Abstract
Background
The roundworms, Parascaris spp., are important nematode parasites of foals and were historically model organisms in the field of cell biology, leading to many important discoveries. According to karyotype, ascarids in Equus are commonly divided into Parascaris univalens (2n = 2) and Parascaris equorum (2n = 4).
Methods
Here, we performed morphological identification, karyotyping and sequencing of roundworms from three different hosts (horses, zebras and donkeys). Phylogenetic analysis was performed to study the divergence of these ascarids based on cytochrome c oxidase subunit I (COI) and internal transcribed spacer (ITS) sequences.
Results
Karyotyping, performed on eggs recovered from worms of three different Equus hosts in China, showed two different karyotypes (2n = 2 in P. univalens collected from horses and zebras; 2n = 6 in Parascaris sp. collected from donkeys). There are some differences in the terminal part of the spicula between P. univalens (concave) and Parascaris sp. (rounded). Additionally, it was found that the egg’s chitinous layer was significantly thicker in Parascaris sp. (> 5 μm) than P. univalens (< 5 μm) (F(2537) = 1967, P < 0.01). Phylogenetic trees showed that the sequences of Parascaris from Equus hosts were divided into two distinct lineages based on sequences of the COI and ITS.
Conclusions
Comparing the differences in roundworms collected from three different Equus hosts, this study describes a Parascaris species (Parascaris sp.) with six chromosomes in donkeys. It is worth noting that the thickness of the chitinous layer in the Parascaris egg may serve as a diagnostic indicator to distinguish the two roundworms (P. univalens and Parascaris sp.). The Parascaris sp. with six chromosomes in donkeys in the present study may be a species of P. trivalens described in 1934, but the possibility that it is a new Parascaris species cannot be ruled out. Both karyotyping and molecular analysis are necessary to solve the taxonomic problems in Parascaris species.
Similar content being viewed by others
Background
The Equidae are important reservoir hosts for various nematode parasites, some of which can cause significant morbidity or mortality if their hosts are untreated. Equine roundworms are large parasitic nematodes that predominantly infect foals and weanlings. Parascaris have a direct life cycle where infective eggs ingested from the environment hatch in the horse’s stomach. The larvae then penetrate the intestinal mucosa, migrating through the liver and lungs, and eventually return to the small intestine to develop into adults and reproduce [1]. Infected with large numbers of adult worms, the hosts often present with coughing, anorexia, lethal intestinal obstruction or rupture, and even death [2]. Larvae in the migrating stages can also cause hepatitis, pneumonitis and associated respiratory disorders [3].
Two species of roundworms, Parascaris univalens and Parascaris equorum, are found infecting Equus hosts [4,5,6]. These two species cannot be easily distinguished morphologically but differ concerning their karyotype. One pair of chromosomes (2n = 2) is found in P. univalens and two pairs (2n = 4) are found in P. equorum [7]. Additionally, P. trivalens, another rare horse roundworm with three pairs of chromosomes, was described in 1934 and 1937 [8, 9]. However, there have been no reports of this species since 1937.
In 2014, Nielsen et al. karyotyped P. univalens and uploaded a complete mitochondrial genome of the worm to the National Center for Biotechnology Information (NCBI) database. Until now, only Goday et al. have karyotyped P. equorum, collected from horses in the 1980s [7, 11, 12]. However, many studies have shown that P. univalens is more prevalent whereas P. equorum cannot be found in horses using cytological methods [1, 6, 10]. Himmelstjerna et al. (2021) concluded that most P. equorum registered in the NCBI database based solely on cytochrome c oxidase subunit I (COI) and internal transcribed spacer (ITS) sequence analysis without karyotyping were actually derived from P. univalens specimens [13]. For this reason, the results based only on analyzing the sequences of horse roundworms are inadequate for identifying P. univalens and P. equorum at the present time.
Although cytological analysis is a useful method for specific identification, it would be desirable to have available genomic markers for polymerase chain reaction (PCR)-based analyses of genetic variation within Parascaris. Combined with cytological analysis, some researchers have generated mitochondrial genome sequence data for P. univalens to provide a reference sequence for this parasite [3, 6]. Here, we analyzed the morphology, karyotype and genetic characteristics of Parascaris in three Equus host populations of horse (E. caballus), zebra (E. zebra) and donkey (E. asinus) in northern China. We present the first report on the cytological analysis of Parascaris populations in donkeys and show that the roundworms in donkeys were a Parascaris species with six chromosomes.
