Abstract
Background
Rodents play an important role in the life cycle of ixodid and argasid ticks, particularly as hosts of larvae and nymphs. The great gerbil (Rhombomys opimus), the preferred prey item of several carnivores (e.g. the red fox and marbled polecat), is the dominant rodent species in the Gurbantunggut Desert in northwestern China. The aim of this study was to investigate tick species associated with different hosts in the habitat of great gerbils, including wildlife and livestock.
Methods
During 2018–2023, ticks were removed from 326 great gerbils, two red foxes (Vulpes vulpes), three marbled polecats (Vormela peregusna), 35 pastured sheep (Ovis aries), and one long-eared desert hedgehog (Hemiechinus auritus) in the Gurbantunggut Desert. Ticks were identified according to standard morphological keys. Then, they were further analyzed by molecular and phylogenic methods based on two mitochondrial markers, 16S rDNA and cytochrome c oxidase subunit I (COI) genes.
Results
A total of 889 ticks were collected, representing five species. These included Hyalomma asiaticum (n = 425: 24 larvae, 79 nymphs and 322 adults), Rhipicephalus turanicus (n = 153: 2 nymphs and 151 adults), Haemaphysalis erinacei (n = 298: 4 larvae, 7 nymphs and 287 adults), Ixodes acuminatus (n = 7: 4 nymphs and 3 adults) and Ornithodoros tartakovskyi (6 adults). Based on COI sequences, molecular and phylogenetic analyses showed that (i) I. acuminatus from great gerbils and marbled polecats clustered with I. acuminatus reported from Europe; (ii) O. tartakovskyi found in northwestern China belonged to an independent clade; (iii) Hy. asiaticum, R. turanicus and Ha. erinacei had 100% sequence identities to conspecific ticks sampled previously in China.
Conclusions
The great gerbil is an important host for the developmental stages of I. acuminatus, O. tartakovskyi, Ha. erinacei, Hy. asiaticum and R. turanicus, thus supporting the life cycle of several tick species which, as adults, parasitize predators (red fox and marble polecat) as well as pastured sheep and hedgehogs in the Gurbantunggut Desert. Ixodes acuminatus and O. tartakovskyi were found for the first time on great gerbil and marbled polecat, respectively.
Graphical Abstract

Similar content being viewed by others
Background
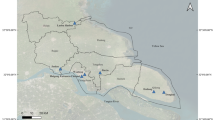
The Gurbantunggut Desert, covering 4.88 × 104 km2, is listed as the second largest desert in China. Its area ranges 84°50ʹ–91°20ʹE and 44°15ʹ–46°50ʹN [1] (Fig. 1). It lies in a typical temperate continental dry climate with annual precipitation of 70 to 150 mm. The annual average temperature ranges between 3 °C–7 °C, and its extreme high and low temperatures can reach > 40 °C and < − 40 °C, respectively. Its altitude ranges from 300 to 600 m a.s.l. The fixed and semi-fixed dunes account for 97% of the entire desert area. Haloxylon ammodendron, Ha. persicum and various ephemeral plants grow and provide food and water supplies for the great gerbils (Rhombomys opimus) [2, 3].
Meanwhile, > 100 animal species, including red fox (Vulpes vulpes), marble polecat (Vormela peregusna), long-eared hedgehog (Hemiechinus auritus) and goitered gazelle (Gazella subgutturosa sairensis) live in the area [4]. The great gerbil, the most dominant rodent species in the Gurbantunggut Desert, was previously reported to carry a variety of tick-borne pathogens, such as Tacheng tick virus 1, Songling virus [5], Karimabad virus [6], Babesia spp., Theileria spp. and Anaplasma ovis [7].
Ticks, such as Hyalomma asiaticum, Rhipicephalus turanicus and Dermacentor marginatus, were reported to be the most important pathogen vectors in the Gurbantunggut Desert [8]. To explore the relationship of local mammals as hosts of tick species shared between great gerbil and its predators or other sympatric mammals, this study aimed to systematically investigate ticks from great gerbils, marbled polecats (V. peregusna), a long-eared desert hedgehog (H. auritus), red foxes (V. vulpes) and pastured sheep (O. aries).
