Abstract
Background
Limited data are currently available on protozoan parasites of the genus Sarcocystis that infect their avian hosts within the order Anseriformes (waterfowl). To date, no Sarcocystis species has been recorded in ducks in China.
Methods
Leg muscles were sampled from 26 domestic ducks (Anas platyrhynchos) in China in 2021. Morphological characteristics of sarcocysts detected in the muscle tissue were described using light microscopy (LM) and transmission electron microscopy (TEM). Genomic DNA was extracted from single sarcocysts obtained from different ducks, and three genetic markers, 18S ribosomal DNA (18S rDNA), 28S ribosomal DNA (28S rDNA) and mitochondrial (mt) cytochrome oxidase subunit 1 (cox1), were amplified and cloned for sequence analyses.
Results
Sarcocysts were observed by LM in only three of the 28 samples (10.7%). These sarcocysts had a thick cyst wall with numerous brush-like villar protrusions (vps) of 3.8–4.3 μm in length (n = 30) on the cyst surface. TEM observation showed that the sarcocysts had lanceolated vps. Each vps narrowed in the stalk and contained a bundle of microtubules that extended into the ground substance. Comparisons of the new sequences with those deposited in GenBank showed that the most similar sequences were those of Sarcocystis halieti in the great cormorant Phalacrocorax carbo and European starling Sturnus vulgaris, and Sarcocystis calchasi in the domestic pigeon (Columba livia) at the 18S rDNA (99.1% identity); Sarcocystis wenzeli from the domestic chicken Gallus gallus at the 28S rDNA (95.9–96.0% identity); and Sarcocystis speeri from the opossum at the mtcox1 (98.2% identity). The new 18S rDNA, 28S rDNA and mitochondrial cox1 sequences shared up to 99.0%, 95.6% and 97.7% identity, respectively, with those of Sarcocystis spp. obtained from Anseriformes avian hosts. Phylogenetic analysis inferred from the sequences of the three genetic markers placed the organism within a group of Sarcocystis spp. obtained from avian or carnivorous intermediate hosts and avian, marsupial or carnivorous definitive hosts. Based on the morphological observation and molecular analyses, the organism found in the Chinese domestic ducks was regarded as a new species and named Sarcocystis platyrhynchosi n. sp.
Conclusions
Based on morphology and sequence analyses, the microcysts diagnosed in the domestic ducks examined in this study were named as a new species. This is the first record of Sarcocystis spp. from waterfowl in China. Sarcocysts of similar morphology occur frequently in different Anseriformes birds, and the relationships among these species need to be further clarified in future studies using more molecular markers.
Graphical Abstract

Similar content being viewed by others
Background
Species of the genus Sarcocystis are cyst-forming intracellular protozoan parasites with an obligate two-host life-cycle between predators (definitive hosts) and their prey animals (intermediate hosts). Current identification and classification of the Sarcocystis species in a given host mainly depend on the ultrastructure of the sarcocyst walls and the nucleotide sequences of a number of molecular markers [1].
Avian species within the order Anseriformes are intermediate hosts of four Sarcocystis species, S. rileyi, S. wobeseri, S. anasi and S. albifronsi. Among these, S. rileyi forms macrocysts that resemble a grain of rice, and the remaining three species form microcysts that are invisible to the naked eye [2,3,4]. Here, we report the morphological and molecular characterization of a new Sarcocystis species that forms microcysts in skeletal muscles of the domestic duck Anas platyrhynchos in China. We also investigated the phylogenetic relationships of the new species with other avian-infecting Sarcocystis spp. using three molecular markers, 18S ribosomal DNA (18S rDNA), 28S ribosomal DNA (28S rDNA) and mitochondrial cytochrome oxidase subunit 1 (mtcox1).
Methods
Morphological observation of sarcocysts in domestic ducks
Muscle samples were collected from the legs of 26 domestic ducks purchased from a rural market located in Shuangtu, Yunyang county, Chongqing municipality, China, in January 2021. These free-range ducks were raised by local farmers. Twenty 3-mm-long pieces were obtained from each muscle sample, and these were pressed and squeezed between two glass slides for observation of sarcocysts under a stereomicroscope. The sarcocysts were then isolated from the muscle fibers using dissection needles and used for light microscopy (LM), transmission electron microscopy (TEM) and DNA studies.