Methods
Sample collection and morphology identification
The roundworms in the present study were collected from Equus hosts after anthelmintic treatment. Four horse roundworm individuals (h1–h4) were collected from a farm in Harbin, Heilongjiang, China. Twelve roundworms from zebras (z1–z12) were obtained from Harbin Northern Forest Zoo, Heilongjiang, China. Fourteen roundworms (d1–d14) from donkeys were collected from a farm in Liaocheng, Shandong, China. Another 10 roundworms from donkeys (d15–d24, karyotyping of these roundworms could not be carried out due to our poor preservation) were collected from a farm in Chifeng, Inner Mongolia, China (Fig. 1). The roundworm information in this study is presented in Additional file 2: Table S1. All 40 specimens were washed extensively in phosphate-buffered saline (PBS, 37 °C) and transported immediately to the parasitology laboratory. Here, the structure of the head, tail and spicula and the length of the body were observed with an Olympus CX43 microscope (Olympus, Tokyo, Japan) using EPview v 3.2 software (Olympus Scientific Solutions, Tokyo, Japan). Additionally, the 20 female individuals (h1–h3, z1−z8 and d1−d9) were carefully dissected, and the gonads and zygotes were collected. The size (length and width) and the chitinous layer of the eggs (20 eggs of each female individual were chosen) were measured. Then the gonads and remaining male roundworms were stored at −80 °C until further use, and zygotes were stored at 4 °C for the next karyotyping.
Karyotyping
We performed karyotyping on the collected zygotes (20 eggs of each female individual were chosen) for analyzing early embryonic mitotic divisions as described previously [6]. Briefly, the appropriate amount of 0.5 M NaOH, 0.4 M KOH and a mixture of 6% hypochlorite and 0.4 M KOH (17:83) were added sequentially to a tube containing the eggs, and after adding each reagent, resting, resuspension, centrifugation and washing with cold distilled water were necessary for the decortication of the eggs. Then the eggs were washed and resuspended in 0.7% saline solution and incubated at 37 °C until they developed into the first or second embryonic mitotic division under the microscope. Before staining the eggs, the saltwater was sucked out, and the tubes were filled with a mixture of methanol, acetic acid and chloroform (6:3:1) and were left for 1 h. Drops of embryo suspension were deposited on slides and left at room temperature for drying. Finally, staining was carried out with 4′,6-diamidino-2-phenylindole (DAPI) for 10 min and then examined under a fluorescence microscope. Additionally, Giemsa banding staining (G-banding) was applied after the eggs were deshelled, as described previously [16].
Nucleic acid isolation and gene amplification
DNA was extracted from all 40 Parascaris samples using the QIAamp DNA Mini Kit (QIAGEN, Germany) following the manufacturer’s instructions. Partial sequences of the gene of mitochondrial DNA (mtDNA), COI and ITS (including partial ITS1, 5.8 s and ITS2) were amplified to explore the genetic characteristics and phylogenetic relationships. Primer F5 (5′-TCATAAGGATATTGGGACC-3′) and primer F6 (5′-GCAAAATGTAAAGGGAAAA-3′) [17] were applied to amply the COI (996-base pair [bp]) gene of each specimen. Primers NC5 (5′-GTAGGTGAACCTGCGGAAGGATCATT-3′) and NC2 (5′-TTAGTTTCTTTTCCTCCGCT-3′) [18] were applied to amply the ITS (~770 bp) sequence of each specimen. Regarding the two PCR reactions, all the volumes were 25 μl, including 12.5 µl Premix Taq (Ex Taq version 2.0 plus dye, Takara, Japan), 8.5 µl double-distilled water ddH2O), 1 µl of each primer and 2 µl of template DNA under the following conditions: initial denaturation at 94 °C for 5 min, then 35 cycles at 94 °C for 30 s (denaturation); annealing at 50 (COI)/55 °C (ITS) for 30 s, extension at 72 °C for 90 s (COI)/60 s (ITS), followed by a final extension at 72 °C for 7 min. The PCR product was examined on a 1.5% agarose gel to verify that the reactions produced single bands, and then was sent to Comate Biosciences Co., Ltd. (Changchun, China) for Sanger sequencing in the forward and reverse directions.
