Abstract
Background
The mosquito Aedes aegypti transmits two of the most serious mosquito-borne viruses, dengue virus (DENV) and Zika virus (ZIKV), which results in significant human morbidity and mortality worldwide. The quickly shifting landscapes of DENV and ZIKV endemicity worldwide raise concerns that their co-circulation through the Ae. aegypti mosquito vector could greatly exacerbate the disease burden in humans. Recent reports have indicated an increase in the number of co-infection cases in expanding co-endemic regions; however, the impact of co-infection on viral infection and the detailed molecular mechanisms remain to be defined.
Methods
C6/36 (Aedes albopictus) cells were cultured in Dulbecco's modified Eagle medium/Mitsuhashi and Maramorosch Insect Medium (DMEM/MM) (1:1) containing 2% heat-inactivated fetal bovine serum and 1× penicillin/streptomycin solution. For virus propagation, the cells were infected with either DENV serotype 2 (DENV2) strain 16681 or ZIKV isolate Thailand/1610acTw (MF692778.1). Mosquitoes (Ae. aegypti UGAL [University of Georgia Laboratory]/Rockefeller strain) were orally infected with DENV2 and ZIKV through infectious blood-feeding.
Results
We first examined viral replication activity in cells infected simultaneously, or sequentially, with DENV and ZIKV, and found interspecies binding of viral genomic transcripts to the non-structural protein 5 (NS5). When we challenged Ae. aegypti mosquitos with both DENV2 and ZIKV sequentially to probe similar interactions, virus production and vector susceptibility to infection were significantly enhanced.
Conclusions
Our results suggest that DENV2 and ZIKV simultaneously establishing infection in the Ae. aegypti mosquito vector may augment one another during replication. The data also implicate the homologous NS5 protein as a key intersection between the flaviviruses in co-infection, highlighting it as a potential target for vector control.
Similar content being viewed by others
Background
Mosquito-borne diseases are one of the most significant public health burdens [1,2,3,4,5]. Human activities, urbanization, and climate change are increasingly bringing more human hosts in contact with disease vectors [2, 6,7,8,9]. Currently, half of the global population is at risk for dengue virus (DENV) infection [2, 8, 10]. In the aftermath of the 2015–2016 Zika virus (ZIKV) outbreak, which exposed more than 130 million people to infection, the virus remains endemic to tropical and subtropical regions [11].
Sharing a common vector in Aedes aegypti, DENV and ZIKV endemicity may expand in concert, resulting in widespread co-circulation [12, 13]. Thus, DENV-ZIKV synergy presents a bleak outlook for the near future with a growing proportion of the global population living under threat of simultaneous infection by both flaviviruses. As DENV-ZIKV co-circulation expands worldwide to affect currently low-risk or virus-free regions, mosquito vectors will have increased opportunities to receive and transmit both viruses [5]. Recent reports of DENV-ZIKV co-infection corroborate this prediction and reflect a proliferating synergy that has been overlooked. This may be the result of systemic underreporting engendered by difficulties in the differential diagnosis and detection of asymptomatic infections [14,15,16].
One cross-sectional study of the ZIKV epidemic in Colombia detected 8.8% of the DENV-ZIKV serotype among 34 co-infection cases [15]. Another study in southern Mexico randomly sampled a cohort of pregnant women during a non-epidemic period and found a relatively high proportion (2%) with DENV-ZIKV co-infection [17]. A previous report revealed that DENV infections occurred during the same period, highlighting the concerning extent of silent transmission of ZIKV with DENV [17, 18]. Besides underreporting, underlying this phenomenon may be antibody-dependent enhancement (ADE) between DENV and ZIKV in co-endemic areas. Studies support ADE of ZIKV infection by anti-DENV antibodies [4, 19,20,21], which not only may increase disease severity, but also may drive ZIKV transmission into primarily DENV-endemic areas. Similar results have been suggested for DENV, in which ZIKV-mediated ADE increases the propensity for severe disease and enables DENV to persist and proliferate in primarily ZIKV-endemic regions [22, 23]. These trends are extremely troubling as they suggest a mutualistic relationship between DENV and ZIKV co-circulating in the Ae. aegypti urban transmission cycle, which could lead to further expansion.
In a mosquito vector simultaneously housing both DENV and ZIKV, either from a single co-infected patient or from separate infectious BMs, the confluence of viral replication and antiviral suppression pathways may produce distinct vector competence phenotypes, possibly to affect enhanced susceptibility and transmissibility. Thus, it is important to clarify DENV-ZIKV co-infection in the mosquito and determine the underlying molecular interactions for the development of effective vector control strategies. To date, only limited studies of arbovirus co-infection have been reported, which demonstrate the susceptibility of Ae. aegypti to DENV-ZIKV co-infection and supported the prospect of transmitting both viruses simultaneously [12, 24]. The specific effect of DENV-ZIKV co-infection on viral replication, however, remains to be elucidated.
In this study, we determined the effects of DENV and ZIKV co-infection on viral replication in Ae. aegypti to identify the specific molecular interactions involved. We first examined viral replication dynamics in cells infected simultaneously or sequentially with DENV and ZIKV. We report interspecies binding of viral genomic transcripts to the non-structural protein 5 (NS5). We then challenged Ae. aegypti mosquitos with both DENV serotype 2 (DENV2) and ZIKV sequentially to identify similar interactions, and found that virus production and vector susceptibility to infection were significantly enhanced. Our results suggest that DENV2 and ZIKV simultaneously establish infection in the Ae. aegypti vector, which may mutually augment one another during replication. The data also implicate the homologous NS5 protein as a key intersection between the flaviviruses in co-infection, highlighting it as a potential target for vector control.
Methods
Mosquitos
Aedes aegypti mosquitos (UGAL [University of Georgia Laboratory]/Rockefeller strain) were maintained at 28 °C and 70% relative humidity under a photoperiod of 12:12 h as described previously [25, 26]. Hatched larvae were transferred to plastic containers filled with water and fed daily with yeast extract. Pupae were collected and transferred to an insect dorm where emerging mosquitos were fed using cotton balls soaked in 10% sucrose solution. Female mosquitos 3–5 days post-eclosion (PE) were used for the experiments, and the sucrose-soaked cotton balls were removed at least 12 h before blood-feeding. Female mosquitos were permitted to feed on an anesthetized Institute of Cancer Research (ICR) strain mouse for 15–30 min. ICR strain mice were anesthetized via intraperitoneal injection of Avertin at a dose of 0.2 ml/10 g body weight. All animal procedures and experimental protocols were approved by the institutional Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International-accredited facility and the Committee on the Ethics of Animal Experiments at the National Taiwan University College of Medicine (IACUC Approval No: 20200210).
Cell culture and viruses
C6/36 (Ae. albopictus) cells were cultured in Dulbecco's modified Eagle medium/Mitsuhashi and Maramorosch Insect Medium (DMEM/MM) (1:1) containing 2% heat-inactivated fetal bovine serum and 1× penicillin/streptomycin solution. For virus propagation, the cells were infected with either DENV2 strain 16681 or ZIKV isolate Thailand/1610acTw (MF692778.1) at 0.01 multiplicity of infection (MOI). The culture supernatant was harvested 7 days post-infection (dpi) and stored at −80 °C. To quantify the viral titer, the supernatant was subjected to examination by plaque assay as described previously [27]. Approximately 1.0 × 107 plaque-forming units (PFU)/ml of DENV2 and ZIKV were used to infect the mosquitos.