For TEM, a total of four sarcocysts isolated from two domestic ducks were fixed first in 2.5% glutaraldehyde in cacodylate buffer (0.1 M, pH 7.4) and then in 1.0% osmium tetroxide, followed by dehydration through an increasing concentration of ethanol and embedding in Durcupan. Ultrathin sections were stained first with uranyl acetate (35 mg/ml) and then with lead citrate (35 mg/ml), followed by examination under a JEM100-CX transmission electron microscope (JEOL Ltd., Tokyo, Japan) at 80 kV. For DNA extraction, individual cysts were stored in sterile water at − 20 °C prior to processing.
DNA isolation, PCR amplification, cloning and sequence analysis
Three sarcocysts, each obtained from a different domestic duck, were separately subjected to genomic DNA extraction using a TIANamp Genomic DNA Kit (Tiangen Biotech Ltd., Beijing, China) according to the manufacturer’s instructions. The primer pairs used for amplification of the three genetic markers were as follows: 18S DSF (5ʹ-CGTGGAAGGGTAGTGTTTA-3ʹ) and 18S DSR (5ʹ-CGGAAACCTTGTTACGACT-3ʹ) for 18S rDNA [5]; KL1 (5ʹ-TACCCGCTGAACTTAAGC-3ʹ) and KL3 (5ʹ-CCACCAAGATCTGCACTAG-3ʹ) for 28S rDNA [5]; SF1 (5ʹ-ATGGCGTACAACAATCATAAAGAA-3ʹ) [6] and CODSR (5ʹ-CCTCTAATCCTACGGTCATC-3ʹ) for mtcox1. The primers 18S DSF, 18S DSR and CODSR used in the study were designed using OLIGO 7.60 primer analysis software (Molecular Biology Insights, Inc., Cascade, CO, USA) according to the highly conserved regions of 18S rDNA and mtcox1 sequences of Sarcocystis spp. from fowls that are available in GenBank.
PCR amplifications were performed as previously described [7]. The resulting PCR products were gel purified using an E.Z.N.A.® Gel Extraction Kit (Omega Bio-Tek, Inc., Norcross, GA, USA) and ligated to the pCE2 TA/Blunt-Zero vector using a 5 min TA/Blunt-Zero Cloning Kit (Vazyme Biotech Co., Ltd. Nanjing, China) according to the manufacturer’s instructions. The ligated vectors were transformed into Trelief® 5α Chemically Competent Cell (Tsingke Biotechnology Co., Ltd., Beijing, China). The positive bacterial clones were sequenced on both directions by an ABI PRISM TM 3730 XL DNA Analyzer (Applied Biosystems, Thermo Fisher Scientific, Waltham, MA, USA).
Phylogenetic analyses were conducted on the nucleotide sequences of the three loci using MEGA X software [8]. Maximum likelihood (ML) trees of the 18S rDNA, 28S rDNA and mtcox1 sequences were constructed with the Kimura 2-parameter, Hasegawa-Kishino-Yano and Hasegawa-Kishino-Yano models, respectively. The reliability of the ML phylograms was tested via the bootstrap method using 1000 replications.
Results
Observation of sarcocysts in domestic ducks by LM and TEM
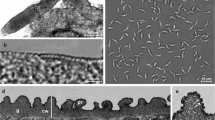
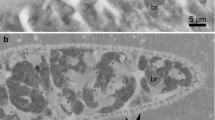
The LM study revealed the presence of sarcocysts in the leg muscle samples from three of the 28 domestic ducks studied (10.7%). The number of sarcocysts was relatively low, and only one to two sarcocysts were found in 20 g of muscle tissues from each of these three ducks. All sarcocysts were morphologically similar. The sarcocysts were microscopic, measuring 980–1694 × 72–140 μm (n = 8) in size and had a thick striated cyst wall with numerous brush-like villar protrusions (vps) of 3.8–4.3 μm in length (n = 30) on the cyst surface (Fig. 1a). They were septate and contained lancet-like bradyzoites of 12.3–14.0 × 1.6–2.3 μm (n = 30) in size (Fig. 1b).
