, L. et al. Anopheles sacharovi in Italy: first record of the historical malaria vector after over 50 years. Parasites Vectors 17, 182 (2024). https://doi.org/10.1186/s13071-024-06252-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-024-06252-2