Abstract
Background
Indoor residual spraying (IRS) capitalizes on the natural behavior of mosquitoes because Aedes aegypti commonly seeks indoor resting sites after a blood meal. This behavior allows mosquitoes to be exposed to insecticide-treated surfaces and subsequently killed. Combinations of deltamethrin and clothianidin with different modes of action have shown promise in IRS, effectively targeting both susceptible and pyrethroid-resistant malaria vectors. However, the effects of this approach on Aedes mosquitoes remain unclear. The present study tested the effects of deltamethrin–clothianidin mixture treatment on behavioral responses and life history traits of Taiwanese and Indonesian populations of Ae. aegypti.
Methods
We adopted an excito-repellent approach to explore the behavioral responses of pyrethroid-resistant Ae. aegypti populations from Indonesia and Taiwan to a deltamethrin–clothianidin mixture used in contact irritancy and non-contact repellency treatments. We further evaluated the life history traits of surviving mosquitoes (i.e., delayed mortality after 7-day post-treatment, longevity, fecundity, and egg hatching) and investigated the potential transgenerational hormetic effects of insecticide exposure (i.e., development rate and survival of immatures and adult mosquitos).
Results
All tested field populations of Ae. aegypti displayed strong contact irritancy responses; the percentage of escape upon insecticide exposure ranged from 38.8% to 84.7%. However, repellent effects were limited, with the escape percentage ranging from 4.3% to 48.9%. We did not observe immediate knockdown or mortality after 24 h, and less than 15% of the mosquitoes exhibited delayed mortality after a 7-day exposure period. However, the carryover effects of insecticide exposure on the survival of immature mosquitoes resulted in approximately 25% higher immature mortality than that in the control. By contrast, we further documented stimulated survivor reproduction and accelerated transgenerational immature development resulting from the sublethal effects of the insecticide mixture. In particular, the number of eggs laid by treated (both treatments) female mosquitoes increased by at least 60% compared with that of eggs laid by control female mosquitoes.
Conclusions
IRS with deltamethrin–clothianidin effectively deters Aedes mosquitoes from entering residential areas and thereby reduces mosquito bites. However, the application rate (deltamethrin: 25 mg/m2; clothianidin: 200 mg/m2) may be insufficient to effectively kill Aedes mosquitoes. Insecticide response appears to vary across mosquito species; their behavioral and physiological responses to sublethal doses have crucial implications for mosquito control programs.
Graphical Abstract

Similar content being viewed by others
Background
Aedes aegypti (Linnaeus) is a key vector of arboviral diseases, including yellow fever, chikungunya, and dengue viruses [1, 2]. No effective dengue vaccine is yet available, thus highlighting the importance of controlling Ae. aegypti populations and reducing dengue fever transmission. Current approaches used to combat these mosquitoes are primarily focused on integrated vector management, encompassing biological, mechanical, and chemical methods [3,4,5,6,7].
Chemical control remains a vital measure for managing anthropophilic disease vectors during outbreaks [8, 9]. Various chemical control methods, including the space spraying of insecticides and the application of larvicides, have limitations in effectively suppressing mosquito populations [9]. For instance, space spraying has a short residual effect, necessitating continuous application for successful mosquito control [10, 11]. Moreover, adult Ae. aegypti mosquitoes typically seek indoor resting sites and hide in cryptic places (e.g., at a height of < 1.5 m, especially in dark areas, under beds, beneath household furniture, or on hanging clothes), with their primary food source being human blood [12, 13]. This behavior makes outdoor spraying methods, such as fogging or ultra-low volume fogging, less effective in controlling the spread of dengue fever [14]. Therefore, alternative control strategies to complement traditional approaches are urgently required.
Indoor residual spraying (IRS) has emerged as an effective method for reducing Ae. aegypti populations and preventing dengue transmission [3, 15,16,17]. IRS leverages the natural behavior of Ae. aegypti, which typically rests indoors after feeding on blood. This behavior exposes mosquitoes to insecticide-treated surfaces, eventually killing them [13, 17, 18].
An insecticide mixture containing clothianidin and deltamethrin complements the existing pyrethroid (deltamethrin)-based IRS. Deltamethrin acts on nerve membranes by delaying the closure of the activation gate for the sodium ion channel. Clothianidin functions as a neonicotinoid insecticide agonist, targeting the nicotinic acetylcholine receptor in the insect central nervous system. The combination of these two active ingredients with different modes of action has shown promise in IRS, effectively targeting malaria vectors both susceptible and resistant to pyrethroid [19,20,21,22,23]. Studies evaluating the insecticidal activity of IRS have often used standard World Health Organization cone bioassays [24]. However, some controversy exists surrounding the use of these bioassays to quantify residual spraying efficacy, because they may not fully represent natural mosquito behavior. Deltamethrin, a neurotoxin, can induce contact irritancy in mosquitoes, prompting avoidance behavior and escape from treated areas within a short time [25, 26]. Yu et al. [27] revealed that at least 3.6–31.2% of field Ae. aegypti mosquitoes exhibited avoidance behavior and escaped deltamethrin-treated areas within 30 min. Furthermore, Kongmee et al. [28] reported that six field populations of Ae. aegypti exhibited strong escape responses (32–78%) within 30 min of contact with deltamethrin. This escaping behavior may affect the mortality of mosquitoes exposed to insecticide-treated surfaces.
Resistant mosquitoes often exhibit delayed mortality. For example, Fongnikin et al. [22] observed that pyrethroid-resistant Anopheles gambiae (sensu lato) exposed to Fludora Fusion (a mixture of deltamethrin and clothianidin) exhibited elevated mortality rates, increasing from approximately 20% after 24 h to 70% after 120 h of exposure, with 30% of all mosquitoes surviving at the end of the experiment. These survivors can mate with individuals of the opposite sex and reproduce, thus contributing to population persistence. However, in most IRS trials, mortality was recorded for a duration of only up to 72 or 96 h, and the life history of exposed mosquitoes beyond the experimental period was not considered [19, 20, 23].