Phylogenetic relationship and genetic structure
Multiple sequence alignments of nucleotide sequences in this study and sequences available from GenBank were generated using ClustalX v2.0 software. DnaSP v5.10 software was applied to establish the sequence haplotypes of different populations. For COI, the sequences of P. univalens samples in horses and zebras were divided into three haplotypes (CHU1–CHU3); the Parascaris sp. in donkeys in Liaocheng were divided into five haplotypes (CHS1–CHS5); the sequences of samples in donkeys in Chifeng were divided into three haplotypes (CH1–CH3). For ITS, the sequences of P. univalens were divided into two haplotypes (IHU1 and IHU2); the Parascaris sp. were divided into one haplotype (IHS1); the sequences of samples in donkeys in Chifeng were divided into two haplotypes (IH1 and IH2) (Additional file 3: Table S2). For phylogenetic analysis, the representative sequences for each haplotype defined in the present study were used to analyze phylogenetic relationships. The Ascaris suum COI (KY045804) and ITS (KY964445) sequences were retrieved from GenBank and used as outgroups to perform phylogenetic analysis, which was hypothesized using maximum likelihood (ML) and Bayesian inference (BI). The best-fitting nucleotide substitution model was selected using Modeltest 3.7 software with the Akaike information criterion (AIC) [19]. For ML, the best models of COI and ITS sequences were HKY+G and T92, respectively. In addition, phylogenetic analysis was conducted using PhyML 3.0 software [20]. Bootstrap branch support values (MLBS) were obtained with 1000 rapid bootstrap inferences, and thereafter searched through ML search on the dataset. For BI, the best models of COI and ITS sequences were GTR+G and HKY, respectively. Phylogenetic analysis was performed using MrBayes v3.2 software [21]. The parameters were set as follows: nst = 6 (COI sequences)/2 (ITS sequences), rates = propinv (COI sequences)/equal (ITS sequences), with four Markov chain Monte Carlo (MCMC) run for two runs from random starting trees for five million generations, and the trees were sampled every 1000 generations. In addition, 25% of generations were discarded as “burn-in,” and the remaining samples were used to calculate Bayesian posterior probabilities (BPP). Phylograms were plotted using FigTree v1.4.2 software.
Statistical analysis
To investigate the differences between the structural morphology (the size and thickness of the chitinous layer) of the Parascaris eggs from different hosts, the data were first tested for normality using the W-test. Then one-way analysis of variance (ANOVA) in R (v4.0.2) was used to analyze the divergence of the data. The "ggpair" function in R was used to perform correlation analysis between the size and the thickness of the chitinous layer of the eggs. The final result when using analytical signals was the complex Pearson correlation coefficient. The P-value was used to show the significance of the difference. Additionally, the “dplyr” package, “patchwork” package and “ggplot2” package in R were used to visualize the results.
Results
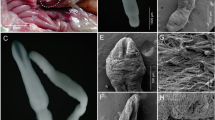
Karyotyping was performed with eggs recovered from worms of three different hosts in China (in total 20 worms). Representative pictures of stained eggs are shown in Fig. 2. The roundworms collected from horses and zebras showed only two large chromosomes and were therefore assigned to the species P. univalens. In comparison, the roundworms (Parascaris sp.) collected from the donkey in Liaocheng showed six large chromosomes. The chromosome breakage occurred during the development of the eggs, and chromatin diminution occurred in pre-somatic cells of Parascaris sp. like A. suum and P. univalens (Fig. 3).
It was found that the spiculae of P. univalens terminate in a truncated and slightly concave form. In contrast, in Parascaris sp., they terminate in a distinctly rounded form (Fig. 2). Additionally, the chitinous layer of Parascaris sp. (> 5 μm) was thicker than that of the P. univalens (< 5 μm, F(2537) = 1967, P < 0.01, Additional file 2: Table S1). Furthermore, the correlation coefficient between the size and the thickness of the chitinous layer of the eggs indicates no correlation (|r|< 0.3, P > 0.05, Additional file 1: Fig. S1). It is worth noting that the eggs in roundworms from the horses and donkeys were significantly larger than those from the zebras (P < 0.01).