Methods
Sample collection and species identification
During 2018–2023, ticks were collected from 326 great gerbils (118 males, 208 females), three marbled polecats (road-killed), one long-eared desert hedgehog (road-killed), two red foxes (road-killed) and 35 pastured sheep (food supply for local oil workers) at 17 sampling sites in Gurbantunggut Desert (Fig. 1 and Additional file 1: Table S1). The great gerbils were captured in live traps according to our previous work [9] and were killed by cervical dislocation under the license of local Public Welfare Forest Reserve Management Station (PWFRMS). The road-killed wildlife were sent to our laboratory under the agreement of local PWFRMS. The pastured sheep were returned to local oil workers after sampling.
The presence of ticks was examined over the entire body of each animal at different intervals, including ears, neck, thorax, armpits, abdomen, interfeminium, crissum and so on. Ticks were collected with forceps and stored in 70% ethanol [7]. The ticks were morphologically observed using a stereo-microscope (Nikon SMZ-25, Japan) and identified to the species level according to the standard morphological keys as previously described [10].
Sequencing and phylogenetic analysis of ticks
Genomic DNA was extracted from 1–15 ticks representing each tick species from each host species, using the TIANamp Genomic DNAKit (TIANGEN, Beijing, China). Morphological identification was confirmed by molecular and phylogenic analyses based on two mitochondrial markers, 16S rDNA and cytochrome c oxidase subunit I (COI) genes [11]. In addition, phylogenetic analysis was performed using maximum likelihood method and 1000 bootstrap replicates with MEGA7.0.
Statistical analysis
The numbers of tick-infested male and female great gerbils were compared according to sampling periods by the Fisher’s exact test (https://www.langsrud.com/fisher.htm). The difference was considered significant if P < 0.05.
Results
Tick identification
A total of 889 ticks were collected, including 425 Hy. asiaticum (24 larvae, 79 nymphs and 322 adults), 153 R. turanicus (2 nymphs and 151 adults), 298 Haemaphysalis erinacei (4 larvae, 7 nymphs and 287 adults), seven Ixodes acuminatus (4 nymphs and 3 adults) and six Ornithodoros tartakovskyi (all adults) (Table 1). Morphological characteristics of tick specimens are shown in Additional file 2: Fig. S1. Larvae and nymphs were only found on female great gerbils during the period between April and June, but ticks infested both female and male great gerbils during September and October. However, this difference was not significant (P = 0.057).
Molecular and phylogenetic analyses
A total of 71 sequences were included in the phylogenetic analysis, and 24 nucleotide sequences from this study had been deposited in the GenBank database (16S rDNA: OR474496.1, OR474529.1, OR475313.1, OR475314.1, OR475315.1, OR475316.1, OR476952.1, OR476978.1, OR478282.1, OR478283.1, OR478284.1, OR478285.1; COI: OR437536.1, OR473062.1, OR473175.1, OR473540.1, OR473542.1, OR473543.1, OR473544.1, OR473541.1, OR473546.1, OR473547.1, OR473545.1 and OR473548.1). Based on COI sequences, molecular and phylogenetic analyses showed that I. acuminatus (OR473062: from R. opimus, China, and OR473543: from Vormela peregusna, China) clustered with I. acuminatus (OR139927 and OR139925: from Neovison vison, Spain, and OL339474: from Anthus pratensis, Malta), and Ixodes redikorzevi (JX394202: unknown host, Romania, and LC508369: unknown host, Portugal) (Fig. 2A). Hyalomma asiaticum, R. turanicus and Ha. erinacei had 100% COI sequence identities to the corresponding tick species reported previously in China (Fig. 2B). Ornithodoros tartakovskyi from great gerbil and marbled polecat had identical COI sequences and formed a sister clade to those of a laboratory strain (ON800883 and NC_067924) (Fig. 2C).