Immunofluorescence assay (IFA)
Aedes albopictus C6/36 cells were dispensed onto a cover glass and cultured in 12-well plates overnight. The virus suspension (MOI = 1 or 10) was then added to each well. Following virus adsorption at 28 °C for 2 h, the suspension was removed and replaced with fresh medium. At 2 dpi, the cover glass was fixed in 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA, USA) for 30 min. The fixative was removed and the cover glass was rinsed in phosphate-buffered saline (PBS), and incubated for 1 h in 0.1% Triton X-100 in PBS for cell permeabilization, and blocked with blocking buffer (1% bovine serum albumin [BSA], 0.5% Triton X-100 in PBS) for 1 h. Monoclonal mouse anti-non-structural protein 1 (NS1) antibody (YH0023) (Yao-Hong Biotechnology Inc., Taipei, Taiwan) and ZIKV-specific envelope protein antibody (GTX133314) were used as the primary antibody (1:1000) to detect DENV and ZIKV antigens in the cells. Cells were then incubated with a 1:500 dilution of goat anti-mouse antibody conjugated to Alexa Fluor 488 fluorochrome (Molecular Probes Inc., Eugene, OR, USA). Finally, the cover glass was mounted with a DAPI-containing medium for confocal microscopy (ZEISS, LSM 510 META confocal microscope).
RNA extraction and reverse transcription
C6/36 cell pellets or homogenized individual mosquitos were collected in 1.5 ml tubes containing 0.5 ml TRIzol Reagent (Invitrogen). Samples were homogenized with a rotor–stator homogenizer and centrifuged at 13,000 rpm for 10 min at 4 °C. The supernatants were then transferred to new tubes each containing 0.1 ml chloroform (J.T.Baker) and mixed thoroughly. After 3 min of incubation on ice, samples were then centrifuged at 13,000 rpm for 15 min at 4 °C, and the supernatants were transferred to new tubes containing 0.25 ml isopropanol (J.T.Baker). Samples were gently mixed and stored at −80 °C for 30 min. After precipitation, the samples were once again centrifuged at 13,000 rpm for 30 min at 4 °C. The supernatants were discarded and the RNA pellets were washed with 0.5 ml 75% ethanol (Taiwan Burnett International Co., Ltd.). The samples were then centrifuged at 8000 rpm for 5 min at 4 °C and the supernatants were discarded. Finally, the RNA pellets were dried in a laminar flow cabinet and dissolved in DEPC-H2O. After Baseline-ZERO™ DNase (Epicentre) treatment, purified RNA samples were stored at −80 °C. The RNA concentrations were quantified using an ultraviolet–visible (UV–Vis) spectrophotometer (NanoDrop 2000, Thermo Fisher) and diluted with DEPC-H2O to a concentration of 1 μg/μl. The RNA (1 μg/μl) was then reverse-transcribed to complementary DNA (cDNA) using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems) and stored at −20 °C.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Quantitative RT-PCR quantification was performed using SYBR Green chemistry. The cDNA samples were quantified with the KAPA SYBR FAST Universal qPCR kit (KAPA). PCR consisted of an initial denaturation at 95 °C for 3 min, then 40 cycles at 94 °C for 3 s each, followed by 40 s at 60 °C. Fluorescence readings were measured at 72 °C after each cycle. The target gene signal was detected and analyzed with the ABI 7900HT Fast Real-Time PCR System and relative quantification results were normalized to the expression of the ribosomal protein S7 gene as an internal control [28,29,30].
Plaque assay
Whole bodies of individual mosquitos were collected in 100 μl of serum-free medium and stored at −80 °C. BHK-21 cells were seeded in a 24-well tissue culture plate and incubated at 37 °C overnight. Homogenized suspensions of individual whole bodies were centrifuged at 18,928×g for 30 min and kept on ice. Cell monolayers were rinsed with PBS and incubated with 200 μl of 10-fold serial diluted homogenized mosquito suspensions for 2 h. Following viral adsorption, 500 μl of 1% methylcellulose cell medium was added to each well and the culture plates were kept in an incubator at 28 °C for 5 days. The plates were then fixed at room temperature with 4% formaldehyde for 1 h and stained with 1% crystal violet for 30 min. Plaques were then quantified manually [27].
Cross-linking and immunoprecipitation followed by reverse transcription and PCR (CLIP-PCR)
C6/36 cells were seeded in a T75 flask and incubated at 28 °C overnight, then the virus suspension was added. Following adsorption at 28 °C for 2 h, the virus suspension was removed and replaced with fresh medium. At 2 dpi, the cells were fixed in 1% paraformaldehyde (Electron Microscopy, Hatfield, PA, USA) for 30 min. The fixative was removed and cells were resuspended in 1 ml of protein lysis buffer (50 mM Tris, pH 7.4, 1% IGEPAL, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid (EDTA), 1 mM phenylmethyl-sulfonylfluoride, 1× protease inhibitor mixture, and 1× phosphatase inhibitor mixture), then homogenized using a rotor–stator homogenizer. The samples were transferred to a QIAshredder™ column (Qiagen) for solubilization of cross-linked complexes. The eluted samples were collected and transferred to new Eppendorf tubes at −80 °C. Protein G-agarose beads (20 μl, packed volume) were coated with a specific antibody for 2 h at 4 °C followed by extensive washing with RIPA buffer containing protease inhibitors. The cell lysate (500 μl) was diluted with RIPA buffer (500 μl), mixed with the antibody-coated beads, and incubated with rotation for 4 h at 4 °C. The beads were collected using a mini-centrifuge at 700×g for 5 min at 4 °C and the supernatant was removed. The antibody-coupled beads were washed three times by adding 1 ml of RIPA buffer and centrifuging at 700×g and 4 °C for 5 min. The beads containing the immunoprecipitated samples were collected, resuspended in 50 μl of TE buffer, and incubated at 70 °C for 45 min to reverse the cross-links. The RNA was extracted from these samples using TRIzol Reagent according to the manufacturer’s protocol (Invitrogen). RNA (1 μg/μl) was then reverse-transcribed to cDNA using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems) and stored at −20 °C.
Oral infection of virus
Female mosquitoes were infected by feeding them with an infectious blood meal prepared by mixing 200 μl of mouse whole blood, 50 μl of 1 mM ATP, and 250 μl of DENV2 16681 strain (2.5 × 106 PFU in 250 μl) using folded Parafilm-M. Prior to the blood meal, the mosquitoes were starved through sugar deprivation for 12 h. After feeding, each mosquito was observed under a stereomicroscope to confirm successful blood intake. The mosquitoes were then kept at 28 °C and 70% relative humidity with a 12 h:12 h light–dark cycle, as described previously [31, 32]. Mosquitos were captured 12 h ahead of day 0 and presented with blood meal (BM), DENV2, ZIKV, or maintained on sugar feeding. On day 5, one group was given a second BM (BM-BM), and others were challenged with DENV2 or ZIKV. At 7 dpi (day 12), whole mosquito bodies were collected and homogenized. Relative viral genome expression of DENV2 and ZIKV was determined by qRT-PCR normalized to the ribosomal S7 protein gene. Infectious viral titers were quantified via plaque assay as described above. Each of the six oral challenge schemes consisted of at least five biologically independent cohorts.
Statistical analysis
All statistical analyses were performed using GraphPad Prism 8 software. One-way analysis of variance (ANOVA) or the Kruskal–Wallis test by ranks was used to compare independent cohorts in each set of experiments. Post hoc analyses were performed for variance tests bearing significance at α = 0.05 using Tukey’s and Dunn’s multiple comparisons tests, respectively.
Graphical illustrations
The graphical abstract and parts of Figs. 2 and 4 were made with Biorender.com. A publication license was obtained for each figure. DENV and ZIKV particles used in the graphical abstract were provided courtesy of David Goodsell (Scripps Research, CA, USA), made publicly available at PDB-101 under a CC BY 4.0 license.
