Abstract
Background
Wild boars (Sus scrofa) may cause substantial damage to crops and can spread zoonotic parasites to domestic animals, posing a risk to health and animal production. Metastrongylus spp. can negatively affect the wild boar population, increasing piglet mortality. In addition to that, studies with Metastrongylus genetic characterization are still scarce in Brazil. The present study aims to characterize Metastrongylus spp. from wild boars hunted in the states of São Paulo, Paraná, and Rio Grande do Sul, Brazil, using traditional morphological description and DNA sequences in an integrative taxonomic approach.
Methods
After nematode collection from 58 wild boars, the parasites were morphologically identified and genetically characterized by the amplification of 18S ribosomal DNA (rDNA), 28S rDNA, internal transcribed spacer (ITS) region, and cox-1 mitochondrial DNA (mtDNA). Descriptors of infection were determined and Pearson's Chi-square test was applied to compare the prevalence of infections among the identified parasite species, host age group (juveniles and adults), and sex. The Mann–Whitney U test was performed to compare the mean intensity between the age groups and sex.
Results
Metastrongylus salmi, Metastrongylus apri, and Metastrongylus pudendotectus were identified in 77.6% (45/58) of the necropsied wild boars. Metastrongylus salmi was the most prevalent and abundant species (70.7%, 11.1), followed by M. pudendotectus (18.9%, 4.3) and M. apri (17.2%, 2.2). Metastrongylus pudendotectus showed the highest mean intensity and range (25.2, 1–93), followed by M. salmi (15.7, 1–58) and M. apri (12.6, 3–27). We found a significantly higher prevalence of Metastrongylus spp. and M. salmi in adult wild boars, probably associated with a more prolonged time of exposure to intermediate host species. The phylogenetic analysis revealed that ITS2 region and cox-1 mtDNA are the most suitable genetic markers for Metastrongylus species characterization. Genetic variability between M. apri and M. salmi isolates was verified.
Conclusions
We expand the knowledge about the Metastrongylus community in the non-captive wild boar population from Brazil as well as the importance of this exotic species in the maintenance of Metastrongylus spp. in its areas of occurrence. The novel genetic sequences obtained may help further studies to understand the genetic diversity in other nematode populations from Brazil and other countries.
Graphical Abstract

Similar content being viewed by others
Background
Wild boars (Sus scrofa) are invasive species widely distributed in Brazil. These animals are related to negative impacts on natural and agricultural environments, in addition to spreading zoonotic parasites [1, 2] posing a risk to the health of domestic animals and the conservation of native species [3]. Furthermore, wild boar populations are negatively affected by Metastrongylus spp. lungworms, increasing piglet mortality in particular [4, 5].
Like other correlated species, Metastrongylus have a heteroxenic life cycle with several earthworm species acting as intermediate hosts [6]. The parasite eggs ingested by earthworms develop into third-stage larvae in their tissues. After wild boars ingest the intermediate host, the parasite migrates through the mesenteric lymph nodes and the right heart to the lungs, reaching the adult form in the lumen of the bronchi and bronchioles [7]. The genus comprises six species: Metastrongylus salmi, M. apri (syn. M. elongatus), M. pudendotectus, M. confusus, M. asymmetricus, and M. madagascariensis, which may occur in mixed infections. All species are described in wild and domestic suids except M. madagascariensis, which is only found in domestic pigs from Madagascar [7]. In Brazil, M. salmi, M. pudendotectus, and M. apri are reported [8].
Currently, Metastrongylus spp. genetic sequences available in databases such as GenBank are still limited, compromising studies that aim to describe the genetic variability or make phylogenetic inferences. Studies focusing on helminth genetic variability are scarce worldwide and almost nonexistent in Brazil [8]. Thus, the present study aims to characterize Metastrongylus spp. from wild boars from the states of São Paulo, Paraná, and Rio Grande do Sul, Brazil, using traditional morphological description and DNA sequences in an integrative taxonomic approach.
Methods
Study areas
The samples were collected from wild boars hunted in rural properties from the municipalities of São Simão, Monte Azul, Paraíso, Colina, Matão, Bebedouro e Monte Alto (São Paulo), Ipiranga (Paraná), and Santo Antônio das Missões (Rio Grande do Sul) (Fig. 1).
The study areas in São Paulo state are located in the transition zone between the Cerrado and the Atlantic Forest biomes. According to the modified Köppen climate classification, the climate is humid subtropical, with an average annual temperature above 18 °C and average annual rainfall between 1200 and 1500 mm. They have 4–5 months of drought in winter, between May and September, and are located about 600 m above sea level [9,10,11]. Agriculture is the main economic activity, with emphasis on sugarcane, soy, corn, peanut, and tomato crops, in addition to beef cattle and poultry [12].
In Paraná State, the Campos Gerais region, where Ipiranga is located, has an average annual rainfall between 1400 and 1800 mm and an average annual temperature between 16 ºC and 20 ºC. The wettest season is from September to March, but frequent precipitation occurs during the winter. The prevailing climate is humid subtropical, according to the Köppen classification, and is located at about 800 m above sea level. The characteristic vegetation is the mixed rainforest. The region is characterized by high-tech crops such as soy, corn, wheat, potatoes, and beans, in addition to dairy cattle [13].
The municipality of Santo Antônio das Missões belongs to the Missões region, northwest of Rio Grande do Sul state, located in the Pampa biome. According to the Köppen classification, the climate is humid subtropical with an average annual temperature of about 17 ºC. January is the hottest month (average 32.7 ºC), and July is the coldest (average 10.5 ºC). Rainfall is about 1900 mm/year, with uneven distribution during the period. The economy is based on agricultural products, with emphasis on soy, rice, wheat, corn, sheep, and beef and dairy cattle, in addition to diversified subsistence production [14].