Maximum likelihood phylogenic tree inferred from the COI sequences of the ticks (A Ixodes acuminatus, B Hyalomma asiaticum, Rhipicephalus turanicus and Haemaphysalis erinacei, C Ornithodoros tartakovskyi) sampled from wildlife and pastured sheep in Gurbantunggut Desert, northwestern China. The new sequences provided by the present study are indicated by black circle/diamond/inverted triangle/square/triangle
Discussion
This study provides, for the first time to our knowledge, the results of a large-scale sampling and molecular-phylogenetic analyses of ticks from wild rodents in the Gurbantunggut Desert, conducted in the frame of a 6-year-long survey.
Among the tick species identified, I. acuminatus is known to prefer rodents as hosts [10]. Based on morphological comparison of female specimens of I. acuminatus from Italy and France, and I. redikorzevi from the former USSR, I. redikorzevi was suggested to be a junior synonym of I. acuminatus [12], as also stated elsewhere [13]. Other literature data attest that both are valid species, until the opposite is proven by extensive morphological-molecular comparisons [14, 15]. In this study, the COI sequence of I. acuminatus (OR473062.1, OR473543.1) shared 98.73–99.39% identities with those of I. acuminatus from Spain and Malta and I. redikorzevi from Romania and Portugal. Interestingly, 100% identity was shown among COI amino acid sequences of ticks that were morphologically identified as either I. acuminatus or I. redikorzevi in distant parts of the Palearctic (Additional file 3: Fig. S2). These data support that I. acuminatus and I. redikorzevi might represent the same species. However, the ultimate conclusion could only be drawn in this context if detailed morphological analyses were possible between ticks that are near-identical in their barcoding gene and/or the corresponding amino acid sequences.
Based on literature data, I. acuminatus is widely distributed in the Palearctic region, including Belgium, France, Germany, UK, Italy, Hungary, Portugal, Spain, Malta and Turkey [10, 14, 16,17,18,19]. This tick species inhabits mainly temperate broad-leaf and mixed forests, where its main hosts are from families Erinaceidae, Mustelidae (including Mustela), Canidae, Felidae, Viverridae, Soricidae, Talpidae, Arvicolidae, Cricetidae (including Microtus), Gliridae, Muridae, Turdidae, Phasianidae, Accipitridae, Sylviidae and Troglodytidae [10, 14, 16,17,18,19]. At the same time, I. redikorzevi was reported to occur in the former USSR, Iran, Pakistan, Afghanistan, Nepal, Israel, Egypt, Kazakhstan, Turkey, Portugal and Romania, where it was collected from Corvidae, Turdidae, Paridae, Passeridae, Ploceidae, Mustelidae (including Mustela), Canidae, Sciuridae, Circetidae (including Microtus), Muridae, Talpidae and Erinaceidae [20,21,22,23,24,25]. Thus, finding seven I. acuminatus specimens in this study on great gerbil (Rhombomys, Circetidae) and marble polecat (Vormela, Mustelidae) in the Gurbantunggut Desert extends the above host range.
Previously, Ha. erinacei was collected from North African hedgehog (Atelerix algirus), European hedgehog (Erinaceus europaeus), white-breasted hedgehog (Erinaceus concolor), long-eared hedgehog (Hemiechinus auritus) and desert hedgehog (Paraechinus aethiopicus) in North Africa, Balkan peninsula, Italy, Pakistan, Afghanistan, Kazakhstan, Saudi Arabia, Turkey, Algeria, Bulgaria, Croatia, Jordan, Iran, Iraq, Israel, Kazakhstan, Kyrgyzstan and Uzbekistan [10]. In China, Ha. erinacei was reported to infest marbled polecat (Vormela peregusna) and red fox (Vulpes vulpes) [26,27,28]. In this study, 298 Ha. erinacei specimens were collected, including four larvae and seven nymphs from great gerbil, as well as 287 adults from marbled polecat, long-eared desert hedgehog and red fox. Based on these results, the marbled polecat was the dominant host of Ha. erinacei, especially in the adult stage of ticks. Interestingly, larvae and nymphs of Ha. erinacei infested great gerbils, which is known to be the most important prey item of marbled polecats [4]. This finding indicates that the co-habitation of Ha. erinacei with both the great gerbil and the marbled polecat is an integral aspect of the development of this tick species.