Results
Simultaneous infection with DENV2 and ZIKV modulates viral NS1 subcellular localization and viral replication in mosquito cells
To investigate DENV-ZIKV co-infection in the mosquito, we infected mosquito cells simultaneously with DENV2 and ZIKV, infecting mosquito C6/36 cells with ZIKV (single infection) and DENV2/ZIKV (co-infection) at an MOI of 10. At 2 dpi, the subcellular localization of viral NS1 protein was characterized via IFA using anti-NS1 antibody (green). Remarkably, simultaneous co-infection (DENV/ZIKV) modulated the subcellular localization of viral NS1 produced by both viruses (Fig. 1A). Departing from our observations in ZIKV single-infected cells, wherein NS1 associated into vesicle-like structures, viral NS1 in DENV2/ZIKV co-infected cells localized in the cytoplasm, as in DENV2 single-infected cells (Additional file 1: Fig. S1a). In addition, the rate of cellular mortality was observed to be greater in cells infected with DENV2 than in those infected with ZIKV (Additional file 1: Fig. S1b). Granted, NS1 detection may not directly correspond to changes in viral genomic replication and localization in vitro.
Replication, subcellular localization, and systematic responses following simultaneous DENV2/ZIKV co-infection of C6/36 mosquito cells. A Immunofluorescence of the flaviviral NS1 (green) in ZIKV single-infected and DENV2/ZIKV co-infected cells, with DAPI (blue)-stained DNA demarcating nuclei. Scale bars, 10 μm. B Relative levels of DENV and ZIKV viral genomes in cell lysate at 2 days post-infection (dpi). C Relative expression of genes encoding catalase and glutathione S-transferase family proteins in cell lysate at 2 dpi. These results are representative of at least three independent experiments. D Relative expression of casp7, dronc, and Mx in cell lysate at 2 dpi. These results are representative of at least three independent experiments. Differences between groups were demonstrated to be statistically significant using Tukey’s multiple comparisons test; *P < 0.05, **P < 0.01, ***P < 0.001
We then quantified the effects of simultaneous DENV2/ZIKV co-infection on viral genomic replication (Fig. 1b). Our results show that, compared with DENV2 single-infected cells, DENV2 viral genome replication in DENV2/ZIKV co-infected cells was significantly higher at equal MOI. Conversely, cellular ZIKV genome replication was drastically suppressed in co-infected cells. Fluorescence imaging corroborates this finding: ZIKV envelope (E) protein was hardly present in DENV2/ZIKV co-infected cells; in ZIKV-infected cells, ZIKV-E was not only present at NS1-localized sites but was also dispersed throughout the cytoplasm (Fig. 1a). Viral E protein serves as an indication of replication activity because it associates with the endoplasmic reticulum membrane at the site of ribonucleocapsid assembly, eventually budding off with prM proteins to line the casing of live viral particles.
In order to gain insight into the underlying mechanisms responsible for the differing viral replication rates and levels of cellular mortality observed in cells infected with DENV2 versus ZIKV, we measured the expression levels of genes associated with stress and apoptosis. We found that transcript abundance of catalase and glutathione-S-transferase (GST), enzymes mediating oxidative stress responses to viral infection in Ae. aegypti, were significantly downregulated in co-infected cells compared with DENV2 single-infected cells (Fig. 1C). Meanwhile, expression of apoptosis-promoting genes casp7 and dronc did not differ significantly between co-infected and DENV-infected cells, with a significant decrease in Mx (Fig. 1D). Overall, we find that co-infection produces a distinct antiviral response profile in terms of oxidative stress, but not apoptotic processes.
Our results demonstrate that, surprisingly, simultaneous DENV2-ZIKV co-infection significantly modulates viral NS1 subcellular localization and viral genomic replication, apparently benefiting DENV2. The fact that viral NS1 co-localizes in co-infected cells raises the interesting possibility that DENV and ZIKV replication may overlap. NS1 proteins serve as scaffolding protein dimers for the flaviviruses’ highly homologous replication complexes (RCs). DENV-ZIKV co-infection presents abundant opportunity for spatio-temporal coordination of viral propagation, with significant implications for viral replication and virus assembly. The divergent outcomes of viral genomic replication observed (DENV2 enhancement, ZIKV attenuation) following co-infection hint at competitive interactions during infection and replication. It is not clear, however, whether the stark contrast between DENV and ZIKV replication in this co-infection model is due to superior engagement of DENV2-NS1, with the cell membrane outcompeting ZIKV-NS1 (Additional file 1: Fig. S1B), or because genomic DENV2 made more efficient use of both DENV2 and ZIKV RCs. To probe this distinction, we employed a sequential co-infection model, which allowed for temporal segregation of DENV2 and ZIKV replication at the level of RC establishment and early genomic replication.
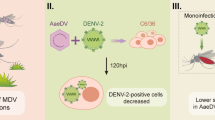
Sequential infection with DENV and ZIKV similarly modulates viral NS1 subcellular localization and viral replication in mosquito cells
To evaluate whether the increase in DENV2 replication efficiency observed in simultaneously co-infected cells resulted from more extensive DENV2 RC establishment during initial infection, we inoculated C6/36 cells first with ZIKV, and then with DENV2 (Fig. 2A). This model of sequential co-infection provides ZIKV with the opportunity to establish infection prior to DENV2 inoculation. Consistent with earlier observations (Fig. 1A), viral NS1 in ZIKV-infected cells tended to associate into vesicle-like subcellular structures, but dispersed throughout the cytoplasm in DENV-infected cells (Fig. 2B). As for cells co-infected sequentially with ZIKV, then DENV2, significant overlap of viral NS1 was observed, with some retention of vesicle-like formations present in ZIKV single-infected cells (Fig. 2B). This presentation is consistent with that for simultaneously co-infected cells, and clearly demonstrates that DENV2-ZIKV co-infection in the mosquito cell, regardless of inoculation sequence, modulates viral NS1 localization to allow for possible extensive overlap of the flaviviruses’ replicative activities.
Sequential co-infection modulates viral NS1 subcellular localization and viral replication in mosquito cells. A Time course of sequential infection with ZIKV and DENV2. B Subcellular localization of flaviviral NS1 (green) and ZIKV-E protein (red) in single-infected and ZIKV → DENV2 sequentially co-infected cells at 2 dpi, with DAPI (blue)-stained DNA demarcating nuclei. Scale bars, 10 μm. C Relative levels (2−(dCt)) of DENV and ZIKV viral genomes in secreted viruses in the supernatant of cell culture medium at 2 dpi. These results are representative of at least three independent experiments. D At 2 dpi, culture supernatants from single-infected and ZIKV → DENV2 sequentially co-infected cells were collected and used in a focus-forming assay. NS1 (green) stained for replicating virus
Assessment of viral RNA extracted from secreted viruses in supernatant of these ZIKV → DENV2 sequentially co-infected cells revealed that both DENV2 and ZIKV replication were significantly enhanced (Fig. 2C). ZIKV replication benefited from a spatio-temporal advantage, allowing it to establish RC and initiate replication first. The fact that DENV2 replication also benefited rules out the possibility that superior membrane integration of DENV2-NS1 and its associated RC allowed it to outcompete ZIKV in the simultaneous co-infection model. Instead, an intriguing prospect arises: interspecies interactions may be at work, whereby replicating viruses engage the complementary RC to its benefit.
