Abstract
Background
Human cercarial dermatitis (HCD) is a clinical disease typically caused by skin-penetrative larvae of avian schistosomes. Its geographical epidemiology is firmly tied with that of infected freshwater intermediate snail hosts. To better understand the current distribution of HCD and its level of nuisance in the UK, we undertook a systematic literature review.
Methods
Following PRIMSA guidelines, PubMed and Scopus databases were searched with keywords “human cercarial dermatitis” OR “swimmer’s itch” AND “United Kingdom”. Articles about imported cases of HCD, or HCD outside the UK, were not formally included.
Results
A total of 30 articles were initially identified. A further two were gained by inspection of all citations. After screening, eight publications were analysed where the location, number of cases and putative avian schistosome species incriminated were tabulated. HCD is mainly found in the south of England, though gaps in evidence and reporting remain across the UK.
Conclusions
Despite its noted recent rise in open water swimmers, published literature on HCD across the UK is sparse; this condition is both overlooked and under-reported. We therefore recommend establishing a national database that raises awareness and encourages self-reporting of this nuisance disease.
Graphical Abstract

Similar content being viewed by others
Background
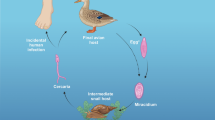
Nearly one hundred years ago, avian schistosome cercariae were first discovered as the causative agent of cercarial dermatitis in the USA [1]. The life cycle of many avian schistosome species is complex [2, 3]. Of particular note is that avian cercariae are attracted to human skin, penetrating the upper dermal layers in response to key fatty acids also found in duck feet skin [4]. Since humans are accidental ‘dead-end’ hosts, these invading avian cercariae die but in so doing stimulate a strong allergic host reaction; this is pathognomonic for human cercarial dermatitis (HCD), for in the 3–48 h period after exposure, a dermal rash will appear with intensely pruritic papules and erythaema. This is followed by intense itching [4], which gives HCD its more common name of ‘swimmer’s itch’. Although the symptoms of HCD are known, data on associated immune responses in human patients are sporadic and incomprehensive; Macháček et al. [5] attempted to correlate symptoms, personal history and time course of HCD with differential cell counts, dynamics of selected cytokines and dynamics and quality of antibody response [5].
Avian schistosomes are distributed worldwide, save on Antarctica [6]. In Europe and North America, HCD is considered an emerging and/or re-emerging disease as cases are increasing [7]. Indeed, monitoring of HCD outbreaks in Europe is now the subject of numerous local and international research projects; various researchers have attempted to better understand risk by examination of snails, inspection of birds and cercariometry of water [8,9,10,11,12,13], alongside introduction of environmental DNA surveillance [14]. Whilst the prevalence of schistosomiasis in European birds can reach 38% [6, 8], in the UK its present epidemiology is poorly understood [6, 7] despite the presence of numerous freshwater bodies that harbour infected snails along major bird migration flyways from Europe [15, 16].
In Scotland, for example, Pennycott et al. [17] found mute swans infected with avian schistosomes that might point towards HCD’s most northerly range [17]. Elsewhere in the UK, notable outbreaks of HCD have occurred, where its detrimental impacts on local economies dependent on tourism can take place [18].
Given shifting snail and bird distribution patterns, alongside climate change, as a foundation step towards better surveillance of HCD in the UK, we conducted a systematic review of the formal literature and later investigated contemporary reports of HCD in the national media.
Methods
Research selection and search criteria
To collate research articles reporting HCD in the UK, literature searches were conducted in two electronic databases (PubMed and Scopus) on 13 March 2023. Manuscripts were selected based on the following inclusion criteria of studies conducted in UK, reports of HCD cases and avian schistosome species implicated.
The detailed search strategies and PRISMA 2020 flow chart are shown in Fig. 1. We focused on keyword of “human cercarial dermatitis” OR "swimmer’s itch” AND “United Kingdom”. The initial results were imported into Mendeley and duplicates removed. The predetermined eligibility criteria were used to exclude any irrelevant articles i.e. imported cases, HCD outside UK and cercariae outside of UK.