Biological samples
The sampling was carried out without biostatistical criteria due to the lack of data regarding the wild boar population in the region. Instead, it relied on the hunting success of our partner hunters. We examined the lungs of 58 wild boars, comprising 33 males and 25 females. The age of the animals was estimated according to dental eruption [15], categorizing them as either juveniles (less than 6 months old), or adults (more than 6 months old). The classification of the two age groups was established based on our fieldwork observations of pregnant wild boars around 6 months old (EGL Hoppe, personal communication, July 17, 2023) probably due to the random crosses between wild boars and domestic pigs. The organs were removed from the thoracic cavity, packed in individually labeled plastic bags, stored in isothermal boxes with ice, and immediately sent to the Laboratory of Parasitic Diseases (LabEPar) at the Department of Pathology, Reproduction and One Health (DPRSU), within the School of Agricultural and Veterinary Studies (FCAV), at the São Paulo State University (Unesp), Jaboticabal, São Paulo, Brazil.
Morphological identification
The trachea and the lungs were slit opened following the airways, from the trachea and main bronchi to the terminal bronchioles. All obtained nematodes were fixed in 70% ethanol and stored in identified flasks. The parasites were clarified with 80% acetic acid and mounted on temporary slides for taxonomic identification, according to Vicente et al. [16] and Gassó et al. [6]. Images and measurements (in millimeters, expressed as mean ± standard deviation, lower and upper values in brackets) were obtained with an Olympus BX-51 microscope attached to a Q-Color 3 camera (Olympus, Tokyo, Japan) and processed using Image-Pro Plus 4 image analyzer software (Media Cybernetics, Rockville, MD, USA). Vouchers were deposited in the collection of the Oswaldo Cruz Institute (CHIOC/Fiocruz, Rio de Janeiro, Brazil), and additional specimens were kept in LabEPar's helminthological collection.
Molecular analysis
DNA extraction
Genomic DNA was extracted from at least two male specimens per municipality studied. Selected specimens were individually washed with sterile phosphate-buffered saline (PBS) pH 7.4 solution and transferred to 1.5 µl microtubes containing 50 µl of tissue lysis buffer (ATL) from the DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany) and macerated with the aid of sterilized plastic rods. Subsequently, glass beads treated with Triton X-100, 130 µl of ATL buffer, and 20 µl of proteinase K were added to the microtubes. The rest of the extraction proceeded according to the manufacturer’s protocol. The analysis of DNA concentration and quality, whose absorbance ratio between the wavelengths of 260 and 280 nm is desirable between 1.8 and 2.0 ng/dl [17], was performed using the NanoDrop One Spectrophotometer (Thermo Fisher Scientific), and the extraction products were stored at −20 °C until amplification by conventional polymerase chain reaction (PCR).
Amplification
Four genetic regions were amplified: 18S ribosomal DNA (rDNA), internal transcribed spacer (ITS), 28S rDNA, and the cytochrome c oxidase subunit I (cox-1) of the mitochondrial DNA (mtDNA). The primers set are expressed in Table 1. The reactions were composed of 1× buffer (KCl 50 mM, TRIS–HCl 200 mM, pH 8,4); 50 mM of MgCl2; 10 mM dNTPs; 0.5 U Platinum Taq [Thermus aquaticus] DNA Polymerase (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA); 5 pmol of each forward and reverse primer; 60 ng of genomic DNA and ultrapure water to complete a final volume of 20 µl. Amplifications were performed in a Nexus thermal cycler (Eppendorf, Hamburg, Germany) programmed to perform one cycle at 95 ºC for 3 min, and 35 cycles at 94 ºC for 40 s; each primer's annealing temperature (Table 1) was kept for 30 s, and 72 ºC for 50 s, followed by a final extension cycle at 72 ºC for 10 min.
To verify the amplification reaction, the PCR products were submitted to electrophoresis in 1% agarose gel, stained with ethidium bromide, and visualized in a Geldoc XR photodocumenter (Bio-Rad®). In the case of low DNA yield, the reamplification was performed using the same protocol and primers cited previously. The products were purified with the Wizard® SV Gel and PCR Clean-Up System kit (Promega, Madison, WI, USA) according to the manufacturer's instruction and submitted to PCR sequencing using the BigDye Terminator v3.1 kit (Applied Biosystems, Waltham, MA, USA), according to manufacturer’s instructions. Sequencing was performed by capillary electrophoresis on an ABI 3130 sequencer (Applied Biosystems, Waltham, MA, USA) according to Sanger's method [18].
Phylogenetic analysis
The electropherograms generated in the sequencing were submitted to the Phred/Phrap/Consed software package [19,20,21] to verify the quality of the bases and trim the sequences considering bases with Phred quality up to 20 or higher. The qualified sequences were compared to others deposited in the National Center for Biotechnology Information (NCBI) database using BLAST (Basic Local Alignment Search Tool) [22]. The sequences from this study and the selected sequences from NCBI's database were aligned using the ClustalW tool [23] on the software BioEdit v. 7.0.5.3 [24].
Phylogenetic trees were obtained by a maximum likelihood analysis using the W-IQ-Tree software [25]. The best evolutionary model was selected according to the Bayesian information criterion (BIC) using the W-IQ-Tree software [26]. The clade stability was evaluated using 1000 bootstrap replicates. The phylograms were graphically edited and rooted on the TreeGraph 2.15.0–887 beta software [27].