Morphological characteristics of Sarcocystis platyrhynchosi n. sp. isolated from the skeletal muscle of domestic ducks. a Light microscopy (LM) micrograph of a sarcocyst (unstained). Note the short brush-like villar protrusions (vps). b LM micrograph of lancet-like bradyzoites (unstained). c Transmission electron microscopy (TEM) micrograph of a sarcocyst. Note the lanceolated villar protrusions (vps) and the bundles of microtubes (mt) within the vps. d TEM micrograph of a sarcocyst. Note the narrowed stalk (arrowhead) of the vps, bundled mt extending into the ground substance (gs) and the smooth electron dense layer (edl) lining the vps
The sarcocyst wall contained numerous lanceolated vps of 2.3–2.5 × 0.3–0.5 μm (n = 10) in size. Each vps narrowed in the stalk, widened in the middle, tapered at the end and was lined by a smooth electron-dense layer. The bundled microtubules were present in the core of the vps, which extended from tips of the vps into the ground substance. A layer of the ground substance of 0.3–0.5 μm in thickness (n = 8) was located immediately beneath the cyst wall (Fig. 1c, d).
Molecular analyses of sarcocysts in domestic ducks
The three selected genetic markers (18S rDNA, 28S rDNA and mtcox1) were successfully amplified and sequenced. One nucleotide sequence was assembled from three clones of each genetic marker for each duck, and the sequences of each of the three genetic markers were determined to be 100% identical among the three ducks. Therefore, only one sequence of each genetic markers was deposited in GenBank under the accession numbers OP480004 for 18S rDNA (1594 bp), OP480005 for 28S rDNA (1558 bp) and OP485287 for mtcox1 (883 bp).
Comparisons of the new sequences with those deposited in GenBank showed that the most similar sequences were those of Sarcocystis halieti in the great cormorant Phalacrocorax carbo and European starling Sturnus vulgaris, and Sarcocystis calchasi in the domestic pigeon (Columba livia) at the 18S rDNA (99.1% identity); Sarcocystis wenzeli from the domestic chicken Gallus gallus at the 28S rDNA (95.9–96.0% identity); and Sarcocystis speeri from the opossum at the mtcox1 (98.2% identity). The new sequences shared no more than 99.0%, 95.6% and 97.7% identity at the 18S rDNA, 28S rDNA and mtcox1, respectively, with those of Sarcocystis wobeseri, S. anasi, S. rileyi and S. albifronsi obtained from Anseriformes avian species (Table 1).
Phylogenetic analysis
Phylogenetic analysis obtained with the 18S rDNA (Fig. 2a), 28S rDNA (Fig. 2b) and mtcox1 (Fig. 2c) sequences placed the organism found in the domestic ducks within a clade encompassing Sarcocystis spp. obtained from avian or carnivorous intermediate hosts and avian, marsupial or carnivorous definitive hosts. All three phylogenetic trees had similar topologies, and the organism described in the present study formed a separate branch, which did not cluster with any of the three main clades of Sarcocystis species using birds as intermediate hosts, i.e. S. calchasi, S. halieti and others (bird–bird life-cycle), S. rileyi, S. wenzeli and others (birds–placental predatory mammals life-cycle), S. falcatula, S. speeri and others (birds–marsupial/specifically opossum life-cycle).