Data extraction
Studies that met our inclusion criteria were subjected to the next phase of screening to extract the following information: month/year of findings; location, main findings such as prevalence if reported, avian schistosome species implicated and year of publication. No time restrictions were placed on publications identified from the systematic literature review.
Results
In total, 30 studies were retrieved from the customized searches. The systematic review identifed eight articles on HCD in UK that met the inclusion and eligibility criteria. While carrying out the full text screen of these eight articles, two further articles not found through the initial search were identified upon inspection of articles’ citations. However, one article was inaccessible with a message being to the book’s author for retrieval of the relevant extract but with no response. One widely known outbreak of HCD in Norfolk, 2004, was mentioned in several papers but no specific evidence could be found through databases.
All published reports describing cercariae and HCD outbreaks in the UK occurred in the summer months, being always connected with paddling or swimming in infested water. There are several species of cercariae which have been detailed in the articles on HCD in the UK. The majority report on Trichobilharzia spp. but with varying species-specific reliability due to a lack of confirmatory molecular analysis. Table 1 shows the key information taken from the selected literature. There appeared to be only a modest number of papers on HCD but these publications frequently noted that as HCD research is increasing there is also a growing number of publications but these were not specifically tied with discrete HCD outbreaks.
Discussion
As shown in Table 1, published literature on HCD in the UK is surprisingly sparse; only eight articles were identified, yet these provide solid epidemiologcial evidence, alongside its underlying putative aetiological agents. All eight reports describing cercariae and HCD outbreaks were observed during summer months only, conclusive of HCD’s well-known seasonality elsewhere [7]. The summer season also influences human behaviour which leads to exposure, outdoor activities such as open water swimming being more common. This clear seasonality can be confirmed with online searches for “swimmer’s itch” in Google, where there are cyclical peaks during summer months each year although such online searches are likely to be by those who wish to know more about what swimmer’s itch actually is (Fig. 2) or whether other animals such as companion pets, for example dogs, are also at risk.
The number of Google searches for “swimmer’s itch” conducted in the UK over the last 5 years. Graph showing the number of the search term “swimmer’s itch” on Google in the UK. There has been increasing popularity in the term over the past 5 years and there are annual peaks in summer months. The term was most popular in July 2021
In terms of freshwater snail species implicated, cercarial shedding from Lymnaea stagnalis was noted. This was positively correlated with temperature and light with these cercariae exhibiting positive phototaxis [12], most often during the afternoon and/or early evening [19]. It is reasonable to speculate that HCD may become more common in the UK because of increasing rise of water temperatures in recent years. For example, Fraser et al. [20] noted an increased incidence of HCD as described occurred during a period of unusually hot weather [20]. Larsen et al. [21] have suggested that a 0.8 ℃ increase in temperature within the last century may be associated with increased prevalence of Trichobilharzia spp. in more northerly parts of Europe [21].
A study conducted in Oxfordshire has also shown that climate change was affecting the migratory behaviour of birds [22]. It is therefore sensible to understand the role of waterfowl on the epidemiology of HCD and to apply appropriate tools to better identify avian schistosome species to assess their risk(s). This is particularly pertinent in view of the distinct lack of knowledge of the diversity of avian flukes and their intermediate freshwater snail hosts in the UK. Fraser et al. [20] identified cercariae morphologically as Trichobilharzia species using a “dichotomous key” but did not go on to make any further species-specific ascertions [20].
Harding [23] identified Trichobilharzia ocellata through consultation with helminthologists at the British Museum [23], while Knight and Worms (1972) reported cercariae of the “Ocellata” group [24]. These descriptions may now be incorrect as the identity of T. ocellata has now been called into question. Rudolfová et al. [25] found that various isolates of T. ocellata from across the globe were genetically dissimilar and that European isolates of this description are actually identical to Trichobilharzia szidati when DNA sequence analysis was conducted [25].