Data analysis
Infection descriptors of prevalence, mean intensity (range of intensity), and mean abundance were based on Bush et al. [28]. Fisher’s exact test was performed to compare parasite prevalence among sex, age group, and states of collection. The Mann–Whitney U test was used to evaluate the differences in mean intensity between the age groups and sex. Differences between the states’ mean intensity were not calculated due to the low parasite burden observed in Paraná state (only one animal was infected). All analyses were performed using the R software version 4.0.4. Values of p < 0.05 were considered statistically significant.
Ethical procedures
All procedures were approved by the Committee on Ethics in the Use of Animals (CEUA) of FCAV/Unesp Jaboticabal protocol no. 3683/20 and Chico Mendes Institute for Biodiversity Conservation (ICMBio), request in the Biodiversity Authorization and Information System (SISBIO) no. 84726–1.
Results
Parasite community
Lungworms were observed in 45 out of 58 animals (77.6%). A total of 1,016 parasites were recovered and three species (M. salmi, M. apri, and M. pudendotectus) were identified. Infection descriptors are shown in Table 2. Metastrongylus salmi was the most prevalent and abundant species (70.7%, 11.1), followed by M. pudendotectus (18.9%, 4.3) and M. apri (17.2%, 2.2). Metastrongylus pudendotectus showed the highest mean intensity and range (25.2, 1–93), followed by M. salmi (15.7, 1–58) and M. apri (12.6, 3–27). In São Paulo state, M. salmi was the only species identified. In the southern states (Paraná and Rio Grande do Sul), the three helminth species were present in mixed infections by two or three of them. Metastrongylus salmi (odds ratio [OR] = 0.1, 95% confidence interval [CI] = 0.02–0.6, P = 0.004) and Metastrongylus spp. (OR = 0.08, 95% CI = 0.01–0.4, P = 0.0005) prevalence was higher in adults than juveniles (Table 3). No significant differences (p > 0.05) were observed between the mean intensities relative to sex and age group (Table 4).
Morphological descriptions
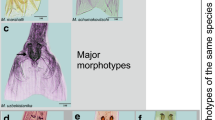
Metastrongylus salmi Gedoelst [29]—Fig. 2a, b, c.
Metastrongylus salmi found in wild boars hunted in São Paulo, Paraná, and Rio Grande do Sul states. a Anterior extremity, showing the trilobated lips (black arrow). Magnification: ×200. Bar: 50 µm. b Female posterior extremity; note the short pre-vulvar swelling (orange arrow). Magnification: ×100. Bar: 100 µm. c Male posterior extremity showing the hook-like form at spicule ending (inset). Magnification: ×40. Bar: 2000 µm
General description: Thin, long, whitish nematodes in vivo. Anterior extremity composed of trilobed lips and claviform esophagus. Males with short copulatory bursa, with some digitiform rays. Intermediate spicules, similar in size, with transverse striations and a single hook-like form at the end. Gubernaculum is absent. Females with the posterior extremity curved ventrally, conical tail, and vulvar opening close to anus covered by a short swelling. Irregular, double-shelled, and embryonated eggs.
Habitat bronchi and bronchioles.
Host Sus scrofa.
Morphometric data (in millimeters).
Males (n = 149).
Total length: 18.7 ± 2.0 (8.9–22.8); width (esophagus–intestinal junction): 0.1 ± 0,0. (0.1–0.2); esophagus: 0.5 ± 0.05 (0.4–0.7); excretory pore (distance for anterior extremity): 0.4 ± 0.04 (0.2–0.5); nerve ring (distance for anterior extremity): 0.3 ± 0.05 (0.2–0.5); large spicule: 2.05 ± 0.1 (1.5–2.6); small spicule: 1.9 ± 0.1 (1.3–2.2).
Females (n = 158).
Total length: 42.2 ± 5.2 (27.8–51.3); width (esophagus–intestinal junction): 0.2 ± 0.02 (0.2–0.4); esophagus: 0.7 ± 0.1 (0.5–0.8); excretory pore (distance for anterior extremity): 0.4 ± 0.1 (0.2–0.6); nerve ring (distance for anterior extremity): 0.4 ± 0.1 (0.2–0.6); vulva–posterior extremity: 0.1 ± 0.02 (0.1–0.2); anus–posterior extremity: 0.1 ± 0.01 (0.1–0.2); eggs (length in micrometers): 49.7 ± 2.9 (43.0–64.0); eggs (width in micrometers): 35.3 ± 2.7 (30.0–40.5).
Metastrongylus pudendotectus Vostokov [30]—Fig. 3(a, b, c).
Metastrongylus pudendotectus found in wild boars hunted in Paraná and Rio Grande do Sul states. a Male posterior extremity; note the anchor-like form at the spicule ending (inset). Magnification: ×100. Bar: 100 μm. b Spicule and gubernaculum (black arrow). Magnification: x400. Bar: 25 μm. c Female posterior extremity; note the prominent swelling surrounding the vulvar opening (orange arrow). Magnification: x100. Bar: 100μm
General description: Thin, long, whitish nematodes in vivo. Mouth with trilobed lips and claviform esophagus. Males with large copulatory bursa and short rays. Short spicules, when compared to other species of the genus, with transverse striations and anchor-like form at the end. Gubernaculum is present. Female with posterior extremity curved ventrally, conical tail, vulvar opening close to the anus covered by a prominent swelling. Irregular, double-shelled, and embryonated eggs.
Habitat bronchi and bronchioles.
Host Sus scrofa.
Morphometric data (in millimeters).
Males (n = 26).
Total length: 19.2 ± 1.7 (16.2–23.5); width (esophagus–intestinal junction): 0.1 ± 0,01 (0.1–0.2); esophagus: 0.5 ± 0.03 (0.5–0.6); excretory pore (distance for anterior extremity): 0.3 ± 0.03 (0.2–0.4); nerve ring (distance for anterior extremity): 0.3 ± 0.03 (0.2–0.4); large spicule: 1.5 ± 0.1 (1.3–1.7); small spicule: 1.4 ± 0.05 (1.3–1.5); gubernaculum (length): 0.04 ± 0.007 (0.03–0.1); gubernaculum (width): 0.02 ± 0.008 (0.01–0.04).