A growing body of evidence suggests that sublethal or low doses of pyrethroids and neonicotinoids affect mosquito physiology and vital behaviors, including host-seeking. For instance, Cohnstaedt and Allan [29] reported significant reductions in activation time to flight and flight direction in Ae. aegypti mosquitoes that survived deltamethrin–permethrin treatment. Rigby et al. [30] demonstrated that exposure to sublethal permethrin doses reduced egg viability, blood avidity, male mating success, and longevity in susceptible Ae. aegypti; this treatment also reduced host-location success by 20–30% in Ae. aegypti [30]. However, insecticide-resistant female mosquitoes exhibited increased reproductive output and mating success rates after exposure, which indicates that exposure to sublethal pyrethroid doses is not necessarily detrimental to mosquitoes [30]. The phenomenon of low-dose stimulation response (also known as hormesis), where exposure to a sublethal insecticide dose exerts a stimulatory effect on certain aspects of the insect’s physiology, has been observed in various insect species [31]. However, the effects of sublethal neonicotinoid doses on the fitness of Aedes mosquitoes remain poorly studied. Therefore, investigating the life history traits of survivors and their offspring is imperative.
In this study, we adopted the excito-repellent (ER) approach to examine the behavioral responses of pyrethroid-resistant Ae. aegypti populations from Indonesia and Taiwan when exposed to a mixture of deltamethrin and clothianidin. The ER approach enabled us to investigate both the voluntary escape behavior triggered by insecticidal contact irritancy (the mosquitoes may be irritated when contacting a treated surface) and non-contact repellency (the mosquito may be repelled by the presence of insecticide before contacting a treated surface), thus facilitating a comprehensive assessment of the insecticide’s effectiveness. In addition, we investigated the life history traits of surviving mosquitoes and the potential transgenerational hormetic effects of exposure to the insecticide mixture.
Methods
Mosquito sampling
We collected 18 populations of Ae. aegypti from Indonesia and Taiwan. In Indonesia, dengue fever caused by dengue virus is a widespread vector-borne disease [32], affecting approximately 93.58% of Indonesia’s regencies/cities in 2019 [33]. By contrast, dengue is not endemic to Taiwan, but sporadic local outbreaks occur due to an increasing number of local travelers visiting dengue-endemic countries. Thermal fogging with pyrethroid insecticides is used as an immediate remedial control measure when the mosquito density is above a certain threshold or a case of dengue fever is reported.
We randomly selected 11 Ae. aegypti populations from regencies/cities located in 11 Indonesian provinces where dengue cases had been reported [34]. Between May and December 2019, Ae. aegypti eggs were obtained using ovitraps from the following areas in Indonesia: Sumatera, Medan, North Sumatra (3°33′20.8″N, 98°37′45.6″E); Pangkal Pinang, Bangka Belitung (2°07′13.3″S, 106°06′17.6″E); Batam, Kepulauan Riau (1°06′20.9″N, 103°57′48.0″E); Java, Kelapa Gading, DKI Jakarta (6°8′53.8″S, 106°54′29.4″E); Semarang, Central Java (6°59′30.7″S,110°25′00.7″E); Kiaracondong, Bandung, West Java (6°55′48.5″S, 107°39′23.8″E); Kalimantan, Kapuas, Central Kalimantan (3°14′40.5″S, 114°23′55.91″E); Samarinda, East Kalimantan (0°28′13.39″S, 117°9′11.13″E); Pontianak, West Kalimantan (0°03′32.1″S, 109°19′44.3″E); Sulawesi, Makassar, South Sulawesi (5°08′35.2″S, 119°26′39.4″E); and Papua, Jayapura, Papua (2°34′12.2″S, 140°41′55.4″E). In addition, seven Ae. aegypti populations were collected from the following areas in southern Taiwan: Tainan, Zhongxi District (22°59′47.3″N, 120°11′38″E); North District (23°00′30.4″N, 120°12′27.7″E); Annan District (23°03′30.6″N, 120°08′08.7″E); Kaohsiung, Sanmin District (22°38′53.9″N, 120°19′35.5″E); Xiaogang District (22°33′18.4″N, 120°21′49.4″E); Lingya District (22°37′24.9″N, 120°19′1.3″E); and Qianzhen District (22°36′2.23″N, 120°18′53.23″E). All 18 populations have been reported to exhibit low to high levels of resistance to deltamethrin [27, 35]
Mosquito rearing
Eggs collected from each location were hatched in plastic containers (29.5 cm × 23.0 cm × 5.0 cm) filled with 1 L of dechlorinated water. The larvae were fed an artificial larval diet consisting of pork liver powder and yeast at a ratio of 1:1. Emerged Ae. aegypti adults were fed with 10% sucrose solution and maintained at a temperature of 25 °C ± 1 °C and a relative humidity of 65 ± 5% under a constant 12-h light/dark photoperiod. Female mosquitoes were provided with pig blood using an artificial feeder device to promote reproduction. Adult Ae. aegypti progenies up to generation F5 were used in the bioassays. A susceptible reference strain, the Bora Bora strain (F35), was used for comparison.
Evaluation of behavioral responses of field Aedes mosquitoes
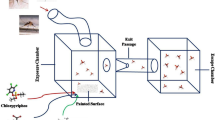
The ER test was conducted to examine the behavioral avoidance (repellency and contact irritancy) [36] of Ae. aegypti toward Fludora Fusion (an insecticide containing 500 g/kg of clothianidin and 62.5 g/kg of deltamethrin). The ER test apparatus comprised four chambers, each containing two treatment and control chambers. In one treatment chamber, the internal lining featured insecticide-impregnated paper to allow mosquitoes to make physical contact (referred to as "contact"); The other treatment chamber had mesh placed in a position to prevent mosquitoes from making physical contact with the test paper surfaces (referred to as "non-contact"). The filter paper (14 cm × 17 cm) was impregnated with the recommended dosages of insecticides (deltamethrin: 25 mg/m2; clothianidin: 200 mg/m2). In the control group, the filter paper was treated with water [36].
To standardize their physiological condition, female mosquitoes were starved of sucrose solution and blood meal for 12 h before analyses. Fifteen 5- to 7-day-old unmated female Ae. aegypti were introduced into each chamber. Before the observation, mosquitoes were allowed to acclimatize for 3 min in the test chamber. The number of mosquitoes escaping from each chamber to the receiving box through an exit hole (designated as escaped mosquitoes) was recorded every minute for 30 min. In addition, the number of mosquitoes remaining inside each chamber (designated as non-escaped mosquitoes) was recorded after the ER bioassay. The experiment was conducted under the aforementioned laboratory conditions and replicated four times for each population.