Phylogenetic trees of selected members of Sarcocystis species. The trees were conducted using 18S rDNA (a), 28S rDNA (b) and mcox1 (c) sequences using maximum likelihood (ML) with the Kimura 2–parameter, Hasegawa–Kishino–Yano and Hasegawa–Kishino–Yano models, respectively. The values between the branches represent bootstrap values per 1000 replicates. Values < 50% are not shown. Besnoitia besnoiti, Cystoisopora suis, Toxoplasam gondii or Hammondia heydorni were selected to root these trees. The newly obtained sequences of the 18S rDNA (OP480004), 28S rDNA (OP480005) and mtcox1 (OP485287) for Sarcocystis platyrhynchosi n. sp. are shown in bold. The phylogenetic trees inferred from the three genes had similar topologies, and Sarocystis platyrhynchosi formed a separate branch within a group encompassing Sarcocystis spp. obtained from avian or carnivorous intermediate hosts and avian marsupial, or carnivorous definitive hosts
On the basis of morphological and molecular characterization of the sarcocysts, the isolate from the domestic ducks from China is regarded as a new species named Sarcocystis platyrhynchosi n. sp.
Family Sarcocystidae Poche, 1913
Sarcocystis platyrhynchosi n. sp.
Diagnosis: The sarcocysts were microscopic, up to 1694 μm long and 140 μm wide. Numerous brush–like vps of 3.8–4.3 μm in length were present on the cyst surface. TEM observation revealed that the sarcocysts had lanceolated vps of 2.3–2.5 × 0.3–0.5 μm, which narrowed in the stalk. Each vps contained bundled microtubules at the core that penetrated diagonally into the ground substance.
Taxonomic summary
Type intermediate host: Domestic duck Anas platyrhynchos.
Type locality: Shuangtu (31°15ʹ59ʺN, 108°94ʹ15ʺE, altitude 495 m a.s.l.), Yunyang County, Chongqing City, China.
Site of infection: Muscular tissues.
Definitive host: Unknown.
Etymology: Latin name of the intermediate hosts is used to name the species.
Molecular characterization: Sequences of the 18S rDNA (OP480004), 28S rDNA (OP480004) and mtcox1 (OP485287) of the new species have been deposited in GenBank. At the 28S rDNA and mtcox1 sequences, S. platyrhynchosi is unambiguously differentiated from Sarcocystis spp. obtained from Anseriformes birds.
Deposited specimens: Formalin–fixed tissues containing cysts of S. platyrhynchosi, as well as photomicrographs from LM and TEM examination of the sarcocysts, have been deposited at the Zoological Specimen Museum of Yunnan University, Kunming, China (collection number Prot202205).
ZooBank registration: To comply with the regulations set out in Article 8.5 of the amended 2012 version of the International Code of Zoological Nomenclature [9], details of the new species have been submitted to ZooBank. The Life Science Identifier (LSID) of the article is urn:lsid:zoobank.org:pub: 4FA38B3B-4505-4EF4-AC87-14067AD9FA7A. The LSID for the new species name Sarcocystis platyrhynchosi is urn:lsid:zoobank.org:act: D1663E66-23C2-4E50-96C5-9E336318C8EE.
Remarks
To date, only four named Sarcocystis species (S. rileyi, S. wobeseri, S. anasi and S. albifronsi) and two unnamed Sarcocystis species have been recorded in avian intermediate hosts of the order Anseriformes (Table 2). Among these, sarcocysts of S. rileyi are macroscopic, and those of the remaining five species are microscopic. The LM study revealed that the sarcocysts of S. rileyi, S. wobeseri and Sarcocystis sp. 2 ex Anser caerulescens have thin and smooth walls, and that those of S. anasi, S. albifronsi and Sarcocystis sp. 1 ex A. caerulescens have thick and striated walls characterized by radial spines- or finger-like vps on the cyst surface. The unltrastructures of the sarcocysts previously described from avian species of Anseriformes are categorized into three TEM wall types according to the classification provided by Dubey et al. [1]. One type includes vps with anastomosing branches that is similar to type 23 in S. rileyi; the second type has minute undulations on the cyst wall that is similar to type 1d for S. wobeseri and Sarcocystis sp. 2 ex A. caerulescens; and the third type has finger-like vps arranged in a palisade fashion that is similar to type 9a for S. anasi, S. albifronsi and Sarcocystis sp. 1 ex A. caerulescens. In our material, S. platyrhynchosi sarcocysts had short, brush–like vps on the cyst surface. The TEM study revealed that the lanceolated vps had a narrow stalk and contained bundled microtubules that penetrated diagonally into the ground substance; this is roughly similar to the TEM wall type 9d or 11a based on outlines of the lanceolated vps or the features of bundled microtubules extended into ground substance, respectively. This TEM wall type differs remarkably from those of Sarcocystis spp. obtained previously from wild mallard ducks and other species of Anseriformes.