Females (n = 30).
Total length: 33.0 ± 5.5 (20.4–52.0); width (esophagus–intestinal junction): 0.2 ± 0.03 (0.1–0.3); esophagus: 0.6 ± 0.1 (0.4–0.8); excretory pore (distance for anterior extremity): 0.3 ± 0.05 (0.2–0.5); nerve ring (distance for anterior extremity): 0.3 ± 0.05 (0.2–0.4); vulva–posterior extremity: 0.2 ± 0.04 (0.1–0.3); anus–posterior extremity: 0.1 ± 0.02 (0.1–0.2); eggs (length in micrometers): 60.7 ± 5.6 (44.3–76.3); eggs (width in micrometers): 44.6 ± 2.3 (37.0–52.0).
Metastrongylus apri Gmelin [31]—Fig. 4 (a, b).
Metastrongylus apri found in wild boars hunted in Paraná and Rio Grande do Sul states. a Male posterior extremity showing the hook-like form at the spicule ending (inset). Magnification: ×40. Bar: 2000 µm. b Female posterior extremity; note the protruding vulvar swelling (blue arrow). Magnification: ×100. Bar: 100 µm
General description: Thin, long, whitish nematodes in vivo. Mouth with two trilobed lips and claviform esophagus. Male with copulatory bursa with broad edges and a rounded lateral external ray in a mushroom-like form. Very long spicules, similar in length, with a single hook-like ending. Gubernaculum is absent. Female with posterior extremity curved ventrally, conical tail, vulvar opening close to the anus covered by a vulvar swelling with an intermediate length between M. salmi and M. pudendotectus. Irregular, double-shelled, and embryonated eggs.
Habitat bronchi and bronchioles.
Host Sus scrofa.
Morphometric data (in millimeters).
Males (n = 33).
Total length: 18.4 ± 1.8 (14.6–21.4); width (esophagus–intestinal junction): 0.1 ± 0.01 (0.1–0.2); esophagus: 0.5 ± 0.1 (0.4–0.6); excretory pore (distance for anterior extremity): 0.4 ± 0.04 (0.3–0.4); nerve ring (distance for the anterior extremity): 0.3 ± 0.04 (0.2–0.4); large spicule 4.5 ± 0.2 (4.2–5.3); small spicule: 4.3 ± 0.2 (3.9–4.7).
Females (n = 33).
Total length: 43.4 ± 6.1 (26.4–51.0); width (esophagus–intestinal junction): 0.2 ± 0.01 (0.2–0.3); esophagus: 0.70 ± 0.1 (0.6–0.9); excretory pore (distance for anterior extremity): 0.5 ± 0.1 (0.3–0.6); nerve ring (distance for anterior extremity): 0.4 ± 0.1 (0.3–0.6); vulva–posterior extremity: 0.1 ± 0.02 (0.1–0.2); anus–posterior extremity: 0.1 ± 0.01 (0.1–0.2); eggs (length in micrometers): 53.3 ± 3.0 (44.9–58.4); eggs (width in micrometers): 39.9 ± 2.1 (34.2–46.3).
PCR and phylogenetic analysis
Metastrongylus salmi and M. apri genetic material were amplified by at least two out of the four genetic markers used. Metastrongylus pudendotectus samples yielded low DNA and could not be used for phylogenetic analysis. The amplicons varied from 784 to 924 base pairs (bp) for 18S rDNA; 623 to 687 for 28S rDNA; 231 to 509 bp for the ITS region, and 626 to 702 bp for cox-1 mtDNA. BLAST analysis revealed disagreements between morphological identification and genetic data (Table 5). The phylogenetic trees for the 18S rDNA, 28S rDNA, ITS region, and cox-1 mtDNA can be seen in Additional file 1, Additional file 2 and Fig. 5, 6.
Maximum-likelihood tree using internal transcribed spacer (ITS) region encompassing Metastrongyloidea helminths. Cystocaulus ocreatus and Umingmakstrongylus pallikuukensis were rooted as outgroups. Sequences obtained from the study are highlighted in red. Metastrongylus sequences downloaded from the GenBank are indicated with accession number, species name, and country. Bootstrap values are shown at the nodes. The best-fit model was the transversion model with equal base frequencies and the discrete Gamma model with four rate categories (TVMe + G4)
Maximum-likelihood tree using cytochrome c oxidase subunit 1 mitochondrial DNA (cox-1 mtDNA) region encompassing Metastrongyloidea helminths. Dirofilaria immitis and Onchocerca lupi were rooted as outgroups. Sequences obtained from the study are highlighted in red. Nucleotide sequences downloaded from the GenBank are indicated with accession number, species name, and country. Bootstrap values are shown at the nodes. The best-fit model was the transition model considering the base frequencies, invariable sites, and discrete Gamma model with four rate categories (TIM + F + I + G4)
Discussion
The prevalence of Metastrongylus spp. observed in the present study was higher when compared with studies with wild boars from commercial breeding [32, 33] and free-ranging animals [34] from Brazil. However, the percentage is lower compared to European countries, where prevalence values are greater than 80% [35]. The different results could be related to animal density, different climatic and terrains of each region, and earthworm distribution. Areas with grain and sugarcane plantations provide abundant food to wild boars, representing an increased risk of infection due to increased final host density and, therefore, a larger population of infected earthworms [36]. In addition, in São Paulo state, food bait is provided in some hunting areas (EGL Hoppe, personal communication, June 24, 2023) and could be related to the high prevalence (77.5%) observed in the region, despite the obvious differences between sample sizes and climate. The construction of fences can also lead to a concentration of animals in a certain area and thus increases the number of infective stages in the environment [37]. A higher prevalence of Metastrongylus spp. was found in areas with high altitude and abundant rainfall, suggesting that such conditions may improve the resistance of parasite eggs and the survival of the intermediate host [38]. The distribution of earthworms is similar to the hosts’ geographical range, but in hot and dry climates, their number could be reduced [39]. The viability of the embryonated egg can be influenced by several climatic factors, and the dryness should be the most important. Moist soil environments can lead to an increased lifespan of the eggs, surviving for 2 years or more [40].