Before probing further possible interspecies DENV-ZIKV interactions during co-infection, we sought to confirm that our model indeed permitted infectious virion production downstream of viral genomic replication. We inoculated uninfected C6/36 cells with supernatant containing viral particles produced by ZIKV → DENV2 sequentially co-infected cells, staining for viral NS1 to confirm infection and replication initiation (Fig. 2D). The extent to which secreted viruses in co-infected supernatant established infection was clearly superior to DENV2 and ZIKV alone, particularly for ZIKV.
Evidently, DENV2/ZIKV co-infection of mosquito cells modulates NS1 cellular localization and significantly enhances virus production. The use of the sequential infection model provides a high level of spatio-temporal resolution that enables us to identify that the observed effects are a result of the viral replication process. The overlap of DENV2-ZIKV replication loci in co-infected cells likely provides for cross-species interactions resulting in mutual enhancement of replication. Accordingly, we probed directly interspecies molecular interactions during DENV-ZIKV co-infection.
The replicating DENV2 genome interacts with the ZIKV-NS5 protein
To identify specific molecular interactions between DENV2 and ZIKV in co-infected cells, we assayed each respective genome against RC-forming non-structural proteins of the complementary virus using CLIP-PCR. We identified a cognate interaction between the replicating DENV2 template single-stranded RNA (ssRNA) and ZIKV-NS5 (Fig. 3), perhaps the prominent DENV-ZIKV interaction contributing to enhanced virus replication in co-infected cells, which overall favored DENV2. This phenotype is striking, and bears important implications for Ae. aegypti vector competence in vivo. Apparently, DENV2 may engage the highly conserved RNA-dependent RNA polymerase ZIKV-NS5 and its RNA-capping methyltransferase activity and, perhaps, vice versa (wherein ZIKV engages DENV2-NS5) to promote its replication, amounting to greater virus production overall. Thus, to determine whether this underlying phenomenon affects vector competence in vivo, we challenged Ae. aegypti females with DENV2 and ZIKV in infectious blood meal.
The replicating DENV2 genome interacts with the ZIKV-NS5 protein in co-infected mosquito cells. ZIKV-NS5 interacting with DENV2 and ZIKV genomic RNA was precipitated from cell lysates at 4 days using CLIP-PCR, followed by RT-PCR with primers specific for DENV and ZIKV genomes. Ribosomal protein S7 was used as a loading control
Co-infection of Ae. aegypti with DENV2 and ZIKV results in differential viral genome expression
Finding significant enhancement of viral replication by DENV2-ZIKV co-infection in vitro possibly arising from cross-species interactions, we challenged adult female Ae. aegypti sequentially with both viruses to quantify the effects of DENV2-ZIKV co-infection on vector competence in vivo. So far, existing studies into DENV-ZIKV co-infection have only challenged Ae. aegypti simultaneously, presenting both viruses in the same blood meal (BM). Contrarily, we elected to challenge mosquitos with DENV2 and ZIKV sequentially to maximize ecological validity. It is much more likely that in a DENV-ZIKV co-endemic area, a female mosquito would acquire co-infection in sequential feeding episodes: by feeding first on a DENV-infected host, then a ZIKV-infected host, or vice versa. The possibility of a mosquito obtaining both viruses from a DENV-ZIKV co-viremic human host through a single BM is much lower. Critically, we also consider the findings of studies reporting that non-infectious BM prior to subsequent viral BM can promote viral replication, as the initial non-infectious BM induces physiological changes in the midgut epithelium, rendering it more permissible to dissemination through the basal lamina [28, 29, 33, 34].
Thus, we included cohorts of mosquitoes presented with an initial naïve BM before challenge with DENV2 or ZIKV to account for this possible confounder in evaluating co-infection effects on viral replication (Fig. 4A). Overall, two co-infection schemes were used in vivo, in which Ae. aegypti were either challenged first with DENV2 and then ZIKV on a second BM (DENV2 → ZIKV), or vice versa (ZIKV → DENV2). These cohorts were compared against mosquitos single-infected with DENV and ZIKV, through either one or two BMs, as well as a mock cohort (BM-BM).
Co-infection of Ae. aegypti mosquitos with DENV and ZIKV results in differential viral genome expression. A Time course of experimental oral challenge with DENV2 and ZIKV. Mosquitos were captured 12 h ahead of day 0 and presented with blood meal (BM), DENV2, ZIKV, or maintained on sugar feeding (Sugar). On day 5, one group was given a second BM (BM-BM), and others were challenged with DENV2 or ZIKV. At 7 dpi (day 12), whole mosquito bodies were collected and homogenized. Relative viral genome expression of B DENV2 and C ZIKV was determined by qRT-PCR analysis, with normalization to the endosomal S7 protein. Each of six oral challenge schemes consisted of at least five biologically independent cohorts, and post hoc comparisons between groups were performed using Tukey’s multiple comparisons test; **P < 0.01; ***P < 0.001; ****P < 0.0001
Collecting mosquitos at 7 dpi, which we previously determined to be an optimal time point for viral genomic analysis, we quantified via qRT-PCR the relative expression of DENV2 and ZIKV genomes, respectively (Fig. 4A). We found that, contrary to our observations in vitro, DENV2 expression was significantly downregulated in both co-infection scenarios, particularly when compared with those challenged twice (DENV2 → DENV2) with DENV2 (Fig. 4B). Also, a significant difference in expression between DENV2 single-infected mosquitos presented with (BM → DENV2), and without (sugar-fed → DENV2), an initial naïve BM suggests that BM-induced modifications do indeed promote viral replication by encouraging dissemination from the midgut. Genomic expression of ZIKV was significantly elevated in both co-infection scenarios, even in comparison to mosquitos infected twice (ZIKV → ZIKV) with ZIKV (Fig. 4C). Between the two co-infection groups, ZIKV → DENV2 mosquitos expressed genomic ZIKV at significantly higher levels than did their DENV2 → ZIKV counterparts. There was also a significant difference in expression between sugar-fed → ZIKV and BM → ZIKV individuals, as with DENV2, again highlighting the initial BM’s ability to promote viral replication in and past the midgut. Moving forward, we evaluated the implications of co-infection on vector competence, quantifying the infectivity of virus particles produced in vivo.
Virus production and vector susceptibility are enhanced in Ae. aegypti challenged with both DENV2 and ZIKV
Having characterized the genomic expression profiles of co-infected mosquitos, we next assessed the quantity and infectivity of viruses produced therein. Performing plaque assays on isolated virus from individuals collected at 7 dpi from each treatment group (Fig. 4A), we found that viral titers were significantly higher in co-infected mosquitos than in the DENV single-infected cohort (Fig. 5A). Notably, these increases were observed for both DENV2 → ZIKV and ZIKV → DENV2 co-infection groups against all three DENV single-infection cohorts: those challenged either once (sugar/BM → DENV2) or twice (DENV2 → DENV2) with DENV2. Accordingly, the corresponding infection rates were markedly higher in co-infected mosquitos, increasing by as much as 36.7% (Fig. 5B). Compared with ZIKV single-infected cohorts, viral titers were also higher in co-infected individuals. Infection rates also saw increases by about 10% except when compared with the sugar → ZIKV cohort, a result likely attributable to individual variability. Despite the unexpectedly high infection rate, the median viral titer for this cohort was at least twofold lower than those of co-infected cohorts. Overall, mosquitos challenged sequentially with DENV and ZIKV produced more infectious virus, which induced a greater extent of infection ex vivo.
