Abstract
Background
A total of 290 mosquito species are recorded in Cambodia among which 43 are known vectors of pathogens. As Cambodia is heavily affected by deforestation, a potential change in the dynamic of vector-borne diseases (VDBs) could occur through alteration of the diversity and density of sylvatic vector mosquitoes and induce an increase in their interactions with humans. Understanding mosquito diversity is therefore critical, providing valuable data for risk assessments concerning the (re)emergence of local VBDs. Consequently, this study mainly aimed to understand the spatial and temporal distribution of sylvatic mosquito populations of Cambodia by determining which factors impact on their relative abundance and presence.
Methods
A study was conducted in 12 sites from four forests in Cambodia. All mosquitoes, collected during the dry and rainy seasons, were morphologically identified. The diversity and relative density of mosquito species in each site were calculated along with the influence of meteorological and geographical factors using a quasi-Poisson generalized linear model.
Results
A total of 9392 mosquitoes were collected belonging to 13 genera and 85 species. The most represented genera were Culex, accounting for 46% of collected mosquitoes, and Aedes (42%). Besides being the most abundant species, Culex pseudovishnui and Aedes albopictus, which are known vectors of numerous arboviruses, were present in all sites during both dry and rainy seasons. The presence of mosquito species reported to be zoo-anthropophilic feeders was also observed in both forested and urban areas. Finally, this study demonstrated that altitude, temperature and precipitation impacted the abundance of mosquitoes but also influenced species community composition.
Conclusion
The results indicate an important diversity of mosquitoes in the four forests and an influence of meteorological and geographical factors on their community. Additionally, this work highlights in parallel the abundance of species considered to be of medical importance and therefore underlines the high risk of pathogen emergence/re-emergence in the region.
Graphical Abstract

Similar content being viewed by others
Background
Vector-borne diseases (VBDs) are a major public health problem worldwide. In 2020, the World Health Organization estimated that they account for > 17% of all infectious diseases worldwide and are responsible for > 700,000 deaths per year, overburdening health systems mainly in the tropical and subtropical areas [1, 2].
Cambodia is affected by VBDs where dengue fever and Japanese encephalitis (JE) are endemic [3, 4]. Specifically, Cambodia has one of the highest dengue infection rates in Southeast Asia, with an average of 103 cases per 10,000 population and a case fatality rate of 1 to 2% since 2000 [5]. Beyond the public health issue it represents, dengue fever is also responsible for a heavy societal burden in Cambodia with a significant cost of illness [6]. JE is the main cause of central nervous system infections leading to encephalitis and other serious clinical complications in Cambodian children [4]. In 2007, the estimated incidence of clinically reported JE in the country was 11.1 cases per 100,000 children < 15 years of age [4]. A recent resurgence of chikungunya was also recorded in the country in 2011, breaking out in the village of Trapeang Roka (Kampong Speu Province) in 2012 [7]; later, in 2020, a nationwide outbreak occurred [8]. Additionally, silent circulation of Zika fever was confirmed in Cambodia [9], and malaria still occurs, accounting for 13.4% of cases in the Southeast Asia region in 2020 [10]. These VBDs are caused by pathogens, namely dengue, Japanese encephalitis, chikungunya and Zika viruses (DENV, JEV, CHIKV and ZIKV, respectively) and Plasmodium, which are transmitted to humans through the bite of vector mosquitoes. To date, 43 confirmed vector species of pathogens have been recorded in Cambodia [11].
Land use change, such as deforestation and urbanization, heavily affects Cambodia: the country lost 65% of its forest coverage from 2006 to 2016 [12,13,14]. This alteration could modify the dynamic of VBDs by potentially changing mosquito communities and abundance. Indeed, several meta-analyses, combining data from different countries, have highlighted that mosquito species can be affected by deforestation, in some cases leading to an increase in their abundance, especially for species associated with VBDs [15, 16]. This potential increase in the abundance of vector mosquitoes directly impacts their vectorial capacity (i.e. the efficiency of the transmission in a specific vector-host relationship in a given environment [17]) and could be multifactorial. This may result from the creation of breeding habitats more favorable for the immature stages [18] or the enhancement of mosquito survival and reproduction due to deforestation-induced microclimate modification [19, 20]. Moreover, deforestation can also result in increased human interaction with wildlife [21] and consequently the likelihood of human-vector contact and (re)emergence of pathogens [22].
In this context, describing the mosquito diversity and relative abundance in Cambodian forests is essential for VBD risk assessments and public health recommendations. Different works have already explored the mosquito fauna in forested areas of Cambodia. Recent studies conducted in the bird sanctuary in Prek Toal flooded forest in Battambang Province and the mangrove forest in Koh Kong have overviewed the overall Culicidae fauna [22, 23]; other works focused only on Anopheles mosquitoes. The first study of Anopheles in Cambodia dates back to 1964 in two villages and the surrounded forests of Pailin Province [24]. Other studies have provided insights into the Anophelinae fauna in different forests or villages inside the forests (or surrounded by forests), sometimes through vector control studies or the evaluation of Anopheles capture methods [25,26,27,28,29,30]. The sites surveyed during these studies were located in Mondulkiri, Pailin, Preah Vihear, Pursat and Ratanak Kiri Provinces.
The extension of these studies to other forests in Cambodia and to the entire Culicidae fauna is strongly recommended to better characterize sylvatic mosquito species. Therefore, the main objective of this work was to examine the spatio-temporal distribution of mosquitoes in Cambodian forests including species vectors of pathogens. The secondary objective was to determine the meteorological and geographical variables that could explain their relative abundance and presence.
Methods
Study sites
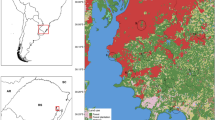
The study was conducted in four different forests located in Kampong Speu, Preah Vihear, Ratanak Kiri and Siemreap (Fig. 1).