Infection with Metastrongylus spp. (OR = 0.1, 95% CI = 0.02–0.6, P = 0.004) and M. salmi (OR = 0.08, 95% CI = 0.01–0.4, P = 0.0005) was significantly higher in adults than in juveniles. However, some evidence demonstrates a higher prevalence of nematodes and digenetic trematodes in younger animals [35, 38, 41, 42], whereas others show no correlation between age and prevalence of lungworms [7, 36, 43]. The higher prevalence observed in the present study may be related to an accumulative effect caused by a greater or more prolonged chance of exposure in older animals over time [44], a well-documented factor in parasites of wild rodents from Europe [45].
Differences in the community of Metastrongylus spp. can change considerably between countries and, within the same country, between regions [5]. A study with wild boars in commercial breeding in southern Brazil observed a predominance of M. apri (52.5%), followed by M. salmi (20%), and M. pudendotectus (7.5%) [33]. In two properties of São Paulo state, wild boars were infected with M. salmi (50% and 15.2%) and M. pudendotectus (5.6% and 3%) [32], and in another survey in wild boars from the municipality of Monte Azul, one of the sampling sites present in this study, M. salmi (82.9%) and M. pudendotectus (11.4%) [34] were recovered. This variation may be restricted to a local level since, in the present study, only M. salmi was found in the São Paulo municipalities, and in other studies, two different species were reported [32, 33]. The absence of M. pudendotectus in the municipalities of São Paulo may also be associated with its low prevalence in the state, making it difficult to detect in small sample sizes.
The different distribution of species observed in São Paulo state (only M. salmi) and the states of southern Brazil (M. salmi, M. apri, and M. pudendotectus) may be associated with the different climatic conditions between these regions or with the low sample size in the studied sites. Wild boars kept in a subtropical climate, present throughout the southern region, may be infected by different species of helminths and demonstrate other infection parameters when compared to animals that live in other climatic conditions [33]. It is important to note that the increase in the host population contributes to the structure of the parasite community in a given region, but its composition can change with the introduction of new parasites in native populations. Such an event may be a consequence, for example, of the translocation of wild boars for hunting purposes [46, 47], one of the practices responsible for expanding the wild boar population in Brazil [48].
Wild boars in southern (Paraná and Rio Grande do Sul) and southeastern (where São Paulo is located) regions may have different origins, and this would be reflected in the different Metastrongylus communities observed in the present study. The second wave of the invasion of wild boars in Brazil came from Uruguay to the south of Rio Grande do Sul [49]. In the 1990s, several commercial breedings were established in the southern and southeastern regions with animals imported from Europe and Canada [3]. Unintentional or deliberated release of half-bred or pure wild boars in a rearing farm located in the municipality of Piedade, São Paulo state, might have contributed to their expansion in the region [3, 50]. Interestingly, one study found M. apri and M. pudendotectus in free-ranging wild boars from the northern region of Uruguay [FN Inzaguirre Pomponi and CS Nuñez de Moraes Gomez, PhD dissertation],Footnote 1 the same two species reported in the southern states.
Despite being the second most important helminth disease in domestic swine breeding, second only to Ascaris suum infection, metastrongylosis in intensive regimes has reduced significantly over the years because the animals are kept on cement floors, which makes contact with the intermediate host improbable. [33]. However, in outdoor farming of domestic swine and wild boar, the disease must be considered, since the environmental conditions and the abundance of hosts favors the maintenance of the nematodes cycle and may cause death or reduced fitness of affected animals, especially when associated with infectious–parasitic agents [51, 52]. The present study describes the presence of infection in free-ranging wild boars, which reinforces the need for effective control measures to avoid the introduction of the helminth in such rearing models.
The phylogenetic analysis revealed that 18S rDNA and 28S rDNA cannot discriminate Metastrongylus spp. species, with ITS region and cox-1 mtDNA proved to be the most suitable markers. A study with domestic pigs in Vietnam was able to separate M. salmi, M. apri, and M. pudendotectus using cox-1 and ITS2 locus [53]. High-resolution restriction fragment length polymorphism (RFLP) and mutation scanning microsatellite assays with ITS2 region found intraspecific and interspecific variation between Metastrongylus isolates, supporting the utility of this marker for nematode species identification [54, 55]. Mitochondrial genes such as cox-1 have a faster evolutionary rate and, for this reason, should be suitable for discriminating closely related species when compared with nuclear ribosomal genes [56]. The partial gene cox-1 was able to properly distinguish M. salmi eggs recovered from pigs of Piaui state, northeastern Brazil [8].
Interestingly, the cox-1 tree shows that M. salmi used in the study can present considerable genetic diversity, with some isolates forming a highly supported clade with species from Australia, France, and Brazil. The ITS2 and cox-1 trees also revealed some close relations between M. apri and others from Europe and Asia but with lower bootstrap values. In the same country of the study, three haplotypes from M. salmi were reported: two new and one for European countries [8]. Further studies should be performed to better understand Metastrongylus genetic diversity in Brazil.