The topology of the phylogenetic trees (Fig. 4) obtained from the ML analysis did not conflict with the BI trees. The COI gene tree showed that all five haplotype sequences (CHS1–CHS5) representing 14 worms from donkeys in Liaocheng formed a distinct clade (clade B). Sequences of the worms isolated from the horses, zebras and donkeys in Chifeng were randomly dispersed within clade A. Meanwhile, the ITS tree did not conflict with the COI trees, and all sequences were divided into two branches (clades C and D).
Phylogenetic relationship among Parascaris spp. based on the COI (a) and ITS (b) sequences using maximum likelihood (ML) and Bayesian inference (BI). Values higher than 50 are displayed on the trees. Bold indicates the sequence of this study. For COI, the sequences of P. univalens samples in horses and zebras were divided into three haplotypes (CHU1–CHU3); the Parascaris sp. in donkeys in Liaocheng were divided into five haplotypes (CHS1–CHS5); the sequences of samples in donkeys in Chifeng were divided into three haplotypes (CH1–CH3). For ITS, the sequences of P. univalens were divided into two haplotypes (IHU1 and IHU2); the Parascaris sp. were divided into one haplotype (IHS1); the sequences of samples in donkeys in Chifeng were divided into two haplotypes (IH1 and IH2)
The roundworms of d1−d14 and d15−d24 were isolated from the donkeys, but they were split into two different clades (Additional file 3: Table S2, Fig. 4), which indicated that there may be more than one species of roundworm in donkeys (P. univalens and Parascaris sp.).
Discussion
In previous work, we performed phylogenetic analysis without karyotyping of roundworms from horses, zebras and donkeys [5]. In this study, the karyotyping of eggs from different stages (1–4-cell stages) of Parascaris sp. showed that the pre-somatic cells underwent chromosome breaks, while the germline cell maintained intact chromosome morphology, consistent with the P. univalens studied by Müller et al. [22].
The only morphological identification study was performed more than 40 years ago by Biocca et al. [23], who found weak morphological differences in the terminal part of the spicula of the two species P. univalens and P. equorum. In this study, we also found differences in the terminal part of the spiculae in P. univalens (concave) and Parascaris sp. (rounded). Additionally, it was found that the egg’s chitinous layer of Parascaris sp. (> 5 μm) was significantly thicker than in P. univalens (< 5 μm) (F(2537) = 1967, P < 0.01). The thickness of the chitinous layer and the size of the eggs were not correlated (|r|< 0.3, P > 0.05, Additional file 1: Fig. S1). Therefore, the thickness of the chitinous layer of eggs may serve as a diagnostic indicator to distinguish these two Parascaris species.
The phylograms showed the relations based on COI (Fig. 4a) and ITS (Fig. 4b), which revealed very close relationships between most of the sequences, regardless of whether they were deposited in GenBank as P. univalens or P. equorum in clades A and C. However, Parascaris sp. was a mono group in clades B and D. The phylogenetic analysis based on these sequences confirmed the karyotype identification results indicating that the worms from the donkeys in Liaocheng were not P. univalens. This study also suggests that some of nucleotide sequences deposited as P. equorum in GenBank were actually derived from P. univalens specimens as Samson-Himmelstjerna has stated [13].
The results of the haplotype information (Additional file 3: Table S2) and phylogenetic tree (Fig. 4) showed that donkeys could be infected not only by Parascaris sp. but also by another species, which may be P. univalens. Unfortunately, karyotyping of the suspected P. univalens in the donkey could not be carried out due to our poor preservation. Further study is necessary to investigate whether donkeys can be infected with different Parascaris species and whether they are reproductively isolated.