Sampling forests were selected to represent different protected areas in the north, northeast and south of Cambodia. These were the forests for which approval from the Cambodian authorities was obtained. Three sites per forest were surveyed corresponding respectively to the depth, middle and edge of the forest. The description of each site is presented in Table 1.
Mosquito sampling and morphological identification
Mosquito sampling in these selected sites was carried out between March 2020 and January 2021. Two field missions were conducted in each forest, one during the dry season and one during the rainy season (with the exception of Kampong Speu where the two missions were conducted during the rainy season for logistical reasons).
Two types of traps were used to collect adult mosquitoes: BG-1 Sentinel™ Mosquito Traps, 7.5–12 volts baited with BG-Lure® (BioQuip, Rancho Dominguez, CA, USA) and CDC Mini Light Traps (BioQuip) with incandescent light. Dry ice was placed in a dry ice dispenser next to each trap. For each mission, these traps were set for 3 consecutive days per site and harvested every 24 h.
Collected mosquitoes were subsequently killed humanely using carbon dioxide. These were morphologically identified by using available identification keys [31,32,33,34].
Meteorological and geographical data
Meteorological data were obtained from app.climateengine.com (accessed on 15 June 2022). The temperature was extracted from CFSR satellite data (19.2 km/28.28 km, daily) and precipitation from CHIRPS satellite data (4.8 km, daily). The meteorological conditions (that could impact the mosquito community) during the year of collection did not differ from those of the previous years (Fig. 2). Moreover, the altitude values at each global positioning system (GPS) data point were obtained directly with Google Earth Pro (version 7.3.6.9345).
Meteorological conditions in the four forests during the year of collection and 2 years before (2018, 2019 and 2020). a Meteorological conditions in Kampong Speu Forest. b Meteorological conditions in Ratanak Kiri Forest. c Meteorological conditions in Siemreap Forest. d Meteorological conditions in Siemreap Forest. Temperatures (red line) were gathered from CFSR satellite data (19.2 km/28.28 km, daily) and precipitation (blue bars) from CHIRPS satellite data (4.8 km, daily)
Data analysis
All the data analyses were performed using R software [35]. First, to assess the composition of the mosquito community in each site, three indices were computed: Shannon’s diversity index (H′) quantifying the species diversity, Simpson index (D) measuring the species dominance and Pielou’s evenness index (Jʹ) calculating whether species are distributed evenly. The equations of these indices are shown here:
Shannon diversity index
where “ni” is the number of specimens belonging to one species and “N” is the total number of specimens from all species in the site.
Simpson index
where Pi is the proportion of specimens belonging to a species and calculated by dividing “ni” by “N.”
Pielou’s evenness index
where “H” is the Shannon diversity index and “H’max” the maximum possible value of H’ if every species is equally likely.
A non-parametric Wilcoxon test was carried out to compare the relative abundance of mosquitoes during the dry and rainy seasons. Then, the correlation between the relative abundance of mosquito species in the different sites was also computed with Pearson tests. Only the species whose number was ≥ 5 was included in the analysis.
Finally, the relationship between mosquito relative abundance and geographical and meteorological factors was evaluated. The meteorological factors (i.e. temperature and precipitations) were classified with a time lag of 1 to 4 weeks prior to collection. One of the distributions commonly used to model count data is the Poisson distribution. However, due to a significant overdispersion of the residuals of the Poisson model, a quasi-Poisson generalized linear model was applied. The collinearity between the different variables was also tested to avoid combining highly correlated variables. An abundance model was performed for all mosquito species whose number was ≥ 40 while a presence model was carried out for mosquito species whose number was < 40. The relative risks (RRs) and 95% confidence intervals (IC95) were calculated to quantify the influence of these factors on relative abundance. The statistical significance threshold for all tests was set at 0.05.
Results
Mosquito diversity and relative abundance
Overall results
A total of 9392 mosquitoes were collected representing 85 species belonging to 13 genera (Table 2). The genera collected were Aedes (17 species), Culex (16 species), Uranotaenia (13 species), Anopheles (11 species), Armigeres (10 species), Heizmannia (5 species), Mansonia, Mimomyia and Tripteroides (3 species each), and Aedeomyia, Coquillettidia, Lutzia and Toxorhynchites (1 species each).
The Culex genus was the most abundant one, accounting for 45.58% (n = 4281) of the total collected mosquitoes, followed by Aedes with 42.01% (n = 3946) of our collection. The third most abundant genus was Uranotaenia accounting for 4.45% (n = 418), while Armigeres and Heizmannia represented 3.49% (n = 328) and 2.40% (n = 225), respectively. In our study, the genus Anopheles represented only 0.7% (n = 66) of the collected specimens.
Overall, two dominant species were observed: Culex pseudovishnui, accounting for 25.11% (n = 2358) of mosquitoes, followed by Ae. albopictus (n = 1394; 14.84%). A total of 21 species are reported to be of medical importance (n = 4132; 43.99%).
Results per forest and per site
With a total of 3130 mosquitoes belonging to 46 species, the Kampong Speu Forest displayed the largest diversity of mosquitoes. In contrast, the forest in Preah Vihear had only a total of 709 mosquitoes belonging to 17 species (Table 2).
Nine mosquito species were common in the four forests, namely (ranked by abundance) Cx. pseudovishnui, Ae. albopictus, Culex brevipalpis, Aedes gardneri imitator, Armigeres subalbatus, Cx. nigropunctatus, Cx. bitaeniorhynchus, Coquillettidia crassipes and Ae. ibis (Table 2). Two of them, Ae. albopictus and Cx. pseudovishnuii, were present in all sites (Additional file 1: Table S1) regardless of the season (Table 2). Two species, Ar. subalbatus and Ae. gardneri imitator, were collected in 11 of the 12 sites in the four forests independently of the season (Additional file 1: Table S1).