Today, molecular DNA identification is the only reliable method to identify the avian cercariae incriminated in HCD as their morphological features are notiously uniform. Upon application of DNA screening, the phylogenic analysis of cercariae by Lawton et al. [7] implicated Trichobilharzia franki. They also noted two novel British lineages of this species which are most closely related to French isolates of T. franki. More recently, and using molecular methods, Juhász et al. [13] uncovered greater and surprising diversity within avian schistosomes in the northwest of England. For example, Bilharziella polonica was noted, a first report in recent years, alongside Trichobilharzia anseri as well as an as of yet divergent lineage within Trichobilharzia [19]. Outside of the UK, Bilharziella is less commonly associated with HCD but is an avian parasite of some general concern [3].
A key gap arising from our systematic literature search is that no official reporting mechanisms in the UK, for example by the UK Health Security Agency (UKHSA), exist. Thus, the true number of people who seek medical attention for HCD is unknown, a worrysome deficit in satisfactory surveillance, particularly as HCD can be confused with other dermatitis for which central guidance from the UK-NHS exists [20]. Nonetheless, national media have reported on worrisome HCD outbreaks, most recently in 2023 in Llanishen Reservoir, Wales (see https://www.bbc.com/news/uk-wales-66328852.amp) and in 2021 in Loch Lomand, Scotland (see https://www.heraldscotland.com/news/homenews/194102211.loch-lomond-wild-swimmers-warned-parasite-skin-reaction/). Each report led to local restrictions in recreational activities and closure of public access. Developing a reporting method where a physician or member of the public may self-report where they have experienced HCD could be valuable. A reporting system, for example, could be styled like that for Lyme disease [26], where people are encouraged to be tick aware. A similar inititiave for HCD, as advertised on wild swimming websites, where water quality and/or sewage contamination is also noted, could help identify at-risk locations. Here, malacological sampling or perhaps environmental DNA inspections for avian schistosomes [27] could be sensibly applied.
Across the UK, certain councils, for example Oxford, already offer HCD advice [28] but such information is fragmented and there is no central advice by the UK-NHS [29]. By contrast, in the USA, where HCD can be particularly common, community initiatives such as the Michigan Swimmer’s Itch Partnership (MISIP) have been developed to help mitigate local risk [30] and recently expanded to attempt to capture reports of HCD in the UK too (see https://swimersitch.info/map-your-itch-2023). Alternatively, in Denmark, an epidemiological baseline with an online national reporting system has been instigated [31].
Conclusions
Despite surprisingly sparse literature, we have identified a clear need for better epidemiological surveillance, with near real-time reporting of HCD, in the UK. To do so, we suggest development of a national database overseen, for example, by the UKHSA or suitably engaged volunteer groups.
Data availability
All data are included as tables and figures within the article.
Abbreviations
- HCD:
-
Human cercarial dermatitis
- UK:
-
United Kingdom
- UKHSA:
-
United Kingdom Health Security Agency
References
Cort WW. Schistosome dermatitis in the United States (Michigan). JAMA. 1989;90:1027–9. https://doi.org/10.1001/jama.1928.02690400023010.
Loker ES, DeJong RJ, Brant SV. Scratching the itch: updated perspectives on the Schistosomes responsible for swimmer’s itch around the World. Pathogens. 2022;11:587. https://doi.org/10.3390/pathogens11050587.
Horák P, Mikeš L, Lichtenbergová L, Skála V, Soldánová M, Brant SV. Avian Schistosomes and outbreaks of cercarial dermatitis. Clin Microbiol Rev. 2015;28:165–90. https://doi.org/10.1128/CMR.00043-14.
Haas W, Schmitt R. Characterization of chemical stimuli for the penetration of Schistosoma mansoni cercariae. I. Effective substances, host specificity. Z Parasitenkd. 1982;66:293–307. https://doi.org/10.1007/BF00925346.
Macháček T, Turjanicová L, Bulantová J, Hrdý J, Horák P, Mikeš L. Cercarial dermatitis: a systematic follow-up study of human cases with implications for diagnostics. Parasitol Res. 2018;117:3881–95. https://doi.org/10.1007/s00436-018-6095-0.
Lashaki EK, Teshnizi SH, Gholami S, Fakhar M, Brant SV, Dodangeh S. Global prevalence status of avian Schistosomes: a systematic review with meta-analysis. Parasite Epidemiol Control. 2020;9:e00142. https://doi.org/10.1016/j.parepi.2020.e00142.