The geographical range of O. tartakovskyi in the Palearctic region covers Kazakhstan, Uzbekistan, Turkmenistan, Kyrgyzstan, Tajikistan, Iran, Czechia and China [29], and its natural hosts include great gerbils and Central Asian tortoise (Testudo horsfieldii) [29]. In this study, five ticks identified morphologically as O. tartakovskyi from great gerbils and marbled polecats shared 93.43% sequence identity to an O. tartakovskyi laboratory strain. Further compared at the level of amino acids, they had 100% identity (Additional file 4: Fig. S3). These findings add marbled polecats to the host range of O. tartakovskyi.
In addition, the immature stages of Hy. asiaticum and R. turanicus were found on great gerbils, and their adults on pastured sheep. This finding indicates that the great gerbil plays an important role in maintaining the larvae and nymphs of Hy. asiaticum and R. turanicus, while their adults use especially ungulates as reproductive hosts in the Gurbantunggut Desert. These results are consistent with our previous work [30].
Lastly, as our sampling sites were located in deep region of Gurbantunggut Desert rather than in the oasis of the Junggar Basin, some tick species, such as Dermacentor marginatus and D. nuttalli [30], were not found in this investigation. Moreover, the marbled polecats, red foxes and great gerbils served as reservoirs for spotted fever Rickettsia, Babesia and novel tick-borne bunyaviruses [5, 26,27,28]. In the future, there will be more work on ticks and tick-borne pathogens in the Gurbantunggut Desert.
In this study, tick larvae and nymphs were mostly found on female great gerbils, which might be related to the following: (i) the breeding population of great gerbils usually consists of an adult male and 1–3 females (sometimes up to 6–7 females), and the latter feed their young in burrows where nidicolous immature ticks stay [31]; (ii) female great gerbils need to eat Haloxylon and ephemeral plants to obtain more food and water supply in stages of fetation and nursing, especially during April–June [2, 3]. However, larvae and nymphs were found on both female and male great gerbils during September and October, which might be related to the availability of more sub-adult gerbils among sampled hosts.
Conclusions
Based on the morphological and molecular-phylogenetic characters five tick species were identified, for which great gerbil acts as the primary host compared to the other desert animals including marbled polecat, long-eared desert hedgehog, red fox and pastured sheep (Additional file 5: Fig. S4). The indigenous status of I. acuminatus in China was confirmed by both morphological and molecular analyses. Two tick species were reported from their hosts for the first time, i.e. I. acuminatus from great gerbil and O. tartakovskyi from marbled polecat.
Availability of data and materials
The sequences obtained and analyzed during the present study were deposited in the GenBank database (16S rDNA: OR474496.1, OR474529.1, OR475313.1, OR475314.1, OR475315.1, OR475316.1, OR476952.1, OR476978.1, OR478282.1, OR478283.1, OR478284.1, OR478285.1; COI: OR437536.1, OR473062.1, OR473175.1, OR473540.1, OR473542.1, OR473543.1, OR473544.1, OR473541.1, OR473546.1, OR473547.1, OR473545.1 and OR473548.1).
Abbreviations
- COI:
-
Cytochrome c oxidase subunit I
References
Wang YT, Zhang DH, Zhang ZS. Spatial distribution and interspecific correlation of Haloxylon persicum and H. ammodendron on fixed dunes of the Gurbantunggut Desert, China. Biodiversity Sci. 2022;30:21280.
Li C, Li Y, Tang L, Ikenaga M, Liu R, Xu G. Soil microbial community shifts explain habitat heterogeneity in two Haloxylon species from a nutrient perspective. Ecol Evol. 2023;13:e9727.
Peng M, He H, Wang Z, Li G, Lv X, Pu X, et al. Responses and comprehensive evaluation of growth characteristics of ephemeral plants in the desert-oasis ecotone to soil types. J Environ Manage. 2022;316:115288.
Ablimiti AQ. Classification and distribution of mammals in Xinjiang. Beijing: Science Press; 2013.