In addition, 12 other species common to the forested and anthropized areas (rural village or ranger station) were found. These were Aedes albolineatus, Ae. desmotes, Ae. gardneri imitator, Armigeres annulitaris, Ar. kesseli, Ar. subalbatus, Cq. crassipes, Culex bitaeniorhynchus, Cx. brevipalpis, Cx. nigropunctatus, Cx. quinquefasciatus and Uranotaenia macfarlanei (Additional file 1: Table S1). Thirty-nine other species were only found at a single site of a single forest (Additional file 1: Table S1).
Also, a strong positive correlation between the relative abundance of different species due to their co-occurrence in the same site was observed (Additional files 2 and 3: Tables S2 and S3, Fig. 3). This was the case for Mansonia uniformis and Cx. brevipalpis mostly collected in site 3 of Siemreap Forest but also for Ae. gardneri imitator, Culex gelidus, Mansonia indiana, Uranotaenia longirostris, Ur. metatarsata, Ur. bimaculiala and Mimomyia hybrida mostly found in site 1 of Siemreap Forest. Aedes aegypti and Cx. quinquefasciatus were mainly collected in site 3 of Kampong Speu Forest while Ae. albolineatus and Cq. crassipes were mainly present across the three sites of Kampong Speu. Six other species, Ae. desmotes, Ar. annulitarsis, Culex bitaeniorhynchus, Cx. cinctellus, Cx. fraudatrix and Uranotaenia koli, were mostly found in sites 1 and/or 2 of the Kampong Speu Forest. Aedes ibis, Ae. ostentatio, Ar. subalbatus, Cx. pseudovishnui, Heizmanni demeilloni, Uranotaenia bicolor and Ur. macfarlanei were mainly present in sites 1 and/or 2 of Ratanak Kiri Forest. Finally, Aedes eldridgei and Culex mimulus were mostly collected in site 1 of Kampong Speu Forest.
Correlation matrix representing Pearson correlation between the relative abundance of species in each site. The size of the circle and color intensity are relative to the correlation coefficients (the values of correlations coefficient are presented in the Additional file 2: Table S2). Negative correlations are shown in red and positive correlations in blue. On the right, the legend shows the corresponding colors and the correlation coefficients. The different boxes represent the different study sites
Seasonal relative abundance and diversity of mosquitoes
The relative abundance of Culicidae increased significantly during the rainy season compared to the dry season in Preah Vihear (Wilcoxon test, P = 0.001) and Siemreap (Wilcoxon test, P = 2.5 × 10–05). Moreover, the number of mosquito species also increased significantly in these two forests during the rainy season. It went from 12 to 17 species in Preah Vihear (Wilcoxon test, P = 0.007) and from 18 to 33 in Siemreap Forest (Wilcoxon test, P = 0.005).
A change in Shannon, Simpson and Pielou’s evenness indices between the dry and rainy seasons was highlighted. A decrease of Shannon index was observed during the dry season in Preah Vihear and Siemreap (Table 2). In contrast, in Ratanak Kiri, a decrease of Shannon and Pielou’s indices was observed during the rainy season with a dominance of Cx. pseudovishnui.
Impact of meteorological and geographical factors on the relative abundance of mosquito species
Altitude, ranging from 75 to 401 m above sea level, showed mainly a slight positive impact on the relative abundance and presence of mosquito species (Table 3 and Additional file 4: Table S4). Specifically, a positive correlation between altitude and the abundance of five Aedes species (Ae. albolineatus, Ae. albopictus, Ae. desmotes, Ae. eldridgei, Ae. gardneri) was found as well as the abundance of Cq. crassipes and three Culex species (Cx. bitaeniorhynchus, Cx. cinctellus, Cx. fraudatrix). In contrast, a negative correlation between the altitude and the relative abundance of Ar. subalbatus andCx. brevipalpis was observed. Moreover, the presence of Ma. uniformis was negatively impacted by altitude while this factor impacted positively on the presence of Ur. koli (Additional file 4: Table S4).
The study also demonstrated that the precipitation impacted the relative abundance and presence of mosquitoes mainly positively (Table 3 and Additional file 4: Table S4). The average precipitation in the first week prior to the collection impacted the abundance of Hz. demeilloni positively and the presence of Ur. longirostris negatively. The average precipitation in the second week prior to the sampling also impacted the relative abundance of Ae. albolineatus, Ae. gardneri, Ar. annulitarsis and Cx. bitaeniorhynchus and the presence of Ur. koli positively. The average precipitation in the third week before the mosquito collection impacted the relative abundance of Ae. eldridgei, Ar. subalbatus and Cx. pseudovishnui and the presence of Ae. ibis positively as well. The average precipitation in the third week prior to the collection had a significant negative impact on the relative abundance of Cx. fraudatrix. Similarly, the average precipitation in the fourth week before the collection impacted the presence of Ae. aegypti negatively.
In contrast, our results demonstrated that for all the time lags, the temperature mainly impacted the relative abundance and presence of mosquitoes negatively (Table 3 and Additional file 4: Table S4). The average temperature in the first week before the sampling impacted the abundance of Ae. eldridgei, Ar. annulitarsis and Cx. bitaeniorhynchus negatively. The average temperature during the second week before the collection impacted the abundance of Ar. subalbatus and Cx. fraudatrix negatively and the presence of Ae. aegypti positively. The temperature in the third week before the collection impacted the relative abundance of Ae. albolineatus and Cx. cinctellus negatively and the abundance of Cx. brevipalpis positively. Finally, the temperature in the fourth week before the collection had a significant positive impact on the relative abundance of Ae. albopictus and Ae. gardneri and on the presence of Ma. uniformis and Ur. longirostris.