Knockdown and mortality
All mosquitoes that escaped or remained inside the test chambers were transferred to respective paper cups (measuring 7.5 cm in diameter and 9.5 cm in height) and provided with 10% sucrose solution. The rate of mortality was determined 24 h after exposure.
Evaluation of post-treatment life history traits
For this study, mosquito populations from the Sanmin, North, and Zhongxi districts in Taiwan were used. Non-escaped survivors from both contact irritancy and spatial repellency treatments were placed in a paper cup and provided with 10% sucrose solution. Each paper cup contained a plastic cup (measuring 4.0 cm in diameter and 2.5 cm in height), which was filled with water and lined with filter paper (13.0 cm in length and 2.0 cm in width) to make it an oviposition site [37]. Each female was paired with a male from the same population. After 7 days of pairing, eggs laid by each female were collected, counted, and dried for further analysis. Blood meals were provided on a weekly basis until all female mosquitoes had died. The numbers of deaths and eggs laid were recorded daily. The experiment was replicated four times for each population.
Egg hatchability assessment
To assess egg hatchability, the batch of eggs laid by each female mosquito was transferred to a 10-cm plastic cup filled with dechlorinated water. The number of eggs that hatched was recorded daily for 3 days.
Development of immature mosquitoes
Newly emerged first-instar larvae were transferred to plastic containers filled with 1 L of dechlorinated water. The larvae were provided with 0.5 g of an artificial diet (ad libitum) containing dried pork liver powder and dried yeast at a ratio of 1:1. The numbers of larvae, pupae, and adult mosquitoes were recorded daily.
Wing length measurement
Newly emerged female mosquitoes were transferred to mosquito cages (measuring 32.5 cm in length, 32.5 cm in width, and 32.5 cm in height) and provided with 10% sucrose solution. Subsequently, 30 5-day-old female mosquitoes from each population were randomly collected and stored at −20°C for wing length measurement. A pair of wings from each female was mounted on a microscope slide (Leica S8 AP0, Leica Microsystems, Heerbrugg, Switzerland). A digital image of the wings was captured using a camera (EI200 HD, OPTI Advanced Imaging Ltd., Taipei City, Taiwan), and the distance between veins R3 and R4+5 was measured from the axillary incision (or alula notch) to the apex of the wing, excluding the fringe scales [37]. The images were analyzed using Helicon Focus Lite version 6.7.1 (Helicon Soft Ltd., Kharkiv, Ukraine).
Data analysis
The observed mean escape percentage for the 30-min exposure was adjusted using the Abbot formula, considering the control mosquitoes. The Kolmogorov–Smirnov Z-test was performed to examine the normal distribution of the data. Data regarding the rates of mosquito escape, knockdown, mortality, egg hatching, and immature survival were subjected to arcsine square root transformation. In addition, data on egg numbers were subjected to log10 transformation. A one-way analysis of variance test, followed by Tukey’s honestly significant difference test, was conducted to compare the mean percentages of escape, knockdown, and mortality among the field populations of Ae. aegypti for both contact irritancy and non-contact repellency treatments. In addition, a probit analysis was performed to calculate the escape time of 50% (ET50) of all mosquitoes in each population. An independent-samples t-test was performed to determine differences in the life history of mosquitoes between the treatment and control groups. Kaplan–Meier survival analysis was performed to estimate the mean lifespan of treated and control female mosquitoes. The log-rank test was used to compare lifespan between treated and control female mosquitoes. Statistical significance for all tests was set at P < 0.05.
Results
Behavioral response
The escape percentage was higher in the contact irritancy treatment (9.2–84.7%) than in the non-contact repellency treatment (4.3–48.9%) (Tables 1 and 2). Significant differences in the escape percentage were observed between the populations exposed to a deltamethrin–clothianidin mixture in the contact irritancy treatment (F(18,57) = 5.057, P < 0.05). In most field populations from Indonesia, the escape percentage after 30 min of exposure ranged from 48.6% to 84.7%, and these values were statistically similar to the escape percentage of the Bora Bora strain (69.0 ± 7.0%; Table 1). However, the field population from Kapuas, which exhibited high resistance to deltamethrin, had a significantly lower escape percentage (9.2 ± 1.8%; Table 1) than the Bora Bora strain. The mean escape time for the Bora Bora strain was 4.81 min (% fiducial limit: 3.3–6.2 min). Out of 11 field mosquito populations from Indonesia, four required < 7 min to escape the treatment chamber; this value did not significantly differ from that of the Bora Bora strain. However, the field populations from Kapuas, Samarinda, and Jayapura exhibited relatively non-responsive behaviors to the insecticide mixture, requiring > 25 min to escape from the treatment chamber (Table 3). Among the seven field strains collected from Taiwan, the Qianzhen population, which showed the highest level of resistance to deltamethrin, exhibited the lowest escape percentage, with 24.1 ± 10.0% escaping within 30 min of exposure. The other six field strains exhibited escape percentages ranging from 38.8% to 75.8% after 30 min of direct contact with treated surfaces (Table 1). All populations from Taiwan required significantly more time to escape from the treatment chambers (ranging from 12 to 48 min) than did the Bora Bora strain, except for Zhongxi, which escaped within 5 min (Table 3).
A post hoc analysis of the results of the non-contact repellency treatment revealed that only the escape percentage of the Zhongxi population (48.9 ± 9.1%) varied significantly from that of the Bora Bora strain (4.2 ± 4.2%; F (18,57) = 2.281, P < 0.05; Table 2). The non-responsiveness of mosquitoes in the non-contact repellency treatment was supported by their delayed escape times, with most field mosquito populations requiring at least 26 min (ET50) to escape from the treatment chamber (Table 3).