Ji N, Wang N, Liu G, Zhao S, Liu Z, Tan W, et al. Tacheng tick virus 1 and songling virus infection in great gerbils (Rhombomys opimus) in Northwestern China. J Wildl Dis. 2023;59:138–42.
Li Y, Wang YN, Tian F, Zhang XL, Zhang JT, Li S, et al. First report of Karimabad virus in Rhombomys opimus in China. One Health. 2022;15:100437.
Song R, Wang Q, Guo F, Liu X, Song S, Chen C, et al. Detection of Babesia spp., Theileria spp. and Anaplasma ovis in Border Regions, northwestern China. Transbound Emerg Dis. 2018;65:1537–44.
Guo LP, Jiang SH, Liu D, Wang SW, Chen CF, Wang YZ. Emerging spotted fever group rickettsiae in ticks, northwestern China. Ticks Tick Borne Dis. 2016;7:1146–50.
Ji N, Chen X, Liu G, Zhao S, Tan W, Liu G, et al. Theileria, Hepatozoon and Taenia infection in great gerbils (Rhombomys opimus) in northwestern China. Int J Parasitol Parasites Wildl. 2021;15:79–86.
Estrada-Peña A, Mihalca AD, Petney TN. Ticks of Europe and North Africa: a guide to species identification. Berlin: Springer; 2017.
Hornok S, Sándor AD, Beck R, Farkas R, Beati L, Kontschán J, et al. Contributions to the phylogeny of Ixodes (Pholeoixodes) canisuga, I. (Ph.) kaiseri, I. (Ph.) hexagonus and a simple pictorial key for the identification of their females. Parasit Vectors. 2017;10:545.
Kolonin GV (2009) Fauna of ixodid ticks of the world (Acari: Ixodidae). http://web.archive.org/web/20100922170628/http://www.kolonin.org/3.html
Pérez-Eid C. Les tiques Identification, biologie, importance médicale et vétérinaire. Coll. Monographies de microbiologie. Paris: Lavoisier; 2007.
Guglielmone AA, Robbins RG, Apanaskevich DA, Petney TN, Agustín EP, Horak IG. The hard ticks of the world (Acari: Ixodida: Ixodidae). Dordrecht Heidelberg New York London: Springer; 2014.
Bursali A, Keskin A, Tekin S. Ticks (Acari: Ixodida) infesting humans in the provinces of Kelkit Valley, a Crimean-Congo Hemorrhagic Fever endemic region in Turkey. Exp Appl Acarol. 2013;59:507–15.
Guglielmone AA, Robbins RG. Hard ticks (Acari: Ixodida: Ixodidae) parasitizing humans-a global overview. Springer International Publishing AG, part of Springer Nature; 2018.
Gilot B, Couatarmanach A, Guiguen C, Beaucournu JC. Biology and ecology of Ixodes acuminatus Neumann, 1901, its hosts, Seasonal activity and distribution in France. Annales De Parasitologie Humaine Et Comparee. 1992;67:19–25.
Petney TN, Moser E, Littwin N, Pfaeffle M, Muders SV, Taraschewski H. Additions to the “annotated checklist of the ticks of Germany”: Ixodes acuminatus and Ixodes inopinatus. Syst Appl Acarol. 2015;20:221–4.
Chen Z, Yang X, Bu F, Yang X, Yang X, Liu J. Ticks (Acari: Ixodoidea: Argasidae, Ixodidae) of China. Exp Appl Acarol. 2010;51:393–404.
Mihalca AD, Dumitrache MO, Magdaş C, Gherman CM, Domşa C, Mircean V, et al. Synopsis of the hard ticks (Acari: Ixodidae) of Romania with update on host associations and geographical distribution. Exp Appl Acarol. 2012;58:183–206.
Koprulu TK, Tekin S, Keskin A, Bursali A. Presence of Rickettsia japonica in Ixodes redikorzevi collected from humans in Tokat province. J Biotechnol. 2012;161:34.
Yeruham I, Hadani A, Aroch I, Galker F, Gilor H, Rodrig S. Cases of apparent tick toxicosis in humans and dogs, caused by Ixodes redikorzevi s.l. Ann Trop Med Parasitol. 2000;94:413–5.