Discussion
The overall mosquito fauna in the four forests was quite diverse but the relative abundance showed a dominance of Culex mosquitoes. The same result has been observed in other Cambodian forests [22, 23] and also in different urban, peri-urban and rural areas of Cambodia [36,37,38]. However, the dominant Culex species changed according to the biotope. In our study, Cx. pseudovishnui was mainly the dominant species regardless of the type of forest. Little is known about the biology of Cx. pseudovishnui [33, 39, 40]. During a previous study, it was found to be more abundant during the rainy season [40], which was confirmed by our study, except in Siemreap Forest where this species was surprisingly more abundant during the dry season. Culex cinctellus was the most abundant Culex species in the bamboo forests of Kampong Speu where the traps were set next to a stream. Interestingly, at this site, this species was only collected in November. These observations confirm the previously described breeding habitat and seasonality of Cx. cinctellus reported in Thailand, where mosquitoes have been collected in a bamboo forest on October and November [39]. Culex quinquefasciatus was the most dominant Culex species in the rural village of Kampong Speu; it is a common species in rural and urban areas [41, 42]. Moreover, Cx. brevipalpis was the dominant Culex species in the zoo of Siemreap Forest. According to the literature, this species is able to colonize different breeding habitats, and humans are not the usual hosts [33, 39].
Aedes was the second most abundant mosquitoe in our study. This genus is the second most diversified in Cambodia [11] but its relative abundance has been found to be less important in other forests [22, 23] and in anthropized areas of Cambodia [36,37,38]. Aedes albopictus was the most abundant Aedes species in this work and was present in all the sites. Its presence and abundance could be explained by its sylvatic origin in the tropical forest areas of Southeast Asia [43] and its preference for shaded areas [44]. In the rural village of Kampong Speu, however, Ae. albolineatus took the lead over Ae. albopictus in terms of relative abundance. This species was only collected in Kampong Speu and only in November. Little is known regarding its biology and behavior. It seems that the coconut husks and small tree holes are the main breeding habitat of this species [45].
Uranotaenia was the third most abundant mosquitoe in our study and was also quite diverse, reaching half of Uranotaenia species currently recorded in Cambodia [11], which is not surprising since this genus is common in forests. Among them, Uranotaenia macfarlanei was the most abundant Uranotaenia species. This species was mostly collected in the semi-evergreen forest of Ratanak Kiri during the dry season. According to the literature, this mosquito lay eggs in small pools of dirty water and can be found at about 900 m above sea level [11, 46], but in our study, the adults were collected between 110 and 300 m above sea level. They are known to mainly feed on frogs, and their vectorial status is still unknown [47].
Our Armigeres collection was also quite diverse, with 10 of the 26 Armigeres species currently present in Cambodia [11]. Interestingly, Ar. subalbatus species was present in almost all the sites and was the dominant species in the ranger station in Ratanak Kiri Forest. This species is known to be ecologically flexible and can be commonly found in rural and peri-urban/urban habitats as well [44]. Larvae of this species are found in different container habitats containing nutrient-rich and polluted water, mostly in banana stumps in Cambodia [11].
Heizmannia was the fifth most abundant genus in our study, reaching half of the current Heizmannia species of Cambodia [11]. Little is known about the biology of Heizmannia. Apparently, females of these mosquitoes mainly lay their eggs in tree holes and bamboo, are active during daytime in forests and readily bite humans. Heizmannia demeilloni was the most abundant Heizmannia species, which was mostly found in in the semi-evergreen forest of Ratanak Kiri and mainly collected during the rainy season. This species is known to breed in bamboo stumps [48].
The mosquitoes belonging to other genera like Aedeomyia, Anopheles, Coquillettidia, Lutzia, Mansonia, Mimomyia, Tripteroides and Toxorhynchites were scarce in our forests, accounting for only 1.96% (n = 184) of our collections. The scarcity of Anopheles was particularly surprising given that previous studies have highlighted high diversity and abundance of these mosquitoes in Cambodian forests, including Preah Vihear and Ratanak Kiri [29, 30]. However, during these previous studies, human- and cow-baited traps were chosen, which might be more efficient for Anopheles sampling than the type of traps used during our work.
This study also provided predictive relationships between abiotic factors and mosquito abundance for a wide range of species including some uncommon or poorly studied ones. The results clearly demonstrated that when the relative abundance of mosquitoes was positively impacted by altitude it was mainly negatively related to temperature at a species-specific time lag. This could be explained by the fact that temperature generally decreases with altitude [49]. The result of our model combined with the observations from previous studies [49, 50] indicate that lowlands are more suitable for Ar. subalbatus occurrence and abundance. Regarding Ae. albopictus abundance, the highlands were more suitable, while it was positively impacted by the temperature during the fourth week before collection. This might be explained by the ability of this species to adapt to various ranges of temperature [51].
Additionally, these abiotic factors have been highlighted as important parameters determining the community composition of mosquito species. For instance, the co-occurrence of Ae. albolineatus, Ar. annulitarsis and Cx. bitaeniorhynchus in the two semi-evergreen forests of Kampong Speu could be explained by the fact that their relative abundance was positively correlated with the altitude and average temperature in the second week prior to the collection. Previous studies demonstrated that mosquito community composition is strongly influenced by landscape [52, 53]. In our case, for logistical reasons, a better characterization of our study site has not been made. This should be undertaken in the future to assess this impact of environmental factors on the mosquito community in these forests.
The forests investigated in this study are located in protected areas of Cambodia. Despite this, many forest-goers rely on timber and non-timber forest products, increasing the deforestation rate, yet to be efficiently regulated in Cambodia. The presence of mosquito species well adapted to living in close vicinity to humans and human settlements indicates the presence of human activities in these areas. The collected mosquitoes that could be indicators of anthropization were Ae. aegypti and Cx. quinquefasciatus, two domestic mosquito species well adapted to the human environment [42, 54, 55]. The presence of Anopheles campestris and An. baimaii, whose females are highly antropophilic [56, 57], could also be evidence of human activities in Kampong Speu where these species were only found. Finally, Cx. gelidus, which feed on large domestic animals [11, 39], and Mansonia annulifera, a highly anthropophilic mosquito biting mainly inside habitations [41], could also be an indicator of anthropization in Siemreap Forest. Surprisingly, despite the human activities observed in Preah Vihear Forest, the mosquito species collected in the three sites were likely either mainly zoophilic or opportunistic. The same finding was observed in Ratanak Kiri Forest.