Knockdown and mortality
In the contact irritancy treatment, the susceptible Bora Bora strain exhibited a knockdown percentage of 27.3 ± 7.2% and 24-h mortality of 27.3 ± 7.2% when exposed to the insecticide mixture for 30 min. No knockdown was observed for mosquitoes that escaped from the treatment chamber during the 30-min exposure period. However, subsequent mortality of 22.9 ± 14.6% was noted among the escaped mosquitoes at 24 h post-exposure. No knockdown or 24-h mortality was recorded in the escaped or non-escaped mosquitoes for any of the field populations of Ae. aegypti (Table 1). In the non-contact repellency treatment, both the susceptible Bora Bora strain and field populations exhibited negligible knockdown and mortality, regardless of whether mosquitoes escaped from the treatment chamber (Table 2).
Delayed mortality and longevity of survivors
On day 7 after exposure to the insecticide mixture, the rate of mortality was significantly higher among female mosquitoes from North (10.1 ± 1.7%) and Zhongxi (11.4 ± 2.3%) than among control female mosquitoes (North: 1.6 ± 1.6%; Zhongxi: 0.0 ± 0.0%) (Table 4). However, no significant difference in delayed mortality was observed between treated and control female mosquitoes from the Sanmin population in the contact irritancy treatment (Table 4). In the non-contact repellency treatment, only the population from North exhibited a significant difference in delayed mortality between treated female mosquitoes (15.5 ± 1.5%) and control female mosquitoes (North: 8.8 ± 1.5%) (Table 5). The Kaplan–Meier survival analysis revealed that the lifespan of treated female mosquitoes was significantly shorter than that of control mosquitoes in both contact irritancy (except for Sanmin and North populations) and non-contact repellency treatments (Table 4 and 5).
Fecundity
Regarding fecundity, in the contact irritancy treatment, the mean number of eggs laid by female mosquitoes from the North, Sanmin, and Zhongxi populations increased by 59%, 130%, and 68%, respectively, compared with the respective control groups (Table 4). However, only the increase in the Zhongxi population was statistically significant (Table 4). In the non-contact repellency treatment, the mean number of eggs laid by female mosquitoes from the North, Sanmin, and Zhongxi populations increased by 65%, 66%, and 71%, respectively, compared with the respective control groups (Table 5). However, no significant difference was noted between treated and control mosquitoes in the North and Sanmin populations (Table 5).
Egg hatching
The rates of egg hatching did not vary significantly between control and treated female mosquitoes in the contact irritancy (Table 4) or non-contact repellency treatment groups (Table 5). However, in the contact irritancy treatment, eggs laid by treated female mosquitoes from the North population hatched 1 day earlier than those laid by control female mosquitoes. For treated mosquitoes, the rates of egg hatching were 67.9 ± 5.8% and 64.4 ± 3.4% on day 2 in the contact irritancy and non-contact repellency treatments, respectively (Fig. 1). No significant difference in the hatching rate was observed between eggs laid by treated female mosquitoes from the Sanmin and Zhongxi populations in either treatment (Fig. 1).
Rates of the hatching of eggs and the development of larvae and pupae produced by female mosquitoes exposed to the deltamethrin–clothianidin mixture (contact) and spatially repelled by the deltamethrin–clothianidin mixture (non-contact). * Indicates that the proportions of progenies produced by treated female mosquitoes were significantly higher than those of progenies produced by control female mosquitoes
Survival and development of immature mosquitoes
In terms of immature survivorship, the proportion of immature mosquitoes that successfully transformed into adults in the progeny produced by female mosquitoes subjected to the contact irritancy treatment was significantly lower than those produced by control female mosquitoes (Table 4). In the non-contact repellency treatment, a significant reduction was observed in immature survivorship in the progeny produced by treated female mosquitoes from the Zhongxi population (Table 5).
The development of larvae and pupae produced by female mosquitoes that came into contact with the insecticide mixture was significantly accelerated (Fig. 1). Similarly, larvae and pupae produced by female mosquitoes in the non-contact repellency treatment exhibited a significantly increased rate of development into adults compared with that noted in the progeny produced by control female mosquitoes (Fig. 1).
Wing length
No significant difference in wing length was observed between the progenies produced by treated and control female mosquitoes in either treatment (Table 4 and 5).
Discussion
Insecticide resistance has been reported in Ae. aegypti populations in Indonesia [35] and Taiwan [27, 38], underscoring the need for strategies for managing insecticide resistance, including the use of mixtures of insecticides with different modes of action. An alternative strategy involves IRS with insecticides, which capitalizes on mosquitoes’ tendency to seek indoor resting sites after a blood meal, thereby enhancing the likelihood of contact with the insecticide. Our study results demonstrated that all the tested field populations of Ae. aegypti displayed a strong contact irritancy response upon exposure to the deltamethrin–clothianidin mixture, whereas repellent effects were limited. Although we did not observe immediate knockdown or mortality, we detected significant but < 10% delayed mortality 7 days after treatment in the North populations. In addition, exposure to the insecticide mixture affected the life history traits of exposed female mosquitoes and the survival and development of their offspring.
The behavioral response of Ae. aegypti from Thailand and Taiwan to deltamethrin has been associated with insecticide resistance and cuticular thickness in field mosquitoes [27, 39]. Resistant field populations typically exhibited a limited response to deltamethrin-treated surfaces, with < 35% of field mosquitoes escaping from such surfaces, compared with the approximately 75% escape observed in laboratory-susceptible mosquitoes [27]. However, in our study, regardless of the insecticide resistance status, all tested Ae. aegypti populations exhibited a high escape rate (ranging from 40 to 80%) upon contact with the insecticide mixture-treated surfaces. This finding indicated that the contact irritancy response of field Ae. aegypti to the insecticide mixture was stronger than that to deltamethrin alone. This finding aligns with the results reported by Fongnikin et al. [22], who investigated the effect of this insecticide mixture by applying the hut trial procedure to a wild, free-flying pyrethroid-resistant An. gambiae (s.l.) population from Cové, Benin. The researchers reported that exposure to the insecticide mixture led to significantly higher levels of early mosquito exiting (55–60%) than did exposure to clothianidin alone (37–38%) [22]. Moreover, the results suggest that the insecticide mixture can keep female mosquitoes out of treated houses, leading to a reduction in mosquito bites on humans.