We could not explain the differences between morphological and genetic data observed in Table 5. This may be related to the scarcity of genetic markers available in the GenBank database or the close genetic relationship between M. apri and M. salmi (interspecific nucleotide variation between 1.3 and 3.6%) [53]. Morphological misidentification should be ruled out because M. salmi can be easily distinguished from M. apri by the length of spicules (shorter than M. apri) and less pronounced pre-vulvar swelling in females [6].
Our results highlight the importance of studying helminths from different localities to explore better the environmental diversity and their influence on the nematode population. Further studies to determine Metastrongylus ecology in other Brazilian biomes would help to assess the relationship between the lungworm communities and the host population in the country.
Conclusions
The present study shows differences in Metastrongylus communities from São Paulo and the southern states and shows for the first time that wild boars can act as a source of Metastrongylus infection for domestic and wild animals. Given the scarcity of the nematode genetic data in databases, we expanded the sequences available for M. salmi in addition to other genetic markers explored, and presented novel sequences from M. apri. These new genetic data will help further studies to understand the genetic variability of Metastrongylus nematodes in different regions.
Availability of data and materials
All data generated during this study are included in the published article.
Notes
Izaguirre Pomponi FN, Nuñez de Moraez Gomes CS. Estudio de la helmintofauna de jabalíes y cerdos asilvestrados (Sus scrofa) de la región norte de Uruguay [doctoral dissertation]. Universidad de la República; 2022.
Abbreviations
- PBS:
-
Phosphate-buffered saline
- ATL:
-
Tissue lysis buffer
- DNA:
-
Deoxyribonucleic acid
- 18S rDNA:
-
18 Subunit of ribosomal deoxyribonucleic acid
- 28S rDNA:
-
28 Subunit of ribosomal deoxyribonucleic acid
- ITS:
-
Internal transcribed spacer
- cox-1 mtDNA:
-
Cytochrome c oxidase subunit 1 of mitochondrial deoxyribonucleic acid
- Taq DNA:
-
Thermus aquaticus deoxyribonucleic acid
- PCR:
-
Polymerase chain reaction
- BLAST:
-
Basic Local Alignment Search Tool
- bp:
-
Base pairs
References
Silva CS, Mendonça TO, Machado DMR, Arias-Pacheco CA, Oliveira WJ, Perin PP, et al. Seropositive wild boars suggesting the occurrence of a wild cycle of Trichinella spp. in Brazil. Animals. 2022;12:462.
Machado DMR, de Barros LD, Nino BDSL, Pollo AS, dos Santos Silva AC, Perles L, et al. Toxoplasma gondii infection in wild boars (Sus scrofa) from the State of São Paulo, Brazil: serology, molecular characterization, and hunter’s perception on toxoplasmosis. Vet Parasitol Reg Stud. 2021;23:100534.
Pedrosa F, Salerno R, Padilha FVB, Galetti M. Current distribution of invasive feral pigs in Brazil: economic impacts and ecological uncertainty. Nat Conserv. 2015;13:84–7.
Houszka M. Metastrongylosis as an agent in the population decrease of wild boars. Med Weter. 2001;57:638–40.
Nosal P, Kowal J, Nowosad B. Structure of Metastrongylidae in wild boars from southern Poland. Helminthologia. 2010;47:212–8.
Gassó D, Rossi L, Mentaberre G, Casas E, Velarde R, Nosa P, et al. An identification key for the five most common species of Metastrongylus. Parasitol Res. 2014;113:3495–500.
Spieler N, Schnyder M. Lungworms (Metastrongylus spp) and intestinal parasitic stages of two separated Swiss wild boar populations north and south of the Alps: similar parasite spectrum with regional idiosyncrasies. Int J Parasitol Parasites Wildl. 2021;14:202–21.
Bacelar PAA, Jaeger LH, Calegar DA, Santos JP, Coronato-Nunes B, Reis ERC, et al. Molecular detection of Metastrongylus salmi eggs from pigs in low-resource communities in the state of Piauí, northeastern Brazil. J Vet Diagn Invest. 2022;34:689–92.
CIIAGRO. Centro Integrado de Informações Agrometeorológicas. Instituto Agronômico. Monitoramento Climático. 2019. http://www.ciiagro.sp.gov.br/ciiagroonline/. Accessed: 16 June 2023.
INMET. Instituto Nacional de Meteorologia. Ministério da Agricultura, Pecuária e Abastecimento. Precipitação Total Anual. 2019. http://www.inmet.gov.br/portal/index.php?r=clima/page&page=desvioChuvaAnual. Accessed: June 16 2023.
IBGE. Instituto Brasileiro de Geografia e Estatística. Mapa de clima do Brasil. 2019. http://mapas.ibge.gov.br/tematicos.html. Accessed 16 June 2023.
São Paulo. Secretaria de Planejamento e Desenvolvimento Regional. Caracterização socioeconômica das regiões do Estado de São Paulo—Região Administrativa de Barretos. 2012. www.planejamento.sp.gov.br/noti_anexo/files/uam/trabalhos/Barretos.pdf. Acesso em: 22 Set 2022.
Tsukahara RY, Caramori PH, Caviglione JH, Martorano LG, Strauch JC, Galdino J. Análise Climática da região de Campos Gerais, PR. In: XIII Congresso Brasileiro de Agrometeorologia, Anais. Santa Maria: SBAGRO; 2003. p. 1119–20.
Neto BS, Stamberg ARP, Oliveira A. Dinâmica do Sistema Agrário e transformações da agricultura familiar do município de Santo Antônio das Missões. RS Cad Ciênc Tec. 2007;24:77–114.