Our study highlighted a high risk of pathogen emergence/re-emergence in our sites due to the presence of mosquito species of medical importance in these areas. One of the most important features is the abundance of Cx. pseudovishnui, a potential vector of JEV [58, 59], which was present in all the sites regardless of the season. Other species collected in this study, Ae. albopictus, Aedes vexans, Ar. subalbatus, Cx. bitaeniorhynchus, Culex fuscocephala, Cx. gelidus, Cx. quinquefasciatus, Cx. sitiens, Ma. annulifera, Ma. indiana and Ma. uniformis, are also reported to be confirmed or potential vectors of JEV [59,60,61,62,63,64,65,66] and can also transmit other pathogens. For instance, Ae. albopictus, the second most abundant mosquito in this work, could transmit several other arboviruses including CHIKV, DENV and ZIKV [67, 68]. This species was also present across the different sites independently of the season. Armigeres subalbatus, which was present in almost all sites, is a potential vector of ZIKV [69] and is implicated in the transmission of filaria [70]. Aedes vexans, Cx. quinquefasciatus and Ma. uniformis could transmit different arboviruses including the Rift Valley fever virus [71]. Also, Ae. aegypti is a vector of several pathogens [72] and is considered a major vector of DENV [73]. Finally, seven Anopheles species, namely An. barbirostris, An. campestris, An. karwari, An. maculatus, An. minimus, An. nivipes and An. philippinensis, are reported to be vectors of Plasmodium [11, 30]. Furthermore, due to their presence in both forested and rural areas in our study and their zoo-anthropogenic behavior [74,75,76,77], Ae. albopictus, Ar. subalbatus, Cx. pseudovishnui and Cx. quinquefasciatus could potentially act as bridge vectors for new emerging pathogens.
The main limitations of the present study are that, for logistical reasons, each site was visited only two times and only two kinds of traps (BG-sentinel and Light trap) were used. Increasing the number of samplings and the diversity of traps in these areas would improve the diversity and density of mosquito fauna.
Conclusion
This study shows the important diversity of mosquitoes as well as the density of the species of medical importance in four forests in Cambodia which responded differently to meteorological and geographical factors. It also highlights the presence of mosquitoes related to human activities in these supposedly protected areas. Additionally, it emphasizes a high risk of re-emergence of pathogens in these areas due to the abundance of mosquito species that are potentially vectors of pathogens. Finally, the potential emergence of new pathogens in these areas is a public heath consideration due to the presence and abundance of mosquitoes displaying zoo-anthropogenic behavior in forested and rural areas. In fact, these could serve as bridge vectors between sylvatic and anthropogenic pathogens. Further studies using next-generation sequencing methods should therefore be conducted to investigate the pathogen diversity among these mosquitoes, providing information on the risk of disease emergence.
Availability of data and materials
Data supporting the conclusions of this article are included within the article and its additional files. Raw data are available from the corresponding authors upon reasonable request.
References
OMS. 2020. Maladies à transmission vectorielle. 2020. Available from: https://www.who.int/fr/news-room/fact-sheets/detail/vector-borne-diseases. Accessed 30 August 2022.
OMS. Global Vector Control Response 2017–2030. 2017. p. 51.
Huy R, Buchy P, Conan A, Ngan C, Ong S, Ali R, et al. National dengue surveillance in Cambodia 1980–2008: epidemiological and virological trends and the impact of vector control. Bull World Health Organ. 2010;88:650–7.
Touch S, Hills S, Sokhal B, Samnang C, Sovann L, Khieu V, et al. Epidemiology and burden of disease from Japanese encephalitis in Cambodia: results from two years of sentinel surveillance. Trop Med Int Heal. 2009;14:1365–73.
Ledien J, Souv K, Leang R, Huy R, Cousien A, Peas M, et al. An algorithm applied to national surveillance data for the early detection of major dengue outbreaks in Cambodia. PLoS ONE. 2019;14:1–11.
Lee JS, Mogasale V, Lim JK, Ly S, Lee KS, Sorn S, et al. A multi-country study of the economic burden of dengue fever based on patient-specific field surveys in Burkina Faso, Kenya, and Cambodia. PLoS Negl Trop Dis. 2019;13:1–15.
WHO. Chikungunya outbreak—Cambodia, February–March 2012. Wkly Epidemiol Rec. 2012;61:358–68.
Rachmat A, Kelly GC, Hontz RD, Supaprom C, Heang V, Hip P, et al. Clinical and epidemiologic evaluation of a 2020 chikungunya outbreak in Cambodia. BMC Infect Dis. 2022;22:1–11. https://doi.org/10.1186/s12879-022-07936-9.
Duong V, Ong S, Leang R, Huy R, Ly S, Mounier U, et al. Low circulation of Zika virus, Cambodia, 2007–2016. Emerg Infect Dis. 2017;23:296–9.
Sovannaroth S, Ngor P, Khy V, Dunn JC, Burbach MK, Peng S, et al. Accelerating malaria elimination in Cambodia: an intensified approach for targeting at-risk populations. Malar J. 2022;21:1–11. https://doi.org/10.1186/s12936-022-04234-2.
Maquart PO, Fontenille D, Rahola N, Yean S, Boyer S. Checklist of the mosquito fauna (Diptera, Culicidae) of Cambodia. Parasite. 2021;28:1–24.