Lawton SP, Lim RM, Dukes JP, Cook RT, Walker AJ, Kirk RS. Identification of a major causative agent of human cercarial dermatitis, Trichobilharzia franki (Müller and Kimmig 1994), in southern England and its evolutionary relationships with other European populations. Parasit Vectors. 2014;7:277. https://doi.org/10.1186/1756-3305-7-277.
Pilz J, Eisele S, Disko R. Zerkariendermatitis (swimmer’s itch). Fallbericht einer Zerkariendermatitis durch Trichobilharzia (Digena, Schistosomatidae) [Cercaria dermatitis (swimmer’s itch). Case report of cercaria dermatitis caused by Trichobilharzia (Digena, Schistosomatidae)]. Hautarzt. 1995;46:335–8. https://doi.org/10.1007/s001050050262.
de Gentile L, Picot H, Bourdeau P, Bardet R, Kerjan A, Piriou M, et al. La dermatite cercarienne en Europe: un problème de santé publique nouveau? [Cercarial dermatitis in Europe: a new public health problem?]. Bull WHO. 1996;74:159–63.
Kolárová L, Skirnisson K, Horák P. Schistosome cercariae as the causative agent of swimmer’s itch in Iceland. J Helminthol. 1999;73:215–20. https://doi.org/10.1017/s0022149x99000335.
Marszewska A, Cichy A, Heese T, Żbikowska E. The real threat of swimmers’ itch in anthropogenic recreational water body of the Polish Lowland. Parasitol Res. 2016;115:3049–56. https://doi.org/10.1007/s00436-016-5060-z.
Al-Jubury A, Kania P, Bygum A, Buchmann K. Temperature and light effects on Trichobilharzia szidati cercariae with implications for a risk analysis. Acta Vet Scand. 2020;62:54. https://doi.org/10.1186/s13028-020-00553-z.
Juhász A, Majoros G, Cech G. Threat of cercarial dermatitis in Hungary: a first report of Trichobilharzia franki from the mallard (Anas platyrhynchos) and European ear snail (Radix auricularia) using molecular methods. Int J Parasitol Parasites Wildl. 2022;18:92–100. https://doi.org/10.1016/j.ijppaw.2022.04.009.
Helmer N, Hörweg C, Sattmann H, Reier S, Szucsich NU, Bulantová J, et al. DNA barcoding of Trichobilharzia (Trematoda: Schistosomatidae) species and their detection in eDNA water samples. Diversity. 2023;15:104. https://doi.org/10.3390/d15010104.
Ashrafi K, Sharifdini M, Darjani A, Brant SV. Migratory routes, domesticated birds and cercarial dermatitis: the distribution of Trichobilharzia franki in Northern Iran. Parasite. 2021;28:4. https://doi.org/10.1051/parasite/2020073.
Lovas-Kiss Á, Martín-Vélez V, Brides K, Wilkinson DM, Griffin LR, Green AJ. Migratory geese allow plants to disperse to cooler latitudes across the ocean. J Biogeogr. 2023. https://doi.org/10.1111/jbi.14674.
Pennycott TW. Lead poisoning and parasitism in a flock of mute swans (Cygnus olor) in Scotland. Vet Rec. 1998;142:13–7. https://doi.org/10.1136/vr.142.1.13.
Leighton BJ, Nervos S, Webster JM. Ecological factors in schistosome transmission, and an environmentally benign method for controlling snails in a recreational lake with a record of schistosome dermatitis. Parasitol Int. 2000;49:9–17. https://doi.org/10.1016/s1383-5769(99)00034-3.
Juhász A, Barlow SEJ, Williams H, Johnson B, Walsh ND, Cunningham LC, et al. A report of Bilharziella polonica cercariae in Knowsley Safari, Prescot, United Kingdom, with notes on other trematodes implicated in human cercarial dermatitis. J Helminthol. 2022;96:e79. https://doi.org/10.1017/S0022149X22000694.
Fraser SJ, Allan SJ, Roworth M, Smith HV, Holme SA. Cercarial dermatitis in the UK. Clin Exp Dermatol. 2008;34:344–6. https://doi.org/10.1111/j.1365-2230.2008.02903.x.