Virus production and vector susceptibility are enhanced in DENV2/ZIKV co-infected mosquitos. A Mosquitos were challenged with virus as described in Fig. 4, and then collected at 7 dpi (day 12) for plaque assay. Geometric means (PFU/ml) are plotted, and each of eight viral challenge schemes comprised at least three biological cohorts. B Sample size n, infection rate, and median PFU/ml corresponding to the experimental groups. Infected samples had positive (> 0) PFU/ml values; uninfected, negative samples are represented on the log scale as positive (PFU/ml = 1) only for visual interpretation. Comparison between groups post hoc was performed using Dunn’s multiple comparisons test; *P < 0.05; **P < 0.01; ***P < 0.001
Mosquitos twice-challenged with ZIKV produced higher viral titers than those co-infected, an unsurprising result following consecutive ZIKV infection. Secondary infection is greatly assisted by the existing RC infrastructure and an infected, compromised midgut epithelium and basal lamina (ZIKV → ZIKV). Indeed, viral titers were higher in co-infected mosquitos compared with those challenged once (sugar/BM → ZIKV). Infection rates of co-infected mosquitos also increased, suggesting greater susceptibility to infection and virus propagation, i.e., enhanced vector competence. As for mosquitos twice-challenged with DENV2, the clear enhancement of viral replication by co-infection is much more readily apparent, as these cohorts were entirely refractory to infection (0% infected, DENV2 → DENV2). Across DENV2 single-infected cohorts, infection rates peaked at 11.1%. The fact that vector infection rates were elevated to 44.1% and 46.7%, respectively, in co-infected cohorts indicates that DENV2-ZIKV co-infection positively, mutually modulates viral replication.
Of note, mosquitos given an initial naïve BM neither produced significantly higher viral titers nor were infected at higher rates than those directly challenged with DENV2/ZIKV. This suggests that while an initial non-infectious BM may correlate with higher viral genomic expression by assisting virus dissemination from the midgut, it ultimately neither enhances the production of viable, infectious particles nor promotes co-infected Ae. aegypti susceptibility to infection.
Taken together, our results show that DENV2-ZIKV co-infection significantly enhances virus production and vector susceptibility to infection. At the cellular level, viral replication is mutually enhanced owing to the overlap of highly homologous flaviviral replication machinery. We observed therein that DENV2 transcripts engage ZIKV-NS5. Overall, our findings raise grave concerns about DENV2 and ZIKV co-circulation, which threatens to strain healthcare resources and exacerbate transmission and disease as mosquitos vector these flaviviruses with greater efficiency.
Discussion
DENV and ZIKV are flaviviruses transmitted by mosquitos of the Aedes genus, primarily Ae. aegypti [35]. They co-circulate in overlapping endemic areas. Consequently, as the spread of DENV and ZIKV expands rapidly, mosquitos will have increased opportunities to acquire simultaneous and/or mixed infections with different types of flaviviruses [35]. This may occur following an infectious BM from a single human co-viremic for DENV and ZIKV, or when mosquitos acquire sequential BMs from two individuals, each infected with a different virus. DENV and ZIKV share a highly conserved non-structural protein repository consisting of five enzymes/subunits (NS1–5), which associate closely to form a tightly-regulated RC. We hypothesized that DENV and ZIKV interact through their homologous RC components during replication, which has significant implications for viral replication and vector competence.
In the present study, we observed significant enhancements in virus production and vector susceptibility following DENV2-ZIKV co-infection in Ae. aegypti, and we demonstrated the cellular and molecular bases of these effects. In co-infected mosquito cells, we found that DENV2 expression was significantly enhanced, whereas ZIKV was coincidentally markedly suppressed and virus production was significantly increased for both. The finding that flaviviral RCs co-localize extensively in the cytoplasm, we determined whether DENV and ZIKV participate in cross-species interactions during replication. We found that the replicating DENV2 genome engages ZIKV-NS5. This surprising interaction readily explains the drastic enhancement of DENV2 expression in vitro, which suggests that DENV2 engages the ZIKV RC competitively. DENV-ZIKV interactions during replication provide a basis for the mutual increase in secretory virus particles observed. Next, we challenged Ae. aegypti adult females with both viruses to determine whether similar DENV2-ZIKV interactions also modulate viral replication in vivo. We found that while genomic expression at 7 dpi of DENV2 was markedly downregulated, with ZIKV upregulated, there was again a mutual enhancement of viral replication as indicated by significantly elevated levels of infectious virions produced in co-infected mosquitos, which were also more susceptible to infection.
Our findings describe for the first time the potent mutualistic outcomes of flaviviral co-infection for viral replication, and suggest that vector competence may be enhanced as a result. Vector competence in the arboviral sylvatic cycle is defined by the extent to which the mosquito permits a virus to utilize its circulatory system. The virus must first establish infection in the midgut and produce virions that disseminate to secondary tissues, which must then sustain replication throughout the organ system until freshly propagated virions breach the salivary glands. We found that viruses produced in DENV-ZIKV co-challenged mosquitos were significantly more abundant compared with those infected with only one virus, particularly DENV2. Moreover, these mosquitos were infected at a higher rate, suggesting that co-infected mosquitos are more susceptible to infection, perhaps resulting from the convergence of immune suppression pathways of both replicating viruses, particularly in the midgut. These results strongly suggest that co-infection enhances vector competence because, at a collection date of 7 dpi, either virus will have already disseminated from the midgut and commenced replication throughout the entire body to produce viable, infectious particles. Although employing more collection points to visualize replication kinetics may allow for a clearer spatio-temporal resolution, for the purpose of understanding co-infection in terms of viral replication and vector competence, it was sufficient to isolate virus from mosquitos at 7 dpi for the plaque assay. The implications of our results for vector competence are somewhat limited, however, absent organ-specific analysis. Specifically, quantifying virus present in the salivary glands may permit more direct observation of outcomes for vector competence, as infectious viral particles must be secreted into the saliva during a BM for transmission [56]. However, the variability of flaviviral infection in vivo among individual mosquitos largely precluded such an investigation, as salivary glands dissected and analyzed individually would likely vary greatly in viral titer. Collecting whole bodies enabled an individual analysis without sacrificing statistical integrity. Additionally, we studied replication dynamics in vivo using a blood meal challenge, opting not to infect mosquitos by intrathoracic injection to preserve the critical barrier to vector competence manifest in the midgut’s physical and immunological fortifications. Quantifying virus propagated through sequential co-infection by oral challenge allowed us to observe the consequences of DENV-ZIKV interaction directly through the entire course of infection within the vector, beginning with ingestion in the midgut. Furthermore, our findings are highly relevant to the evolving landscape of DENV-ZIKV endemicity, as our sequential oral infection model is closely aligned with actual vector activity. Mosquitos are much more likely to acquire flaviviral co-infection sequentially from individual hosts than from a single host viremic for both DENV and ZIKV. Individual variability in feeding opportunity, i.e., extent of engorgement, however, likely negates many real differences in virus intake between laboratory and field.
To date, exploratory studies into arboviral co-infection have only established that Ae. aegypti are susceptible to infection by more than one type of virus and they may simultaneously transmit multiple viruses. One study reported that Ae. aegypti simultaneously challenged with a combination of two or three arboviruses including DENV2, ZIKV, and chikungunya (CHIKV), were frequently double- or triple-infected, which indicated that the mosquitos are susceptible to co-infection. Inoculation of saliva in vitro confirmed the potential for co-transmission of all three viruses [12]. Another study found that mosquitos simultaneously co-infected with DENV and ZIKV preferably transmit the latter [24]. The co-infection and co-transmission potential of ZIKV-CHIKV [36, 37] and DENV-CHIKV [38] have also been supported. With respect to arboviral co-infection, our study contributes novel insight into its implications for viral propagation in Ae. Aegypti and demonstrates that DENV-ZIKV co-infection mutually enhances viral replication. Our results also suggest that vector competence may be enhanced following DENV-ZIKV co-infection, as indicated by increased infection rates. We also provide the first account of molecular mechanisms underlying co-infection effects by reporting that DENV engages ZIKV-NS5 during replication.