Davis KF, Yu K, Rulli MC, Pichdara L, D’Odorico P. Accelerated deforestation driven by large-scale land acquisitions in Cambodia. Nat Geosci. 2015;8:772–5.
Grogan K, Pflugmacher D, Hostert P, Mertz O, Fensholt R. Unravelling the link between global rubber price and tropical deforestation in Cambodia. Nat Plants. 2019;5:47–53. https://doi.org/10.1038/s41477-018-0325-4.
Kong R, Diepart JC, Castella JC, Lestrelin G, Tivet F, Belmain E, et al. Understanding the drivers of deforestation and agricultural transformations in the northwestern uplands of Cambodia. Appl Geogr. 2019;102:84–98. https://doi.org/10.1016/j.apgeog.2018.12.006.
Perrin A, Glaizot O, Christe P. Worldwide impacts of landscape anthropization on mosquito abundance and diversity: a meta-analysis. Glob Chang Biol. 2022;23:6857–71.
Burkett-Cadena ND, Vittor AY. Deforestation and vector-borne disease: forest conversion favors important mosquito vectors of human pathogens. Basic Appl Ecol. 2021;176:1–16.
Garrett-Jones C, Grab B. The assessment of insecticidal impact on the malaria mosquito’s vectorial capacity, from data on the proportion of parous females. Bull World Health Organ. 1964;31:71–86.
Vittor AY, Willim P, Gilman RH, Tielsch J, Glass G, Shields T, et al. Linking deforestation to malaria in the amazon: characterization of the breeding habitat of the principal malaria vector, Anopheles darlingi. Am J Trop Med Hyg. 2013;81:5–12.
Afrane YA, Zhou G, Lawson BW, Githeko AK, Yan G. Life-table analysis of Anopheles arabiensis in western Kenya highlands: effects of land covers on larval and adult survivorship. Am J Trop Med Hyg. 2007;77:660–6.
Kweka EJ, Kimaro EE, Munga S. Effect of deforestation and land use changes on mosquito productivity and development in western Kenya highlands: Implication for malaria risk. Front Public Heal. 2016;4:1–9.
Sehgal RNM. Deforestation and avian infectious diseases. J Exp Biol. 2010;213:955–60.
Maquart P-O, Sokha C, Boyer S. Mosquito (Diptera: Culicidae) diversity and medical importance in Koh Kong mangrove forests, Cambodia. Asian Biomed. 2022;16:121–9.
Maquart PO, Sokha C, Boyer S. Mosquito diversity (Diptera: Culicidae) and medical importance, in a bird sanctuary inside the flooded forest of Prek Toal, Cambodia. J Asia Pac Entomol. 2021;24:1–7.
Eyles DE, Wharton RH, Cheong WH, Warren M. Studies on Malaria and Anopheles balabacensis in Cambodia. Bull World Health Organ. 1964;30:7–21.
Sochantha T, Van Bortel W, Savonnaroth S, Marcotty T, Speybroeck N, Coosemans M. Personal protection by long-lasting insecticidal hammocks against the bites of forest malaria vectors. Trop Med Int Heal. 2010;15:336–41.
Van Bortel W, Trung HD, Sochantha T, Keokenchan K, Roelants P, Backeljau T, et al. Eco-ethological heterogeneity of the members of the Anopheles minimus complex (Diptera: Culicidae) in Southeast Asia and its consequences for vector control. J Med Entomol. 2004;41:366–74.
Trung HD, Van Bortel W, Sochantha T, Keokenchanh K, Quang NT, Cong LD, et al. Malaria transmission and major malaria vectors in different geographical areas of Southeast Asia. Trop Med Int Heal. 2004;9:230–7.
Durnez L, Mao S, Denis L, Roelants P, Sochantha T, Coosemans M. Outdoor malaria transmission in forested villages of Cambodia. Malar J. 2013;12:1–14.
St Laurent B, Oy K, Miller B, Gasteiger EB, Lee E, Sovannaroth S, et al. Cow-baited tents are highly effective in sampling diverse Anopheles malaria vectors in Cambodia. Malar J. 2016;15:1–11.
Vantaux A, Riehle MM, Piv E, Farley EJ, Chy S, Kim S, et al. Anopheles ecology, genetics and malaria transmission in northern Cambodia. Sci Rep. 2021;11:1–17. https://doi.org/10.1038/s41598-021-85628-1.
Rattanarithikul R, Harbach RE, Harrison BA, Panthusiri P, Coleman RE. Illustrated keys to the mosquitoes of Thailand V. Genera Orthopodomyia, Kimia, Malaya, Topomyia, Tripteroides, and Toxorhynchites. Southeast Asian J Trop Med Public Health. 2007;38:1–65.
Rattanarithikul R, Harbach RE, Harrison BA, Panthusiri P, Coleman RE, Richardson JH. Illustrated keys to the mosquitoes of Thailand. VI. Tribe Aedini. Southeast Asian J Trop Med Public Health. 2010;41:1–225.
Rattanarithikul R, Harbach RE, Harrison BA, Panthusiri P, Jones JW, Coleman RE. Illustrated keys to the mosquitoes of Thailand II. Genera Culex and Lutzia. Southeast Asian J Trop Med Public Health. 2005;36:1–96.
Rattanarithikul R, Harrison BA, Harbach RE, Panthusiri P, Coleman RE. Illustrated keys to the mosquitoes of Thailand IV. Anopheles. Southeast Asian J Trop Med Public Health. 2006;37:1–26.
R core team. 2021. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria. 2021. https://www.R-project.org/. Accessed 1 Dec 2022.
Boyer S, Durand B, Yean S, Brengues C, Maquart PO, Fontenille D, et al. Host-feeding preference and diel activity of mosquito vectors of the Japanese encephalitis virus in rural Cambodia. Pathogens. 2021;10:1–14.