The early escape of mosquitoes from treated surfaces may lead to reduced contact time with the insecticide, resulting in reduced insecticide intake and increased survival rates after treatment. A study demonstrated that field Ae. aegypti mosquitoes from Taiwan and Thailand exhibited negligible mortality rates upon exposure to deltamethrin (application rate: 25 mg active ingredient (a.i.)/m2) alone in contact irritancy (< 11%) and non-contact repellency (< 6%) treatments [27]; even the addition of clothianidin did not enhance the 24-h mortality rate in the present study. Our results indicated that neither knockdown nor mortality occurred in either treatment, regardless of whether the mosquitoes escaped from the test chambers. However, we observed that adult female mosquitoes exposed to the insecticide mixture exerted sublethal carryover effects on immature mosquitoes in the contact irritancy treatment, resulting in an immature mortality rate of approximately 25%.
In the present study, we observed limited delayed mortality; the mortality of female mosquitoes significantly increased to 10% after 7 days of post-exposure compared with the rate noted in the control group from North populations. This finding differs from that of the hut trial, where the mortality of pyrethroid-resistant An. gambiae increased from approximately 25% after 24 h to 69% after 120 h [22]. Despite methodological differences, the mortality of Aedes mosquitoes in this study was considerably lower than that of Anopheles mosquitoes [22]. The lower mortality of Aedes mosquitoes can be attributed to several factors, including differences in insecticide susceptibility between Aedes and Anopheles mosquitoes. For example, the LD50 of deltamethrin for susceptible Ae. aegypti larvae is substantially higher (0.770 mg/l) than that for susceptible An. gambiae larvae (0.0068 mg/l) [40, 41]. The LD50 of imidacloprid (a neonicotinoid) for susceptible Ae. aegypti adults (7.7 × 10−4 μg/mg) is higher than that for susceptible Anopheles quadrimaculatus (3.8 × 10−4 μg/mg) [42]. This finding suggests that a higher insecticide dose is required to ensure similar levels of mortality in Ae. aegypti and Anopheles mosquitoes. Overall, the discrepancy in mortality rates between Aedes and Anopheles mosquitoes highlights the complexity of insecticide responses across mosquito species and warrants further investigation to fully elucidate the underlying mechanisms. Moreover, it underscores the importance of carefully considering the appropriate application rate and insecticide mixtures when designing mosquito control strategies to effectively combat insecticide-resistant Aedes mosquitoes.
We observed that the lifespan of treated female mosquitoes was significantly reduced by 2 to 4 days compared with that of control female mosquitoes after both contact irritancy and non-contact repellency treatments. The survivors remained reproductively active and could mate with male mosquitoes. We initially anticipated that although female mosquitoes survived the treatment, their reproductive output would be significantly reduced due to the sublethal effects of deltamethrin and clothianidin, as observed in other insects [43,44,45,46]. However, we found that the mean number of eggs produced per surviving female increased by at least 60% compared with that produced by control mosquitoes. This unexpected finding suggests a low-dose stimulation response of insecticides on female mosquitoes, regardless of whether they were irritated or spatially repelled by the insecticide. Our results align with those reported by Choi et al. [47], who observed that transfluthrin-exposed mosquitoes were more attracted to oviposition sites, resulting in increased egg-laying, than were non-exposed mosquitoes. Similarly, Rigby et al. [30] reported a 26% increase in eggs per female and a 37% increase in male mating success in resistant Ae. aegypti following exposure to sublethal doses of permethrin. These increases were partly attributed to changes in the mosquitoes’ blood avidity and host-locating ability, indicating a behavioral shift following exposure to sublethal permethrin doses [30]. Bong et al. [48] conducted a study where Ae. aegypti female mosquitoes were exposed to oviposition sites containing chlorpyrifos. The researchers observed that chlorpyrifos-exposed mosquitoes laid significantly more eggs than did control mosquitoes, indicating the stimulatory effects of sublethal insecticide doses on egg production.
Transgenerational hormesis is common in insects [49]. This phenomenon refers to the effect where the exposure of one parental generation of insects to a sublethal dose of an insecticide results in beneficial effects or enhancements in its progenies. This transgenerational hormetic effect has been observed in various aspects of insect offspring, including growth [50], survival [51,52,53], body size [51], lifespan [52], and reproduction [54,55,56,57]. In the present study, we did not observe any differences in the rate of egg hatching in the progeny of the treated female mosquitoes. However, the egg hatching and development of immature mosquitoes were accelerated. This is probably the first evidence of transgenerational hormesis in mosquitoes.
Another study investigated the effects of sublethal exposure to thiamethoxam on both the parental generation (F0) and the subsequent progeny generation (F1) of Aphis gossypii Glover. The researchers [58] reported that when adult aphids in the F0 generation were exposed to a sublethal dose of thiamethoxam (LC15), the resulting F1 progeny exhibited stimulatory effects on the pre-adult stage, longevity, and fertility. Moreover, they observed significant increases in the expression levels of key genes involved in the production of vitellogenin and ecdysone receptors as well as cytochrome P450 enzymes in the F1 generation; these increases resulted from parental exposure to thiamethoxam [58]. The mechanisms underlying the reproduction of female Aedes mosquitoes and the growth rate of immature mosquitoes (progenies) remain to be elucidated. The changes in gene expression levels likely play crucial roles in mediating the observed improvements in reproduction and immature mosquito growth. This topic deserves further investigation.
Conclusions
We demonstrated that Ae. aegypti mosquitoes exhibit avoidance behavior when exposed to a mixture of deltamethrin and clothianidin, as evidenced by their high percentage of escape from treated surfaces. This finding suggests that IRS with the insecticide mixture effectively deters Aedes mosquitoes from entering residential areas, thus reducing mosquito bites. Although we observed delayed mortality and carryover effects of insecticide exposure on the survival of immature mosquitoes, the rate of application might have been insufficient to effectively kill Aedes mosquitoes. This finding is unexpected when compared with the results of previous studies using World Health Organization cone bioassays, which reported relatively high mortality rates within 72 h of exposure to residual spraying with the insecticide mixture [19,20,21,22,23, 59]. Notably, we observed stimulated mosquito reproduction and accelerated transgenerational immature development resulting from sublethal doses of the insecticide mixture. This finding indicates that when female mosquitoes survive exposure, their reproductive output may increase, and immature mosquitoes may develop rapidly. These changes in the life history of mosquitoes can have far-reaching consequences, potentially affecting vector management strategies and increasing disease transmission risks. Our findings serve as cautionary notes, highlighting the need to avoid assuming that the recommended application rate of an insecticide product will be universally effective against all mosquito vectors. Each mosquito species may respond differently to insecticides, and their behavioral and physiological responses to sublethal doses can have substantial implications for mosquito control programs.