Magnell O, Carter R. The chronology of tooth development in wild boar—a guide to age determination of linear enamel hypoplasia in prehistoric and medieval pigs. Vet ir Zootech. 2007;40:43–8.
Vicente JJ, Rodrigues HO, Gomes DC, Pinto RG. Nematoides do Brasil. Parte V: Nematoides de mamíferos Zool. 1994;14:1–452.
Sambrook J, Russell DW. Molecular cloning: a laboratory manual. Londres: CSHL Press; 2001.
Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci. 1977;74:5463–7.
Green P. Phrap documentation. 1996. http://bozeman.mbt.washington.edu/phrap.docs/phrap.html Acesso em: 26 Set 2022.
Ewing B, Green P. Base-calling of automated sequencer traces using PHRED II Error probabilities. Genome Res. 1998;8:186–94.
Gordon D, Abajian C, Green P. Consed: a graphical tool for sequence finishing. Genome Res. 1998;8:195–202.
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–10.
Thompson JD, Higgins DG, Gibson TJ. Clustal W: improving the sensitivity of progressive multiple sequence alignments through sequence weighting, position-specific gap penalties, and weight matrix choice. Nucleic Acids Res. 1994;22:4673–80.
Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95–8.
Nguyen LT, Schmidt HA, Von Haeseler A, Minh BQ. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 2015;32:268–74.
Posada D, Buckley TR. Model selection and model averaging in phylogenetics: advantages of Akaike information criterion and Bayesian approaches over likelihood ratio tests. Syst Biol. 2004;53:793–808.
Stöver BC, Müller KF. TreeGraph 2: Combining and visualizing evidence from different phylogenetic analyses. BMC Bioinformatics. 2010;11:1–9.
Bush AO, Lafferty K, Lotz J, Shostak AW, et al. Parasites meet ecology on its own terms Margolisrevisited. J Parasitol. 1997;83:83.
Le GL. genre Metastrongylus Molin, 1861. Bull Soc Pathol Exot. 1923;16:622–30.
Vostokov V. Die Strongyliden der Lungen bei den Haussäugetieren von Charikov. Sbornik trudov Khar’kov Vet Inst. 1905;7:1–17.
Gmelin B. Caroli a Linné, systema naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. 13rd ed. Lipsae: G. E. Beer; 1790.
Gomes RA, Bonuti MR, Almeida KS, Nascimento AA. Infecções por helmintos em javalis (Sus scrofa scrofa) criados em cativeiro na região Noroeste do Estado de São Paulo. Brasil Cienc Rural. 2005;35:625–8.
Da Silva D, Müller G. Parasites of the respiratory tract of Sus scrofa scrofa (wild boar) from commercial breeder in southern Brazil and its relationship with Ascaris suum. Parasitol Res. 2013;112:1353–6.
Perin PP, Lapera IM, Arias-Pacheco CA, Mendonça TO, Oliveira WJ, Pollo AS, et al. Epidemiology and integrative taxonomy of helminths of invasive wild boars. Brazil Pathogens. 2023;12:175–92.
Humbert JF, Henry C. Studies on the prevalence and the transmission of lung and stomach nematodes of the wild boar (Sus scrofa) in France. J Wildl Dis. 1989;25:335–41.
Poglayen G, Marchesi B, Dall’oglio G, Barlozzari G, Galuppi R, Morandi B. Lung parasites of the genus Metastrongylus Molin, 1861 (Nematoda: Metastrongilidae) in wild boar (Sus scrofa L, 1758) in Central-Italy: An eco-epidemiological study. Vet Parasitol. 2016;217:45–52.
Anderson RM. Modern parasitology: a textbook of parasitology. 2nd ed. Oxford: Blackwell Publishing Ltd; 1993.
García-González AM, Pérez-Martín JE, Gamito-Santos JA, Calero-Bernal R, Alonso MA, Carrión EMF. Epidemiologic study of lung parasites (Metastrongylus spp.) in wild boar (Sus scrofa) in Southwestern Spain. J Wildl Dis. 2013;49:157–62.
Baubet E, Ropert-Coudert Y, Brandt S. Seasonal and annual variations in earthworm consumption by wild boar (Sus scrofa scrofa L). Wildl Res. 2003;30:179–86.
Rose JH. Metastrongylus apri, the pig lungworm. Observations on the free-living embryonated egg and the larva in the intermediate host. Parasitol. 1959;49:439–47.
Rajkovic-Janje R, Bosnic S, Rimac D, Dragicevic P, Vinkovic B. Prevalence of helminths in wild boar from hunting grounds in eastern Croatia. Z Jagdwiss. 2002;48:261–70.
Senlik B, Cirak VY, Girisgin O, Akyol CV. Helminth infections of wild boars (Sus scrofa) in the Bursa province of Turkey. J Helminthol. 2011;85:404–8.
Magi M, Bertani M, Dell’Omodarme M, Prati MC. Epidemiological study of the intestinal helminths of wild boar (Sus scrofa) and mouflon (Ovis gmelini musimon) in Central Italy. Parassitologia. 2002;44:203–5.
Junker K, Spickett A, Swanepoel M, Krasnov BR, Boomker J, Hoffman LC. Gastrointestinal helminths from the common warthog, Phacochoerus africanus (Gmelin) (Suidae), in KwaZulu-Natal Province, South Africa, with comments on helminths of Suidae and Tayassuidae worldwide. Parasitol. 2019;146:1541–9.
Behnke JM, Lewis JW, Zain SM, Gilbert FS. Helminth infections in Apodemus sylvaticus in southern England: interactive effects of host age, sex and year on the prevalence and abundance of infections. J Helminthol. 1999;73:31–44.
Pence DB, Warren RJ, Ford CR. Visceral helminth communities of an insular population of feral swine. J Wildl Dis. 1988;24:105–12.