Parascaris trivalens with three pairs of chromosomes collected from horses was first described in 1934 [9]. The roundworm Parascaris sp. found in the donkey in the present study also has three pairs of chromosomes and could be recognized as P. trivalens. However, P. trivalens was found in horses nearly a century ago. There was no molecular biology information and only some hand-drawn figures of karyotypes without chromosome breaks [8, 9]. Therefore, the Parascaris sp. with six chromosomes in the donkey in the present study may be the species of P. trivalens described in 1934, but the possibility that it is a new Parascaris species cannot be ruled out.
Li posited that the P. trivalens he studied may be a six-chromosome polyploidy, the P. equorum is a tetraploid, and the P. univalens is amphiploid [9]. There were two possible rationales for this case. One was that higher polyploidy series were derived from lower ones by duplication; another way to explain the origin of the various types of Parascaris was by eliminating pairs of chromosomes, and the P. trivalens was the most primitive form [8, 9]. The P. trivalens has not been described in the literature since 1937, and karyotype identification of P. equorum with certainty in the horse has been absent since 1989 [24]. Meanwhile, P. univalens with two chromosomes have been recorded continuously [6]. If P. trivalens is a hexaploid and P. equorum is a tetraploid, why has P. trivalens not been recorded for nearly a century and why has P. equorum been absent since 1989 after it was karyotyped? If the P. trivalens and P. equorum were different Parascaris species and not the polyploidy of P. univalens, had they become extinct as endangered species in history? These phenomena deserve further exploration in the future.
Conclusions
This study is the first report to describe a Parascaris species with six chromosomes in donkeys. It is worth noting that the thickness of the chitinous layer of the Parascaris egg may serve as a diagnostic indicator to distinguish the two ascarids (P. univalens and Parascaris sp.). The Parascaris sp. with six chromosomes in donkeys in the present study may be the species of P. trivalens described in 1934, but the possibility that it is a new Parascaris species cannot be ruled out.
Availability of data and materials
The data that support the findings of this study are openly available in GenBank numbers OP745976, OP745979, OQ517636, OP745988, OP745989, OP745991, OQ517637 OP745984, OQ628067, OQ628069, OQ628068 (COI) and OP747659, OP747663, OP747671, OP747667, OP747668 (ITS).
Abbreviations
- DAPI:
-
4′,6-Diamidino-2-phenylindole
- G-banding:
-
Giemsa banding staining
References
Martin F, Höglund J, Bergström TF, Karlsson Lindsjö O, Tydén E. Resistance to pyrantel embonate and efficacy of fenbendazole in Parascaris univalens on Swedish stud farms. Vet Parasitol. 2018;264:69–73.
Morsy K, Bashtar AR, Al Quraishy S, Adel S. Description of two equine nematodes, Parascaris equorum Goeze 1782 and Habronema microstoma Schneider 1866 from the domestic horse Equus ferus caballus (Famisly: Equidae) in Egypt. Parasitol Res. 2016;115:4299–306.
Jabbar A, Littlewood DT, Mohandas N, Briscoe AG, Foster PG, Müller F, et al. The mitochondrial genome of Parascaris univalens–implications for a “forgotten” parasite. Parasit Vectors. 2014;7:428.
Han L, Lan T, Lu Y, Zhou M, Li H, Lu H, et al. Equus roundworms (Parascaris univalens) are undergoing rapid divergence while genes involved in metabolic as well as anthelminic resistance are under positive selection. BMC Genomics. 2022;23:489.
Peng Z, Shen D, Zhang D, Li X, Wang L, Zhai Q, et al. Genetic characteristics and phylogenetic relationship of Parascaris spp. from Equus zebra, E. caballus, and E. asinus. Vet Parasitol. 2019;271:76–9.
Nielsen MK, Wang J, Davis R, Bellaw JL, Lyons ET, Lear TL, et al. Parascaris univalens–a victim of large-scale misidentification? Parasitol Res. 2014;113:4485–90.
Goday C, Ciofi-Luzzatto A, Pimpinelli S. Centromere ultrastructure in germ-line chromosomes of Parascaris. Chromosoma. 1985;91:121–5.
Li JC. A six-chromosome ascaris in Chinese horses. Science. 1937;86:101–2.
Li JC. A six-chromosome ascaris found in Chinese horses. Peking Nat Hist Bull. 1934;9:131–2.