Hoogstraal H, Traylor MA, Gaber S, Malakatis G, Helmy I. Ticks (Ixodidae) on migrating birds in Egypt. Spring and Fall; 1962.
Begum F Jr, Wissemen CL, Casals J. Tick-borne viruses of West Pakistan. II. Hazara virus, a new agent isolated from Ixodes redikorzevi ticks from the Kaghan Valley, W. Pakistan. Am J Epidemiol. 1970;92:192–4.
Perfilyeva YV, Shapiyeva ZZ, Ostapchuk YO, Berdygulova ZA, Dmitrovskiy AM. Tick-borne pathogens and their vectors in Kazakhstan—a review. Ticks Tick Borne Dis. 2020;11:101498.
Guo LP, Mu LM, Xu J, Jiang SH, Wang AD, Chen CF, et al. Rickettsia raoultii in Haemaphysalis erinacei from marbled polecats, China-Kazakhstan border. Parasit Vectors. 2015;8:461.
Liu X, Yang M, Liu G, Zhao S, Yuan W, Xiao R, et al. Molecular evidence of Rickettsia raoultii, “Candidatus Rickettsia barbariae” and a novel Babesia genotype in marbled polecats (Vormela peregusna) at the China-Kazakhstan border. Parasit Vectors. 2018;11:450.
Liu G, Zhao S, Tan W, Hornok S, Yuan W, Mi L, et al. Rickettsiae in red fox (Vulpes vulpes), marbled polecat (Vormela peregusna) and their ticks in northwestern China. Parasit Vectors. 2021;14:204.
Chen Z, Liu JZ. A review of argasid ticks and associated pathogens of China. Front Vet Sci. 2022;9:865664.
Wang YZ, Mu LM, Zhang K, Yang MH, Zhang L, Du JY, et al. A broad-range survey of ticks from livestock in Northern Xinjiang: changes in tick distribution and the isolation of Borrelia burgdorferi sensu stricto. Parasit Vectors. 2015;8:449.
Zhao TB, Zhou LZ, Zhang ZB, Jin FH, Wu JP, Ning SL. A study on the age structure dynamics and reproductive status of Great Gerbil’s population. Chin J Zool. 2005;40:108–13.
Acknowledgements
The authors appreciate the contributions by the staff at the School of Medicine, Shihezi University.
Funding
This work was supported by the National Key Research & Development Program of China (2022YFC2304000), National Natural Science Foundation of China (82260410, 82260414 and 82260399), Natural Science Key Project of Xinjiang Uygur Autonomous Region (2022B03014), Key Scientific and Technological Projects in Key Areas of XPCC (2022AB014) and High-Level Talent Initiative Foundation of Shihezi University (RCZK202369).
Author information
Authors and Affiliations
Contributions
GL, WT and YW conceived and designed the study and wrote the manuscript. GL, WT, HW, XH, SZ, SW, LM and MY performed the experiments, analyzed the data. SH contributed to study design and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Animal Ethics Committee of Shihezi University (Approval No. AECSU2015-11 and A2018-143-01).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Information on sampled hosts and ticks in Gurbantunggut Desert.
Additional file 2: Figure S1.
Morphological characteristics of Hyalomma asiaticum, Rhipicephalus turanicus, Ixodes acuminatum, Haemaphysalis erinacei and Ornithodoros tartakovskyi.
Additional file 3: Figure S2.
Nucleic acid (A) and amino acid (B) sequence comparison of Ixodes acuminatus and Ixodes redikorzevi.
Additional file 4: Figure S3.
Nucleic acid (A) and amino acid (B) sequence comparison of Ornithodoros tartakovskyi.
Additional file 5: Figure S4.
Schematic diagram illustrating the connectedness of tick hosts in the Gurbantunggut Desert.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, G., Tan, W., Wang, H. et al. The great gerbil (Rhombomys opimus) as a host for tick species in Gurbantunggut Desert. Parasites Vectors 17, 55 (2024). https://doi.org/10.1186/s13071-024-06160-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-024-06160-5