Availability of data and materials
The data supporting the conclusions of this study are provided within the article. All datasets generated and analyzed during this study are available from the corresponding author upon reasonable request.
References
Souza-Neto JA, Powell JR, Bonizzoni M. Aedes aegypti vector competence studies: a review. Infect Genet Evol. 2019;67:191–209. https://doi.org/10.1016/j.meegid.2018.11.009.
Campbell LP, Luther C, Moo-Llanes D, Ramsey JM, Danis-Lozano R, Peterson AT. Climate change influences on global distributions of dengue and chikungunya virus vectors. Philos Trans R Soc Lond B Biol Sci. 2015;370:1665. https://doi.org/10.1098/rstb.2014.0135.
Dunbar MW, Correa-Morales F, Dzul-Manzanilla F, Medina-Barreiro A, Bibiano-Marín W, Morales-Ríos E, Vadillo-Sánchez J , López-Monroy B, Ritchie SA, Lenhart A, Manrique-Saide P, Vazquez-Prokopec GM. Efficacy of novel indoor residual spraying methods targeting pyrethroid-resistant Aedes aegypti within experimental houses. PLoS Negl Trop Dis. 2019;13(2):e0007203. https://doi.org/10.1371/journal.pntd.0007203.
Lee HL, Rohani A, Khadri MS, Nazni WA, Rozilawati H, Nurulhusna AH, et al. Dengue vector control in Malaysia-challenges and recent advances. IIUM Med J Malays. 2015. https://doi.org/10.31436/imjm.v14i1.448.
Samuel M, Maoz D, Manrique P, Ward T, Runge-Ranzinger S, Toledo J, et al. Community effectiveness of indoor spraying as a dengue vector control method: a systematic review. PLoS Negl Trop Dis. 2017;11:e0005837. https://doi.org/10.1371/journal.pntd.0005837.
Ooi EE, Goh KT, Gubler DJ. Dengue prevention and 35 years of vector control in Singapore. Emerg infect Dis. 2006;12:887–93. https://doi.org/10.3201/10.3201/eid1206.051210.
Rahayu A, Saraswati U, Supriyati E, Kumalawati DA, Hermantara R, Rovik A, et al. Prevalence and distribution of dengue virus in Aedes aegypti in Yogyakarta city before deployment of Wolbachia infected Aedes aegypti. Int J Environ Res Public Health. 2019;16:1742. https://doi.org/10.3390/ijerph16101742.
van den Berg H, da Silva Bezerra HS, Al-Eryani S, Chanda E, Nagpal BN, Knox TB, et al. Recent trends in global insecticide use for disease vector control and potential implications for resistance management. Sci Rep. 2021;11:23867. https://doi.org/10.1038/s41598-021-03367-9.
WHO. Global insecticide use for vector-borne disease control. World Health Organization, Geneva, Switzerland. 2021.
Bowman LR, Donegan S, McCall PJ. Is Dengue vector control deficient in effectiveness or evidence? Systematic review and meta-analysis. PLoS Negl Trop Dis. 2016;10:e0004551. https://doi.org/10.1371/journal.pntd.0004551.
World Health Organization. Dengue: guidelines for diagnosis, treatment, prevention and control. Geneva: World Health Organization; 2009.
Tainchum K, Polsomboon S, Grieco JP, Suwonkerd W, Prabaripai A, Sungvornyothin S, et al. Comparison of Aedes aegypti (Diptera: Culicidae) resting behavior on two fabric types under consideration for insecticide treatment in a push-pull strategy. J Med Entomol. 2013;50:59–68.
Dzul-Manzanilla F, Ibarra-Lopez J, Bibiano Marin W, Martini-Jaimes A, Leyva JT, Correa-Morales F, et al. Indoor resting behavior of Aedes aegypti (Diptera: Culicidae) in Acapulco. Mexico J Med Entomol. 2017;54:501–4. https://doi.org/10.1093/jme/tjw203.
Esu E, Lenhart A, Smith L, Horstick O. Effectiveness of peridomestic space spraying with insecticide on dengue transmission; systematic review. Trop Med Int Health. 2010;15:619–31.
Hladish TJ, Pearson CAB, Patricia Rojas D, Gomez-Dantes H, Halloran ME, Vazquez-Prokopec GM, et al. Forecasting the effectiveness of indoor residual spraying for reducing dengue burden. PLoS Negl Trop Dis. 2018;12:e0006570. https://doi.org/10.1371/journal.pntd.0006570.
World Health Organization. Indoor Residual Spraying. Geneva: World Health Organization; 2015.
Chadee DD. Resting behaviour of Aedes aegypti in Trinidad: with evidence for the re-introduction of indoor residual spraying (IRS) for dengue control. Parasit Vectors. 2013;6:255. https://doi.org/10.1186/1756-3305-6-255.
Ngufor C, Fongnikin A, Rowland M, N’Guessan R. Indoor residual spraying with a mixture of clothianidin (a neonicotinoid insecticide) and deltamethrin provides improved control and long residual activity against pyrethroid resistant Anopheles gambiae sl in Southern Benin. PLoS ONE. 2017;12:e0189575. https://doi.org/10.1371/journal.pone.0189575.
Fuseini G, Phiri WP, von Fricken ME, Smith J, Garcia GA. Evaluation of the residual effectiveness of Fludora fusion WP-SB, a combination of clothianidin and deltamethrin, for the control of pyrethroid-resistant malaria vectors on Bioko Island. Equatorial Guinea Acta Trop. 2019;196:42–7. https://doi.org/10.1016/j.actatropica.2019.05.006.
Gueye M, Dia I, Diedhiou S, Samb B, Kane Dia A, Diagne M, et al. Evaluation of the efficacy of Fludora((R)) Fusion WP-SB 56.25 (mixture of clothianidin and deltamethrin) against Anopheles coluzzii laboratory and An. arabiensis wild colonies. Trop Med Infect Dis. 2022;7(10): 316. https://doi.org/10.3390/tropicalmed7100316.