Boyer S, Marcombe S, Yean S, Fontenille D. High diversity of mosquito vectors in Cambodian primary schools and consequences for arbovirus transmission. PLoS ONE. 2020;15:1–13. https://doi.org/10.1371/journal.pone.0233669.
Boyer S, Peng B, Pang S, Chevalier V, Duong V, Gorman C, et al. Dynamics and diversity of mosquito vectors of Japanese encephalitis virus in Kandal province, Cambodia. J Asia Pac Entomol. 2020;23:1048–54. https://doi.org/10.1016/j.aspen.2020.08.018.
Bram RA. Contribution to the mosquito fauna of Southeast Asia II. The genus Culex in Thailand (Diptera: Culicidae). Contrib Am Entomol Inst. 1967;2:1–293.
Hati AK, Bhattacharya S. Biosystematics of Culex vishnui and Culex pseudovishnui based on ecobehavioural pattern. Proc Anim Sci. 1987;96:629–36.
Philip Samuel P, Arunachalam N, Hiriyan J, Thenmozhi V, Gajanana A, Satyanarayana K. Host-feeding pattern of Culex quinquefasciatus Say and Mansonia annulifera (Theobald) (Diptera: Culicidae), the major vectors of filariasis in a rural area of South India. J Med Entomol. 2004;41:442–6.
Wilke ABB, Vasquez C, Carvajal A, Moreno M, Fuller DO, Cardenas G, et al. Urbanization favors the proliferation of Aedes aegypti and Culex quinquefasciatus in urban areas of Miami-Dade County, Florida. Sci Rep. 2021;11:1–12. https://doi.org/10.1038/s41598-021-02061-0.
Paupy C, Delatte H, Bagny L, Corbel V, Fontenille D. Aedes albopictus, an arbovirus vector: from the darkness to the light. Microbes Infect. 2009;11:1177–85. https://doi.org/10.1016/j.micinf.2009.05.005.
Tangena J-AA, Thammavong P, Malaithong N, Inthavong T, Ouanesamon P, Brey PT, et al. Diversity of mosquitoes (Diptera: Culicidae) attracted to human subjects in rubber plantations, secondary forests, and villages in Luang Prabang Province Northern, Lao PDR. J Med Entomol. 2017;54:1589–604.
Paine RW, Edwards FW. Mosquitos from the Solomon islands. Bull Entomol Res. 1929;20:303–16.
Blanford W. The Fauna of British India including Ceylon and Burma, vol. 5. London: Taylor Francis; 1934. p. 453.
Miyagi I, Toma T, Tamashiro M, Higa Y, Kinjyo T, Takara T. Colonization and biology of the frog-feeding mosquito Uranotaeinia macfarlanei in the Ryukyu archipelago. J Am Mosq Control Assoc. 2010;26:99–102.
Mattingly PF. Contributions to the mosquito fauna of Southeast Asia. VI. The genus Heizmannia Ludlow in Southeast Asia. Contrib Am Entomol Inst. 1970;5:1–104.
Chaves LF, Imanishi N, Hoshi T. Population dynamics of Armigeres subalbatus (Diptera: Culicidae) across a temperate altitudinal gradient. Bull Entomol Res. 2015;105:589–97.
Suman D. Efficacy of diurnal BG-Sentinel traps to capture nocturnal adult Armigeres subalbatus mosquitoes and impact of altitudinal variations in forests. Asian Pac J Trop Med. 2019;12:512–9.
Sherpa S, Tutagata J, Gaude T, Laporte F, Kasai S, Ishak IH, et al. Genomic shifts, phenotypic clines, and fitness costs associated with cold tolerance in the Asian tiger mosquito. Mol Biol Evol. 2022;39:1–14.
Ferraguti M, Martínez-De La Puente J, Roiz D, Ruiz S, Soriguer R, Figuerola J. Effects of landscape anthropization on mosquito community composition and abundance. Sci Rep. 2016;6:1–9. https://doi.org/10.1038/srep29002.
Mayi MPA, Bamou R, Djiappi-Tchamen B, Fontaine A, Jeffries CL, Walker T, et al. Habitat and seasonality affect mosquito community composition in the west region of Cameroon. Insects. 2020;11:1–17.
Powell JR, Tabachnick WJ. History of domestication and spread of Aedes aegypti—a review. Mem Inst Oswaldo Cruz. 2013;108:11–7.
Duvallet G, Fontenille D, Robert V. Entomologie médicale et vétérinaire. Marseille: IRD Edition; 2017. p. 687.
Apiwathnasorn C, Prommongkol S, Samung Y, Limrat D, Rojruthai B. Potential for Anopheles campestris (Diptera: Culicidae) to transmit malaria parasites in Pa Rai subdistrict (Aranyaprathet, Sa Kaeo Province). Thailand J Med Entomol. 2002;39:583–6.
Prakash A, Bhattacharyya DR, Mohapatra PK, Mahanta J. Malaria transmission risk by the mosquito Anopheles baimaii (formerly known as An. dirus species D) at different hours of the night in North-east India. Med Vet Entomol. 2005;19:423–7.
Mourya DT, Mishra AC, Soman RS. Transmission of Japanese encephalitis virus in Culex pseudovishnui & C. tritaeniorhynchus mosquitoes. Indian J Med Res. 1991;93:250–2.
Auerswald H, Maquart PO, Chevalier V, Boyer S. Mosquito vector competence for Japanese encephalitis virus. Viruses. 2021;13:1–26.
Chen WJ, Dong CF, Chiou LY, Chuang WL. Potential role of Armigeres subalbatus (Diptera: Culicidae) in the transmission of Japanese encephalitis virus in the absence of rice culture on Liu-Chiu Islet. Taiwan J Med Entomol. 2000;37:108–13.
Muangman D, Edelman R, Sullivan MJ, Gould DJ. Experimental transmission of Japanese Encephalitis Virus by Culex fuscocephala. Am J Trop Med Hyg. 1972;21:482–6.