Larsen AH, Bresciani J, Buchmann K. Increasing frequency of cercarial dermatitis at higher latitudes. Acta Parasitol. 2004;49:217–21.
Cotton PA. Avian migration phenology and global climate change. Proc Natl Acad Sci USA. 2003;100:12219–22. https://doi.org/10.1073/pnas.1930548100.
Harding JR. Cardiff’s tropical disease: cercarial dermatitis. Med Hist. 1978;22:83–8. https://doi.org/10.1017/s0025727300031768.
Knight R, Worms MJ. An outbreak of cercarial dermatitis in Britain. Trans R Soc Trop Med Hyg. 1972;66:21. https://doi.org/10.1016/0035-9203(72)90037-5.
Rudolfová J, Hampl V, Bayssade-Dufour C, Lockyer AE, Littlewood DT, Horák P. Validity reassessment of Trichobilharzia species using Lymnaea stagnalis as the intermediate host. Parasitol Res. 2005;95:79–89. https://doi.org/10.1007/s00436-004-1262-x.
Gov.uk (2012) ’Guidance, Tick awareness and the Tick Surveillance Scheme’ [online]. https://www.gov.uk/guidance/tick-surveillance-scheme
McPhail BA, Froelich K, Reimink RL, Hanington PC. Simplifying schistosome surveillance: using molecular cercariometry to detect and quantify cercariae in water. Pathogens. 2022;11:565. https://doi.org/10.3390/PATHOGENS11050565.
Fisd.oxfordshire.gov.uk. (2016)’ Safe swimming in the UK’ [online]. https://fisd.oxfordshire.gov.uk/kb5/oxfordshire/directory/advice.page?id=KlW4yOU5qt4#:~:text=%22Swimmer's%20itch%22%20(cercarial%20dermatitis,into%20the%20skin%20of%20swimmers.
Nhs.uk (2023) ‘Overview-Contact dermatitis’ [online]. https://www.nhs.uk/conditions/contact-dermatitis/.
Reimink R. Progress in battle against swimmer’s itch. MISIP Fall Conference. 2017; Hagerty Center, Traverse City, Michigan.
Tracz ES, Al-Jubury A, Buchmann K, Bygum A. Outbreak of swimmer’s itch in Denmark. Acta Derm Venereol. 2019;99:1116–20. https://doi.org/10.2340/00015555-3309.
Fewtrell L, Godfree A, Jones F, Kay D, Merrett H. Pathogenic microorganisms in temperate environmental waters. London: Samara Publishing Ltd.; 1994.
Communicable Disease Surveillance Centre. Epidemiology report from the PHLS communicable disease surveillance centre. Br Med J. 1998;296:779.
Acknowledgements
We thank Dr. Gábor Majoros for helpful suggestions that have improved this manuscript. We are also grateful to colleagues at Knowsley Safari, Prescot, who helped foster our local interest in avian schistosomiasis within their property.
Funding
This work forms part of the BSc research dissertation of Ms Orla Kerr and received no specific grant from any funding agency, commercial or not-for-profit sectors. Alexandra Juhasz and Sam Jones receive salary support from the Wellcome Trust and, in part, by the National Institute for Health Research (NIHR) (using the UK’s Official Development Assistance (ODA) Funding) and Wellcome Trust [220818/Z/20/Z] under the NIHR-Wellcome Partnership for Global Health Research. The views expressed are those of the authors and not necessarily those of Wellcome, the NIHR or the Department of Health and Social Care.
Author information
Authors and Affiliations
Contributions
This work reported here forms part of the BSc research dissertation of OK. JRS initiated and supervised the study. AJ, OK and SJ wrote the draft manuscript and prepared figures and tables. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
The manuscript has been approved by Liverpool School of Tropical Medicine, Department of Tropical Disease Biology.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kerr, O., Juhász, A., Jones, S. et al. Human cercarial dermatitis (HCD) in the UK: an overlooked and under-reported nuisance?. Parasites Vectors 17, 83 (2024). https://doi.org/10.1186/s13071-024-06176-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-024-06176-x