Agossa FR, Padonou GG, Fassinou A, Odjo EM, Akuoko OK, Salako A, et al. Small-scale field evaluation of the efficacy and residual effect of Fludora((R)) Fusion (mixture of clothianidin and deltamethrin) against susceptible and resistant Anopheles gambiae populations from Benin, West Africa. Malar J. 2018;17:484. https://doi.org/10.1186/s12936-018-2633-6.
Fongnikin A, Houeto N, Agbevo A, Odjo A, Syme T, N’Guessan R, et al. Efficacy of Fludora(R) Fusion (a mixture of deltamethrin and clothianidin) for indoor residual spraying against pyrethroid-resistant malaria vectors: laboratory and experimental hut evaluation. Parasit Vectors. 2020;13:466. https://doi.org/10.1186/s13071-020-04341-6.
Animut A, Horstmann S. Residual efficacy of Fludora Fusion against Anopheles arabiensis in simple huts in Ethiopia. PLoS ONE. 2022;17:e0263840. https://doi.org/10.1371/journal.pone.0263840.
World Health Organization. Guidelines For Laboratory and Field-testing of Long-lasting Insecticidal nets. Geneva: World Health Organization; 2013.
Manda H, Arce LM, Foggie T, Shah P, Grieco JP, Achee NL. Effects of irritant chemicals on Aedes aegypti resting behavior: is there a simple shift to untreated “safe sites”? PLoS Negl Trop Dis. 2011;5:e1243. https://doi.org/10.1371/journal.pntd.0001243.
Kongmee M, Nimmo D, Labbé G, Beech C, Grieco J, Alphey L, et al. Irritant and repellent behavioral responses of Aedes aegypti male populations developed for RIDL disease control strategies. J Med Entomol. 2010;47:1092–8. https://doi.org/10.1603/me10046.
Yu JJ, Bong LJ, Panthawong A, Chareonviriyaphap T, Neoh KB. Repellency and contact irritancy responses of Aedes aegypti (Diptera: Culicidae) against deltamethrin and permethrin: a cross-regional comparison. J Med Entomol. 2021;58:379–89. https://doi.org/10.1093/jme/tjaa172.
Kongmee M, Prabaripai A, Akratanakul P, Bangs MJ, Chareonviriyaphap T. Behavioral responses of Aedes aegypti (Diptera: Culicidae) exposed to deltamethrin and possible implications for disease control. J Med Entomol. 2004;41:1055–63. https://doi.org/10.1603/0022-2585-41.6.1055.
Cohnstaedt LW, Allan SA. Effects of sublethal pyrethroid exposure on the host-seeking behavior of female mosquitoes. J Vector Ecol. 2011;36:395–403. https://doi.org/10.1111/j.1948-7134.2011.00180.x.
Rigby LM, Johnson BJ, Peatey CL, Beebe NW, Devine GJ. The impact of sublethal permethrin exposure on susceptible and resistant genotypes of the urban disease vector Aedes aegypti. Pest Manag Sci. 2021;77:3450–7. https://doi.org/10.1002/ps.6398.
Cutler GC. Insects, insecticides and hormesis: evidence and considerations for study. Dose Response. 2013;11:154–77. https://doi.org/10.2203/dose-response.12-008.Cutler.
Budi H. Indonesia dengue fever: Status, Vulnerability, and Challenges. In: Alfonso JR-M, editor. Current Topics in Tropical Emerging Diseases and Travel Medicine. Rijeka: IntechOpen; 2018. p. Ch. 5.
Kementerian Kesehatan Republik Indonesia. Profil Kesehatan Indonesia Tahun 2019. Jakarta: Kementerian Kesehatan RI; 2020. https://www.kemkes.go.id/app_asset/file_content_download/Profil-Kesehatan-Indonesia-2019.pdf
Kementerian Kesehatan Republik Indonesia. Profil Kesehatan Indonesia Tahun 2018. Jakarta: Kementerian Kesehatan RI; 2019. https://www.kemkes.go.id/app_asset/file_content_download/profil-kesehatan-indonesia-2018.pdf?utm_medium=email&utm_source=transaction
Silalahi CN, Tu WC, Chang NT, Singham GV, Ahmad I, Neoh KB. Insecticide resistance profiles and synergism of field Aedes aegypti from Indonesia. PLoS Negl Trop Dis. 2022;16:e0010501. https://doi.org/10.1371/journal.pntd.0010501.
Chareonviriyaphap T, Prabaripai A, Sungvornyothrin S. An improved excito-repellency test chamber for mosquito behavioral tests. J Vector Ecol. 2002;27:250–2.
Bong LJ, Tu WC, Neoh KB. Interpopulation variations in life history traits and reproductive tactics in Aedes aegypti: a test on populations 50 km apart. Acta Trop. 2021;213:105750. https://doi.org/10.1016/j.actatropica.2020.105750.
Chung HH, Tsai CH, Teng HJ, Tsai KH. The role of voltage-gated sodium channel genotypes in pyrethroid resistance in Aedes aegypti in Taiwan. PLoS Negl Trop Dis. 2022;16:e0010780. https://doi.org/10.1371/journal.pntd.0010780.
Yu JJ, Bong LJ, Panthawong A, Chareonviriyaphap T, Liu WT, Neoh KB. Effects of piperonyl butoxide synergism and cuticular thickening on the contact irritancy response of field Aedes aegypti (Diptera: Culicidae) to deltamethrin. Pest Manag Sci. 2021;77:5557–65. https://doi.org/10.1002/ps.6597.
Morales D, Ponce P, Cevallos V, Espinosa P, Vaca D, Quezada W. Resistance status of Aedes aegypti to deltamethrin, malathion, and temephos in Ecuador. J Am Mosq Control Assoc. 2019;35:113–22. https://doi.org/10.2987/19-6831.1.
Nkya TE, Akhouayri I, Poupardin R, Batengana B, Mosha F, Magesa S, et al. Insecticide resistance mechanisms associated with different environments in the malaria vector Anopheles gambiae: a case study in Tanzania. Malar J. 2014;13:28. https://doi.org/10.1186/1475-2875-13-28.