As for the apparent conflict between our in vitro and in vivo results in which DENV2 replication appeared be competitively promoted in co-infected cells (Figs. 1B, 2C), but drastically suppressed in the mosquito (Fig. 4), it is important to note that markedly reduced DENV2 expression in co-infected mosquitos reflected only the viral genome content at 7 dpi, not the amount of infectious DENV virions produced by the vector. Indeed, virus production was significantly higher in co-infected mosquitos than in those single-infected with DENV. In addition, co-infected cohorts were much more susceptible to infection. Low DENV2 genomic expression suggests that there may be competitive engagement between ZIKV and DENV2 due to interaction between ZIKV genomic transcripts with DENV2 RC proteins. We showed that DENV2 utilizes ZIKV-NS5 for transcription; thus, it is likely that ZIKV may reciprocally utilize DENV2-NS5 to its advantage. Another intriguing possibility is that flaviviral NS5 may have a dual purpose in the convergence of DENV-ZIKV co-infection by cross-species capping of the respective RNA genomes by N-terminus methyltransferases to assist in evasion of the host immune response. This is an alternative (and not mutually exclusive) molecular premise for the observed increases in virus production and vector susceptibility in co-infected mosquitos. Further biochemical studies regarding the DENV-ZIKV interaction via NS5, a possible target for vector control and vaccine development, is warranted as the co-circulation of DENV and ZIKV broadens globally.
The clinical and epidemiological implications of expanding flaviviral co-circulation remain largely unexplored. Though it is not clear whether co-infected patients develop more severe disease [39,40,41,42], increased co-circulation and transmission of multiple flaviviruses will surely pose significant problems for diagnosis and surveillance because of the common clinical presentation, asymptomatic response, and cross-reactivity. Although progress is being made on the diagnostics front [43,44,45], effective vector control remains the most effective approach to managing and eliminating mosquito-borne diseases [3]. Central to vector control is a clear understanding of the pathogen-vector relationship as it evolves in real-time, with expanding arboviral co-circulation being a worrying trend that we have addressed. As the periphery of DENV-ZIKV co-circulation expands, it also encompasses other arboviruses, such as CHIKV [46]. As we demonstrated, co-infection with DENV and ZIKV mutually enhances viral replication within the vector. Further interaction with other arbovirus, such as yellow fever virus or CHIKV, may result in unknown synergistic effects. This may similarly threaten to facilitate widespread circulation and transmission of multiple deadly viruses by modulating the vector response. The interplay among these arboviruses in Ae. aegypti and its effects on viral replication and vector competence require further study. Future work in this area could incorporate the study of viral interactions with the mosquito microbiota [47, 48] and diverse RNA responses [49,50,51,52] of the mosquito. Whether differential interactions arise between the DENV serotypes in co-infection scenarios is also worth pursuing, as all four DENV serotypes (DENV1–4) are spreading throughout Asia, Africa, and the Americas [53]. It also remains to be determined whether arbovirus co-infection influences virus selection pressure and recombination events [54, 55]. In conclusion, this study presents the novel finding that DENV-ZIKV co-infection mutually enhances viral replication within the mosquito. This threatens to increase the disease burden in co-endemic areas, drive the “silent” transmission of strains not predominantly circulating, and introduce flaviviruses into communities not yet seen. With arboviral co-endemicity on the rise globally, the rapidly shifting vector–pathogen relationship must be further investigated, in which the pathogen itself bears many faces.
Conclusions
In this study, we determined the effects of DENV and ZIKV co-infection on viral replication in Ae. aegypti and to identify the specific molecular interactions involved. We first examined viral replication dynamics in cells infected simultaneously or sequentially with DENV and ZIKV. We report the interspecies binding of viral genomic transcripts to NS5. We then challenged Ae. aegypti mosquitos with both DENV2 and ZIKV sequentially to identify similar interactions, and found that virus production and vector susceptibility to infection were significantly enhanced. Our results suggest that DENV2 and ZIKV simultaneously establish infection in the Ae. aegypti vector, which may mutually augment one another during replication. The data also implicate the homologous NS5 protein as a key intersection between the flaviviruses in co-infection, highlighting it as a potential target for vector control.
Data availability
All data generated or analyzed during this study are included in this published article.
Abbreviations
- BM:
-
Blood meal
- BSA:
-
Bovine serum albumin
- dpi:
-
Days post-infection
- DENV:
-
Dengue virus
- ICR:
-
Institute of Cancer Research
- IFA:
-
Immunofluorescence assay
- MOI:
-
Multiplicity of infection
- PE:
-
Post-eclosion
- RCs:
-
Replication complexes
- ZIKV:
-
Zika virus
References
Wilder-Smith A, Ooi EE, Horstick O, Wills B. Dengue. Lancet. 2019;393:350–63. https://doi.org/10.1016/S0140-6736(18)32560-1.
Wellekens K, Betrains A, De Munter P, Peetermans W. Dengue: current state one year before WHO 2010–2020 goals. Acta Clin Belg. 2020. https://doi.org/10.1080/17843286.2020.1837576.
Wilson AL, Courtenay O, Kelly-Hope LA, Scott TW, Takken W, Torr SJ, et al. The importance of vector control for the control and elimination of vector-borne diseases. PLoS Negl Trop Dis. 2020;14:e0007831. https://doi.org/10.1371/journal.pntd.0007831.
Wang WH, Urbina AN, Wu CC, Lin CY, Thitithanyanont A, Assavalapsakul W, et al. An epidemiological survey of the current status of Zika and the immune interaction between dengue and Zika infection in Southern Taiwan. Int J Infect Dis. 2020;93:151–9. https://doi.org/10.1016/j.ijid.2020.01.031.
Achee NL, Grieco JP, Vatandoost H, Seixas G, Pinto J, Ching-Ng L, et al. Alternative strategies for mosquito-borne arbovirus control. PLoS Negl Trop Dis. 2019;13:e0006822. https://doi.org/10.1371/journal.pntd.0006822.
Shepard DS, Undurraga EA, Halasa YA, Stanaway JD. The global economic burden of dengue: a systematic analysis. Lancet Infect Dis. 2016;16:935–41. https://doi.org/10.1016/S1473-3099(16)00146-8.
Hiscott J, Wilder-Smith A. Editorial overview: the challenge to defeat dengue. Curr Opin Virol. 2020;43:iii–v. https://doi.org/10.1016/j.coviro.2020.10.004.
Cattarino L, Rodriguez-Barraquer I, Imai N, Cummings DAT, Ferguson NM. Mapping global variation in dengue transmission intensity. Sci Transl Med. 2020;12:528. https://doi.org/10.1126/scitranslmed.aax4144.
Stanaway JD, Shepard DS, Undurraga EA, Halasa YA, Coffeng LE, Brady OJ, et al. The global burden of dengue: an analysis from the Global Burden of Disease Study 2013. Lancet Infect Dis. 2016;16:712–23. https://doi.org/10.1016/S1473-3099(16)00026-8.
Brady OJ, Gething PW, Bhatt S, Messina JP, Brownstein JS, Hoen AG, et al. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis. 2012;6:e1760. https://doi.org/10.1371/journal.pntd.0001760.
Attaway DF, Waters NM, Geraghty EM, Jacobsen KH. Zika virus: Endemic and epidemic ranges of Aedes mosquito transmission. J Infect Public Health. 2017;10:120–3. https://doi.org/10.1016/j.jiph.2016.09.008.