Bullini L, Nascetti G, Carrè S, Rumore F, Biocca E. Ricerche cariologiche ed elettroforetiche su Parascaris univalens e Parascaris equorum. 1978;1:151–6.
Goday C, Pimpinelli S. Cytological analysis of chromosomes in the two species Parascaris univalens and P. equorum. Chromosoma. 1986;94:1–10.
Goday C, Pimpinelli S. Chromosome organization and heterochromatin elimination in Parascaris. Science. 1984;224:411–3.
von Samson-Himmelstjerna G, Janssen IJI, Ramünke S, Goday C, Borges FA, Koudela B, et al. Very low intraspecific sequence variation in selected nuclear and mitochondrial Parascaris univalens genes. Infect Genet Evol. 2021;95:105035.
Gao JF, Zhang XX, Wang XX, Li Q, Li Y, Xu WW, et al. According to mitochondrial DNA evidence, Parascaris equorum and Parascaris univalens may represent the same species. J Helminthol. 2019;93:383–8.
Tydén E, Engström A, Morrison DA, Höglund J. Sequencing of the β-tubulin genes in the ascarid nematodes Parascaris equorum and Ascaridia galli. Mol Biochem Parasitol. 2013;190:38–43.
Liu J, Zhang L, Wang G. Cytogenetic studies on Ovomermis sinensis. Plant Prot. 2013;39 02:109-11+27 (In Chinese).
Franssen F, Xie K, Sprong H, van der Giessen J. Molecular analysis of Baylisascaris columnaris revealed mitochondrial and nuclear polymorphisms. Parasit Vectors. 2013;6:124.
Zhu XQ, D’Amelio S, Palm HW, Paggi L, George-Nascimento M, Gasser RB. SSCP-based identification of members within the Pseudoterranova decipiens complex (Nematoda: Ascaridoidea: Anisakidae) using genetic markers in the internal transcribed spacers of ribosomal DNA. Parasitology. 2002;124:615–23.
Posada D, Crandall KA. MODELTEST: testing the model of DNA substitution. Bioinformatics. 1998;14:817–8.
Guindon S, Delsuc F, Dufayard JF, Gascuel O. Estimating maximum likelihood phylogenies with PhyML. Methods Mol Biol. 2009;537:113–37.
Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–4.
Müller F, Tobler H. Chromatin diminution in the parasitic nematodes Ascaris suum and Parascaris univalens. Int J Parasitol. 2000;30:391–9.
Biocca E, Nascett G, Iori A, Costantini R, Bullini L. Descrizione di Parascaris univalens, parassita degli equini, e suo differenziamento da Parascaris equorum. Atti della Accademia Nazionale dei Lincei Classe di Scienze Fisiche, Matematiche e Naturali Rendiconti. 1978;65:133–40.
Pimpinelli S, Goday C. Unusual kinetochores and chromatin diminution in Parascaris. Trends Genet. 1989;5:310–5.
Acknowledgements
Thanks to Tianlu Liu for improving the syntax and grammar throughout the manuscript.
Funding
This work was funded by the Fundamental Research Funds for the Central Universities of China (Grant No. 2572022AW17).
Author information
Authors and Affiliations
Contributions
MCZ, YXL, LH, QL and ZJH designed the project; acquired, analyzed, interpreted the results and wrote the manuscript, with contributions from the other authors. MLL, HTL and DHC contributed to experimental design and performed the experiments. HJL, YLY, LZ and LHT contributed to the design and collected the samples. CYG and JY collected the samples. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
We ensure that our practices conform with Chinese animal ethics guidelines. All experimental designs and animal handling were approved by the Institutional Animal Care and Use Committee of Northeast Forestry University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Correlation analysis of the size and chitinous layer thickness of eggs in different populations of Parascaris spp. a Roundworms from horse. b Roundworms from zebra. c Roundworms from donkey.
Additional file 2: Table S1.
The morphological information of samples in this study.
Additional file 3: Table S2.
The molecular information of samples in this study and sequences in GenBank.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhou, M., Lu, Y., Han, L. et al. Exploration of Parascaris species in three different Equus populations in China. Parasites Vectors 16, 202 (2023). https://doi.org/10.1186/s13071-023-05768-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-023-05768-3