Fernandez-de-Mera IG, Gortazar C, Vicente J, Höfle U, Fierro Y. Wild boar helminths: risks in animal translocations. Vet Parasitol. 2003;115:335–41.
Rosa CA, Wallau MO, Pedrosa F. Hunting as the main technique used to control wild pigs in Brazil. Wildl Soc Bull. 2018;42:111–8.
Deberdt AJ, Scherer SB. O javali asselvajado: ocorrência e manejo da espécie no Brasil. Nat Conserv. 2007;5:31–44.
Hegel CGZ, Faria GMM, Ribeiro B, Salvador CH, Rosa C, Pedrosa F, et al. Invasion and spatial distribution of wild pigs (Sus scrofa L) in Brazil. Biol Invasions. 2022;24:3681–92.
Marruchella G, Paoletti B, Speranza R, Di Guardo G. Fatal bronchopneumonia in a Metastrongylus elongatus and porcine circovirus type 2 co-infected pig. Res Vet Sci. 2012;93:310–2.
Oba P, Dione MM, Wieland B, Mwiine FN, Erume J. Correlations between lung pneumonic lesions and serological status for key respiratory pathogens in slaughtered pigs in northern Uganda. Porc Health Manag. 2021;7:53.
Tuyen NV, Lan NTK, Doahn PN. Molecular phylogenetic relationships of Metastrongylus nematodes with emphasis on specimens from domestic pigs in Vietnam. J Helminthol. 2021;95:e52.
Conole JC, Chilton NB, Jarvis T, Gasser RB. Intraspecific and interspecific variation in the second internal transcribed spacer (ITS-2) sequence for Metastrongylus (Nematoda: Metastrongyloidea) detected by high resolution PCR-RFLP. Int J Parasitol. 1999;29:1935–40.
Conole JC, Chilton NB, Jarvis T, Gasser RB. Mutation scanning analysis of microsatellite variability in the second internal transcribed spacer (precursor ribosomal RNA) for three species of Metastrongylus (Strongylida: Metastrongyloidea). Parasitol. 2001;122:195–206.
Chan AHE, Chaisiri K, Morand S, Saralamba N, Thaenkham U. Evaluation and utility of mitochondrial ribosomal genes for molecular systematics of parasitic nematodes. Parasit Vectors. 2020;13:364.
Holterman M, Wurff A, Elsen S, Megen H, Bongers T, Holovachov O, et al. Phylum-Wide analysis of SSU rDNA reveals deep phylogenetic relationships among nematodes and accelerated evolution toward crown clades. Mol Biol Evol. 2006;23:1792–800.
De Ley P, Félix MA, Frisse LM, Nadler SA, Sternberg PW, Thomas WK. Molecular and morphological characterization of two reproductive species with mirror-image anatomy (Nematoda: Cephalobidae). Nematol. 1999;1:591–612.
Gasser RB, Chilton NB, Hoste H, Beveridge I. Rapid sequencing of rDNA from single worms and eggs of parasitic helminths. Nucleic Acids Res. 1993;21:2525–6.
Kanzaki N, Futai K. A PCR primer set for determination of phylogenetic relationships of Bursaphelenchus species within the xylophilus group. Nematology. 2002;4:35–44.
Acknowledgements
We wish to acknowledge the exotic fauna management team for their support in the wild boar sample collection.
Funding
The Coordination for the Improvement of Higher Education Personnel (CAPES)—Finance Code 001 partially funded this study. EGLH is a researcher sponsored by CNPq (National Council for Scientific and Technological Development; Productivity Grant no. 311063/2022–5 and Research Grant no. 407965/2021-1).
Author information
Authors and Affiliations
Contributions
WJO, PPP, CAAP, TOM, NOZ, LOM, JSG, VMSS, and AFMF supported wild boar necropsy. ASP and PPP performed molecular assays. WJO and RBA performed phylogenetic analysis. WJO and EGLH were responsible for writing and original draft preparation.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures were approved by the Committee on Ethics in the Use of Animals (CEUA) of FCAV/Unesp Jaboticabal protocol no. 3683/20 and Chico Mendes Institute for Biodiversity Conservation (ICMBio), request in the Biodiversity Authorization and Information System (SISBIO) no. 84726–1. The hunters voluntarily consented to participate and are registered in the Brazilian Institute of the Environment and Renewable Natural Resources, Brazilian Ministry of Environment.
Consent for publication
Not applicable.
Competing interests
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Maximum-likelihood tree using 18S ribosomal DNA region encompassing Metastrongyloidea superfamily helminths. Syngamus trachea and Necator americanus were rooted as outgroups. Sequences obtained from the study are highlighted in red. Metastrongylus sequences downloaded from the Genbank are indicated with accession number, species name, and country. Bootstrap values are shown at the nodes. The best-fit model was Tamura two parameters considering the base frequencies and invariable sites (TPM2+F+I).
Additional file 2:
Maximum-likelihood tree using 28S ribosomal DNA region encompassing Metastrongyloidea superfamily helminths. Toxocara vitulorum and Toxocara canis were rooted as outgroups. Sequences obtained from the study are highlighted in red. Metastrongylus sequences downloaded from the Genbank are indicated with accession number, species name, and country. Bootstrap values are shown at the nodes. The best-fit model was the transversion model with equal base frequencies and the discrete Gamma model with four rate categories (TVMe+G4).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Oliveira, W.J., Perin, P.P., Arias Pacheco, C.A. et al. Integrative taxonomy of Metastrongylus spp. in wild boars from Brazil. Parasites Vectors 16, 449 (2023). https://doi.org/10.1186/s13071-023-06047-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-023-06047-x