Ruckert C, Weger-Lucarelli J, Garcia-Luna SM, Young MC, Byas AD, Murrieta RA, et al. Impact of simultaneous exposure to arboviruses on infection and transmission by Aedes aegypti mosquitoes. Nat Commun. 2017;8:15412. https://doi.org/10.1038/ncomms15412.
Sukhralia S, Verma M, Gopirajan S, Dhanaraj PS, Lal R, Mehla N, et al. From dengue to Zika: the wide spread of mosquito-borne arboviruses. Eur J Clin Microbiol Infect Dis. 2019;38:3–14. https://doi.org/10.1007/s10096-018-3375-7.
Pessoa R, Patriota JV, Lourdes de Souza M, Felix AC, Mamede N, Sanabani SS. Investigation into an outbreak of dengue-like illness in Pernambuco, Brazil, revealed a Cocirculation of Zika, Chikungunya, and Dengue virus type 1. Medicine (Baltimore). 2016;95:e3201. https://doi.org/10.1097/MD.0000000000003201.
Mercado-Reyes M, Acosta-Reyes J, Navarro-Lechuga E, Corchuelo S, Rico A, Parra E, et al. Dengue, chikungunya and zika virus coinfection: results of the national surveillance during the zika epidemic in Colombia. Epidemiol Infect. 2019;147:e77. https://doi.org/10.1017/S095026881800359X.
Estofolete CF, Terzian ACB, Colombo TE, de Freitas Guimaraes G, Ferraz HCJ, da Silva RA, et al. Co-infection between Zika and different Dengue serotypes during DENV outbreak in Brazil. J Infect Public Health. 2019;12:178–81. https://doi.org/10.1016/j.jiph.2018.09.007.
Eligio-Garcia L, Crisostomo-Vazquez MDP, Caballero-Garcia ML, Soria-Guerrero M, Mendez-Galvan JF, Lopez-Cancino SA, et al. Co-infection of Dengue, Zika and Chikungunya in a group of pregnant women from Tuxtla Gutierrez, Chiapas: preliminary data 2019. PLoS Negl Trop Dis. 2020;14:e0008880. https://doi.org/10.1371/journal.pntd.0008880.
Sharp TM, Fischer M, Munoz-Jordan JL, Paz-Bailey G, Staples JE, Gregory CJ, et al. Dengue and Zika virus diagnostic testing for patients with a clinically compatible illness and risk for infection with both viruses. MMWR Recomm Rep. 2019;68:1–10. https://doi.org/10.15585/mmwr.rr6801a1.
Paul LM, Carlin ER, Jenkins MM, Tan AL, Barcellona CM, Nicholson CO, et al. Dengue virus antibodies enhance Zika virus infection. Clin Transl Immunol. 2016;5:e117. https://doi.org/10.1038/cti.2016.72.
Castanha PMS, Nascimento EJM, Braga C, Cordeiro MT, de Carvalho OV, de Mendonca LR, et al. Dengue virus-specific antibodies enhance Brazilian Zika virus infection. J Infect Dis. 2017;215:781–5. https://doi.org/10.1093/infdis/jiw638.
Olawoyin O, Kribs C. Coinfection, altered vector infectivity, and antibody-dependent enhancement: the Dengue-Zika interplay. Bull Math Biol. 2020;82:13. https://doi.org/10.1007/s11538-019-00681-2.
Tang B, Xiao Y, Sander B, Kulkarni MA, Radam-Lac Research T, Wu J. Modelling the impact of antibody-dependent enhancement on disease severity of Zika virus and dengue virus sequential and co-infection. R Soc Open Sci. 2020;7:191749. https://doi.org/10.1098/rsos.191749.
Katzelnick LC, Narvaez C, Arguello S, Lopez Mercado B, Collado D, Ampie O, et al. Zika virus infection enhances future risk of severe dengue disease. Science. 2020;369:1123–8. https://doi.org/10.1126/science.abb6143.
Chaves BA, Orfano AS, Nogueira PM, Rodrigues NB, Campolina TB, Nacif-Pimenta R, et al. Coinfection with Zika virus (ZIKV) and Dengue virus results in preferential ZIKV transmission by vector bite to vertebrate host. J Infect Dis. 2018;218:563–71. https://doi.org/10.1093/infdis/jiy196.
Weng SC, Shiao SH. Frizzled 2 is a key component in the regulation of TOR signaling-mediated egg production in the mosquito Aedes aegypti. Insect Biochem Mol Biol. 2015;61:17–24. https://doi.org/10.1016/j.ibmb.2015.03.010.
Chang CH, Liu YT, Weng SC, Chen IY, Tsao PN, Shiao SH. The non-canonical Notch signaling is essential for the control of fertility in Aedes aegypti. PLoS Negl Trop Dis. 2018;12:e0006307. https://doi.org/10.1371/journal.pntd.0006307.
Sri-In C, Weng SC, Chen WY, Wu-Hsieh BA, Tu WC, Shiao SH. A salivary protein of Aedes aegypti promotes dengue-2 virus replication and transmission. Insect Biochem Mol Biol. 2019;111:103181. https://doi.org/10.1016/j.ibmb.2019.103181.
Chen TH, Lo YP, Yang CF, Chen WJ. Additive protection by antioxidant and apoptosis-inhibiting effects on mosquito cells with dengue 2 virus infection. PLoS Negl Trop Dis. 2012;6:e1613. https://doi.org/10.1371/journal.pntd.0001613.
Chen TH, Tang P, Yang CF, Kao LH, Lo YP, Chuang CK, et al. Antioxidant defense is one of the mechanisms by which mosquito cells survive dengue 2 viral infection. Virology. 2011;410:410–7. https://doi.org/10.1016/j.virol.2010.12.013.
Weng SC, Zhou YX, Shiao SH. A flavivirus-inducible gene expression system that modulates broad-spectrum antiviral activity against dengue and Zika viruses. Insect Biochem Mol Biol. 2022;142:103723. https://doi.org/10.1016/j.ibmb.2022.103723.
Weng SC, Tsao PN, Shiao SH. Blood glucose promotes dengue virus infection in the mosquito Aedes aegypti. Parasit Vectors. 2021;14:376. https://doi.org/10.1186/s13071-021-04877-1.
Sri-In C, Weng SC, Shiao SH, Tu WC. A simplified method for blood feeding, oral infection, and saliva collection of the dengue vector mosquitoes. PLoS ONE. 2020;15:e0233618. https://doi.org/10.1371/journal.pone.0233618.
Dong S, Balaraman V, Kantor AM, Lin J, Grant DG, Held NL, et al. Chikungunya virus dissemination from the midgut of Aedes aegypti is associated with temporal basal lamina degradation during bloodmeal digestion. PLoS Negl Trop Dis. 2017;11:e0005976. https://doi.org/10.1371/journal.pntd.0005976.
Armstrong PM, Ehrlich HY, Magalhaes T, Miller MR, Conway PJ, Bransfield A, et al. Successive blood meals enhance virus dissemination within mosquitoes and increase transmission potential. Nat Microbiol. 2020;5:239–47. https://doi.org/10.1038/s41564-019-0619-y.
Vogels CBF, Ruckert C, Cavany SM, Perkins TA, Ebel GD, Grubaugh ND. Arbovirus coinfection and co-transmission: a neglected public health concern? PLoS Biol. 2019;17:e3000130. https://doi.org/10.1371/journal.pbio.3000130.
Goertz GP, Vogels CBF, Geertsema C, Koenraadt CJM, Pijlman GP. Mosquito co-infection with Zika and chikungunya virus allows simultaneous transmission without affecting vector competence of Aedes aegypti. PLoS Negl Trop Dis. 2017;11:e0005654. https://doi.org/10.1371/journal.pntd.0005654.