Okuno T, Mitchell CJ, Chen PS, Hsu S, Ryu E. Experimental transmission of Japanese encephalitis virus by Culex tritaeniorhynchus and C. fuscocephalus. Ann Trop Med Parasitol. 1975;69:203–6.
Sudeep AB, Ghodke YS, George RP, Ingale VS, Dhaigude SD, Gokhale MD. Vectorial capacity of Culex gelidus (Theobald) mosquitoes to certain viruses of public health importance in India. J Vector Borne Dis. 2015;52:153–8.
Huang YJS, Harbin JN, Hettenbach SM, Maki E, Cohnstaedt LW, Barrett ADT, et al. Susceptibility of a North American Culex quinquefasciatus to Japanese Encephalitis Virus. Vector-Borne Zoonotic Dis. 2015;15:709–11.
Van Den Hurk AF, Nisbet DJ, Hall RA, Kay BH, Mackenzie JS, Ritchie SA. Vector competence of Australian mosquitoes (Diptera: Culicidae) for Japanese encephalitis virus. J Med Entomol. 2003;40:82–90.
de Wispelaere M, Desprès P, Choumet V. European Aedes albopictus and Culex pipiens are competent vectors for Japanese Encephalitis Virus. PLoS Negl Trop Dis. 2017;11:1–19.
Gratz NG. Critical review of the vector status of Aedes albopictus. Med Vet Entomol. 2004;18:215–27.
Gloria-Soria A, Payne AF, Bialosuknia SM, Stout J, Mathias N, Eastwood G, et al. Vector competence of Aedes albopictus populations from the Northeastern United States for Chikungunya, Dengue, and Zika Viruses. Am J Trop Med Hyg. 2021;104:1123–30.
Yang W, Zhao S, Xie Y, Liu T, Kong L, Guo Y, et al. Armigeres subalbatus is a potential vector for Zika virus but not dengue virus. Infect Dis Poverty. 2022;11:1–9.
Intarapuk A, Bhumiratana A. Investigation of Armigeres subalbatus, a vector of zoonotic Brugia pahangi filariasis in plantation areas in Suratthani, Southern Thailand. One Health. 2021;13:1–8. https://doi.org/10.1016/j.onehlt.2021.100261.
Ndiaye EH, Fall G, Gaye A, Bob NS, Talla C, Diagne CT, et al. Vector competence of Aedes vexans (Meigen), Culex poicilipes (Theobald) and Cx. quinquefasciatus Say from Senegal for West and East African lineages of Rift Valley fever virus. Parasites Vectors. 2016;9:1–9. https://doi.org/10.1186/s13071-016-1383-y.
Souza-Neto JA, Powell JR, Bonizzoni M. Aedes aegypti vector competence studies: a review. Infect Genet Evol. 2019;67:191–209. https://doi.org/10.1016/j.meegid.2018.11.009.
Jansen CC, Beebe NW. The dengue vector Aedes aegypti: what comes next. Microbes Infect. 2010;12:272–9. https://doi.org/10.1016/j.micinf.2009.12.011.
Reuben R, Thenmozhi V, Samuel PP, Gajanana A, Mani TR. Mosquito blood feeding patterns as a factor in the epidemiology of Japanese Encephalitis in southern India. Am J Trop Med Hyg. 1992;46:654–63.
Delatte H, Desvars A, Bouetard A, Bord S, Gimonneau G, Vourc’h, G., Fontenille, D. Blood-feeding behavior of Aedes albopictus, a vector of chikungunya on la Réunion. Vector-Borne Zoonotic Dis. 2010;10:249–58.
Boonserm R, Jantorn R, Phumee A, Sor-Suwan S, Jariyapan N, Tiawsirisup S, et al. Identification of blood meal from field collected filarial vector mosquitoes, Armigeres subalbatus by multiplex PCR. Thai J Vet Med. 2019;49:155–60.
Alencar J, Silva JDS, De Oliveira LCM, Marcondes CB, Morone F, Lorosa ES. Feeding patterns of Culex quinquefasciatus (Diptera: Culicidae) from Eastern Santa Catarina State. Brazil J Med Entomol. 2012;49:952–4.
Acknowledgements
The authors thank Hélène Guis PhD (CIRAD, Cambodia) for the help with statistical analysis. POM appreciates the Calmette & Yersin Post-doctoral Grant for the funding of his research.
Funding
This study was by the Defense Advanced Research Projects Agency (DARPA).
Author information
Authors and Affiliations
Contributions
SB and POM designed the study. POM and CS collected mosquitoes. AR and CF analyzed the data. AR wrote the first draft. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This research was approved by Cambodian authority and allowed by the Ministry of Environment (permit no, 1443, issued on 15 November 2018), and by the Directorate General of Nature Conservation and Protection (permit no. 300 issued on 21 May 2020 for Kampong Speu, no. 0157 issued on 11 March 2020 for Preah Vihear, no. 057 issued on 14 February 2019 for Ratanak Kiri and no. 036, issued on 26 February 2020 for Siemreap).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
: Table S1 Number of mosquitoes collected per forest and per site.
Additional file 2
: Table S2 Pearson correlation between the relative abundance of mosquito species with relative abundance ≥ 5.
Additional file 3
: Table S3 P-value of the Pearson correlation between the relative abundance of mosquito species with relative abundance ≥ 5.
Additional file 4
: Table S4 Result of regression model showing the correlation between the presence of species and meteorological/geographical variables. Mosquito species having a relative abundance between 10 and 39 were included in the analysis.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Rakotonirina, A., Maquart, PO., Flamand, C. et al. Mosquito diversity (Diptera: Culicidae) and medical importance in four Cambodian forests. Parasites Vectors 16, 110 (2023). https://doi.org/10.1186/s13071-023-05729-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-023-05729-w