Pridgeon JW, Pereira RM, Becnel JJ, Allan SA, Clark GG, Linthicum KJ. Susceptibility of Aedes aegypti, Culex quinquefasciatus Say, and Anopheles quadrimaculatus Say to 19 pesticides with different modes of action. J Med Entomol. 2008;45:82–7. https://doi.org/10.1093/jmedent/45.1.82.
Ding J, Zhao Y, Zhang Z, Xu C, Mu W. Sublethal and hormesis effects of clothianidin on the black cutworm (Lepidoptera: Noctuidae). J Econ Entomol. 2018;111:2809–16. https://doi.org/10.1093/jee/toy254.
Lee CY, Yap HH, Chong NL. Sublethal effects of deltamethrin and propoxur on longevity and reproduction of German cockroaches. Blattella germanica Entomol Exp Appl. 1998;89:137–45.
Fang Y, Wang J, Luo C, Wang R. Lethal and Sublethal effects of clothianidin on the development and reproduction of Bemisia tabaci (Hemiptera: Aleyrodidae) MED and MEAM1. J Insect Sci. 2018;18:37. https://doi.org/10.1093/jisesa/iey025.
Shang J, Yao YS, Chen LL, Zhu XZ, Niu L, Gao XK, et al. Sublethal exposure to deltamethrin stimulates reproduction and alters symbiotic bacteria in Aphis gossypii. J Agric Food Chem. 2021;69:15097–107. https://doi.org/10.1021/acs.jafc.1c05070.
Choi DB, Grieco JP, Apperson CS, Schal C, Ponnusamy L, Wesson DM, et al. Effect of spatial repellent exposure on dengue vector attraction to oviposition sites. PLoS Negl Trop Dis. 2016;10:e0004850. https://doi.org/10.1371/journal.pntd.0004850.
Bong LJ, Tu WC, Neoh KB, Huang CG, Ting RX. The effect of insecticidal stress on reproductive output of susceptible and field strains of Aedes aegypti (Diptera: Culicidae). J Med Entomol. 2017;55:36–42. https://doi.org/10.1093/jme/tjx191.
Agathokleous E, Guedes RNC, Calabrese EJ, Fotopoulos V, Azevedo RA. Transgenerational hormesis: What do parents sacrifice for their offspring? Curr Opin Environ Sci Health. 2022;29:100380. https://doi.org/10.1016/j.coesh.2022.100380.
Wei Y, Su Y, Han X, Guo W, Zhu Y, Yao Y. Evaluation of transgenerational effects of sublethal imidacloprid and diversity of symbiotic bacteria on Acyrthosiphon gossypii. Insects. 2023;14:427. https://doi.org/10.3390/insects14050427.
Margus A, Piiroinen S, Lehmann P, Tikka S, Karvanen J, Lindstrom L. Sublethal pyrethroid insecticide exposure carries positive fitness effects over generations in a pest insect. Sci Rep. 2019;9:11320. https://doi.org/10.1038/s41598-019-47473-1.
Fuciarelli TM, Rollo CD. Trans-generational impacts of paternal irradiation in a cricket: damage, life-history features and hormesis in F1 offspring. Dose Response. 2020;18:1559325820983214. https://doi.org/10.1177/1559325820983214.
Shaw B, Brain P, Wijnen H, Fountain MT. Implications of sub-lethal rates of insecticides and daily time of application on Drosophila suzukii lifecycle. Crop Prot. 2019;121:182–94. https://doi.org/10.1016/j.cropro.2019.04.006.
Feng WB, Bong LJ, Dai SM, Neoh KB. Effect of imidacloprid exposure on life history traits in the agricultural generalist predator Paederus beetle: Lack of fitness cost but strong hormetic effect and skewed sex ratio. Ecotoxicol Environ Saf. 2019;174:390–400. https://doi.org/10.1016/j.ecoenv.2019.03.003.
Ayyanath MM, Cutler GC, Scott-Dupree CD, Sibley PK. Transgenerational shifts in reproduction hormesis in green peach aphid exposed to low concentrations of imidacloprid. PLoS ONE. 2013;8:e74532. https://doi.org/10.1371/journal.pone.0074532.
Chen XD, Seo M, Ebert TA, Ashfaq M, Qin W, Stelinski LL. Hormesis in the brown citrus aphid, Toxoptera citricida (Kirkaldy) (Hemiptera: Aphididae) exposed to sublethal doses of imidacloprid. Fla Entomol. 2020;103:337–43. https://doi.org/10.1653/024.103.0305.
Rix RR, Cutler GC. Low Doses of a neonicotinoid stimulate reproduction in a beneficial predatory insect. J Econ Entomol. 2020;113:2179–86. https://doi.org/10.1093/jee/toaa169.
Ullah F, Gul H, Tariq K, Desneux N, Gao X, Song D. Thiamethoxam induces transgenerational hormesis effects and alteration of genes expression in Aphis gossypii. Pestic Biochem Physiol. 2020;165:104557. https://doi.org/10.1016/j.pestbp.2020.104557.
Kamaraju R, Pant CS, Uragayala S, Baharia RK, Srivastava HC, Yadav RS. Small-scale field evaluation of the entomological efficacy and the residual activity of Fludora((R)) Fusion WP-SB indoor residual spraying against Anopheles culicifacies s.l. in Gujarat, India. Trop Med Int Health. 2021;26(4):469–77. https://doi.org/10.1111/tmi.13549.
Acknowledgements
We thank Envu for providing Fludora Fusion.
Funding
This study was partially supported by grants from the National Science and Technology Council, Taiwan (112-2313-B-005-025).
Author information
Authors and Affiliations
Contributions
CNS and YA performed the experiments. KBN conceived and designed the study. CNS and YA analyzed and interpreted the data. CNS and YA drafted the manuscript. MEC, AI, and KBN substantially revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Silalahi, C.N., Yasin, A., Chen, ME. et al. Behavioral responses and life history traits of Taiwanese and Indonesian populations of Aedes aegypti surviving deltamethrin–clothianidin treatment. Parasites Vectors 17, 117 (2024). https://doi.org/10.1186/s13071-024-06189-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-024-06189-6