Magalhaes T, Robison A, Young MC, Black WC, Foy BD, Ebel GD, et al. Sequential infection of Aedes aegypti mosquitoes with Chikungunya virus and Zika virus enhances early Zika virus transmission. Insects. 2018;9:4. https://doi.org/10.3390/insects9040177.
Nuckols JT, Huang YJ, Higgs S, Miller AL, Pyles RB, Spratt HM, et al. Evaluation of simultaneous transmission of Chikungunya virus and Dengue virus type 2 in infected Aedes aegypti and Aedes albopictus (Diptera: Culicidae). J Med Entomol. 2015;52:447–51. https://doi.org/10.1093/jme/tjv017.
Dejnirattisai W, Jumnainsong A, Onsirisakul N, Fitton P, Vasanawathana S, Limpitikul W, et al. Cross-reacting antibodies enhance dengue virus infection in humans. Science. 2010;328:745–8. https://doi.org/10.1126/science.1185181.
Pantoja P, Perez-Guzman EX, Rodriguez IV, White LJ, Gonzalez O, Serrano C, et al. Zika virus pathogenesis in rhesus macaques is unaffected by pre-existing immunity to dengue virus. Nat Commun. 2017;8:15674. https://doi.org/10.1038/ncomms15674.
Bardina SV, Bunduc P, Tripathi S, Duehr J, Frere JJ, Brown JA, et al. Enhancement of Zika virus pathogenesis by preexisting antiflavivirus immunity. Science. 2017;356:175–80. https://doi.org/10.1126/science.aal4365.
Senaratne UTN, Murugananthan K, Sirisena P, Carr JM, Noordeen F. Dengue virus co-infections with multiple serotypes do not result in a different clinical outcome compared to mono-infections. Epidemiol Infect. 2020;148:e119. https://doi.org/10.1017/S0950268820000229.
Li CJ, Huang PH, Chen HW, Chang SC. Development and characterization of mouse monoclonal antibodies targeting to distinct epitopes of Zika virus envelope protein for specific detection of Zika virus. Appl Microbiol Biotechnol. 2021;105:4663–73. https://doi.org/10.1007/s00253-021-11364-1.
Bosch I, de Puig H, Hiley M, Carre-Camps M, Perdomo-Celis F, Narvaez CF, et al. Rapid antigen tests for dengue virus serotypes and Zika virus in patient serum. Sci Transl Med. 2017;9:409. https://doi.org/10.1126/scitranslmed.aan1589.
Rockstroh A, Moges B, Barzon L, Sinigaglia A, Palu G, Kumbukgolla W, et al. Specific detection of dengue and Zika virus antibodies using envelope proteins with mutations in the conserved fusion loop. Emerg Microbes Infect. 2017;6:e99. https://doi.org/10.1038/emi.2017.87.
Amraoui F, Failloux AB. Chikungunya: an unexpected emergence in Europe. Curr Opin Virol. 2016;21:146–50. https://doi.org/10.1016/j.coviro.2016.09.014.
Monteiro VVS, Navegantes-Lima KC, de Lemos AB, da Silva GL, de Souza GR, Reis JF, et al. Aedes-Chikungunya virus interaction: key role of vector midguts microbiota and its saliva in the host infection. Front Microbiol. 2019;10:492. https://doi.org/10.3389/fmicb.2019.00492.
Caragata EP, Tikhe CV, Dimopoulos G. Curious entanglements: interactions between mosquitoes, their microbiota, and arboviruses. Curr Opin Virol. 2019;37:26–36. https://doi.org/10.1016/j.coviro.2019.05.005.
Campbell CL, Harrison T, Hess AM, Ebel GD. MicroRNA levels are modulated in Aedes aegypti after exposure to Dengue-2. Insect Mol Biol. 2014;23:132–9. https://doi.org/10.1111/imb.12070.
Bryant B, Macdonald W, Raikhel AS. microRNA miR-275 is indispensable for blood digestion and egg development in the mosquito Aedes aegypti. Proc Natl Acad Sci USA. 2010;107:22391–8. https://doi.org/10.1073/pnas.1016230107.
Eng MW, Clemons A, Hill C, Engel R, Severson DW, Behura SK. Multifaceted functional implications of an endogenously expressed tRNA fragment in the vector mosquito Aedes aegypti. PLoS Negl Trop Dis. 2018;12:e0006186. https://doi.org/10.1371/journal.pntd.0006186.
Pompon J, Manuel M, Ng GK, Wong B, Shan C, Manokaran G, et al. Dengue subgenomic flaviviral RNA disrupts immunity in mosquito salivary glands to increase virus transmission. PLoS Pathog. 2017;13:e1006535. https://doi.org/10.1371/journal.ppat.1006535.
Guzman MG, Halstead SB, Artsob H, Buchy P, Farrar J, Gubler DJ, et al. Dengue: a continuing global threat. Nat Rev Microbiol. 2010;8:S7-16. https://doi.org/10.1038/nrmicro2460.
Cologna R, Armstrong PM, Rico-Hesse R. Selection for virulent dengue viruses occurs in humans and mosquitoes. J Virol. 2005;79:853–9. https://doi.org/10.1128/JVI.79.2.853-859.2005.
King CC, Chao DY, Chien LJ, Chang GJ, Lin TH, Wu YC, et al. Comparative analysis of full genomic sequences among different genotypes of dengue virus type 3. Virol J. 2008;5:63. https://doi.org/10.1186/1743-422X-5-63.
Black WC IV, Bennett KE, Gorrochótegui-Escalante N, et al. Flavivirus susceptibility in Aedes aegypti. Arch Med Res. 2002;33:379–88. https://doi.org/10.1016/s0188-4409(02)00373-9.
Acknowledgements
We thank the Second Core Laboratory of the Department of Medical Research of National Taiwan University Hospital and the National Laboratory Animal Center for technical assistance. This work was supported by the Ministry of Science and Technology (Taiwan) Grant (MOST 109-2327-B-400-004) and (MOST 109-2320-B-002-062-MY3) to SHS, and NTUH UN109-018 and NTUH 112-UN0035 to PNT and SHS. This work was also supported by the National Taiwan University (NTU) Grant 111L2033-09 to SHS.
Funding
This work was supported by the Ministry of Science and Technology (Taiwan) Grant (MOST 109-2327-B-400-004) and (MOST 109-2320-B-002-062-MY3) to SHS, and NTUH UN109-018 and NTUH 112-UN0035 to PNT and SHS. This work was also supported by the National Taiwan University (NTU) Grant 111L2033-09 to SHS.
Author information
Authors and Affiliations
Contributions
DCL, SCW, and SHS designed and performed the experiments, derived experimental models, and analyzed the data. PNT, JJHC, and SHS helped supervise the project. DCL, SCW, and SHS wrote the manuscript in consultation with PNT and JJHC. All authors discussed the results and commented on the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All animal procedures and experimental protocols were approved by an AAALAC-accredited facility and the Committee on the Ethics of Animal Experiments of the National Taiwan University College of Medicine (IACUC approval No. 20200210).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Figure S1. DENV2 and ZIKV single-infected cells are phenotypically distinct. A Cells inoculated with either DENV2 or ZIKV at MOI = 1 were stained for DAPIand flaviviral NS1at 2 dpi. B Cell density imaged under bright field revealed that DENV2-infected cells were less numerous than ZIKV-infected cells. Representative images shown.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lin, D.CD., Weng, SC., Tsao, PN. et al. Co-infection of dengue and Zika viruses mutually enhances viral replication in the mosquito Aedes aegypti. Parasites Vectors 16, 160 (2023). https://doi.org/10.1186/s13071-023-05778-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-023-05778-1