Discussion
Sarcocysts have been found in the muscles of at least 20 avian species within the order Anseriformes in Europe and North and South America [2,3,4, 10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25]. In studies carried out during the previous century in birds in North America, macrocysts were observed in Anseriformes avian hosts by Riley in 1869, and all were subsequently designated as S. rileyi by Stiles in 1893 (see [2]). Microcysts were subsequently observed using LM in five named species of Anseriformes in North America [23]. These were classified into five cyst categories, namely 1a, 1b, 2a, 2b and 2c, based on the thickness of the cyst wall [23]. Microcysts were also found in 15 species of Anseriformes in Europe by LM, and these were divided into four microcysts types, I–IV [26]. Type I cyst found in 10 species had cyst walls up to 1.2 μm in thickness and no clear protrusions on the surface. The walls of type II cysts from four species were surrounded by palisade-like protrusions (up to 1.5 μm long) on the surface, and the type III cysts from four species had teat- or finger-like protrusions (up to 2.4 μm long) on the surface of the cyst walls. Type IV cysts found in two species had a wavy cyst wall. However, no species names for these microcysts were proposed by these authors at that time, mainly due to these sarcocysts being morphologically indistinguishable in different intermediate hosts.
Characterization of the ultrastructures of sarcocysts is a reliable method to identify and determine Sarcocystis species within a given host. The ultrastructure of cyst walls has been categorized into 42 types, with several subgroups [1]. The ultrastructures of macrocysts in the shoveler duck Anas clypeata [2] and mallard duck [10] were characterized for the first time in this century. The morphologically similar sarcocysts in these two hosts were designated as S. rileyi by the authors. Subsequently, mainly based on the ultrastructures of the microcysts, the type I cysts in the barnacle geese (Branta leucopsis) and type IV cysts in the mallard ducks were shown to be morphologically similar, resulting in them being named as one species, S. wobeseri [3]. However, the type II cysts ex mallard ducks and type III cysts ex white–fronted geese Anser albifrons were named S. anasi and S. albifronsi [4], respectively, even though they were also found to be morphologically similar, and classified into TEM wall type 9a [4, 18, 21]. In our materials, the ultrastructures of microcysts obtained from domestic ducks were roughly similar to the TEM wall type 9d or 11a, and were completely different from the those of Sarcocystis spp. reported in wild mallard ducks and wild geese.
Morphologically similar species occur frequently in avian species of Anseriformes, such as the macrocysts found in numerous duck species within the genera Aix, Anas, Aythya, Bucephala and Melanitta [2], leading to confusion as to whether they represent a same species of Sarcocystis in different intermediate hosts. In recent decades, molecular analysis has been used as a primary or supplemental tool to delineate or identify species of Sarcocystis, and different genetic markers have shown different levels of intra- or interspecific sequence diversity [6]. Based on nucleotide sequence analysis, all macrocysts found in the mallard duck, Eurasian wigeon Anas penelope, common teal Anas crecca and the common eider Somateria mollissima are considered to be S. rileyi [11, 12], and the intermediate hosts of S. wobeseri were revealed to be mallard ducks, barnacle geese and herring gulls Larus argentatus [3, 19, 20]. TEM wall type 9a sarcocysts in mallard ducks and white-fronted geese were specified as S. anasi and S. albifronsi, respectively, based on divergence of the internal transcribed spacer sequences (90.8% identity) and differences in the shapes and sizes of the bradyzoites [4]. Bradyzoites of 13.0–16.1 × 1.8–2.5 μm in S. anasi were slightly bent with blunt ends, widening toward one end [18], whereas those of S. albifronsi were almost straight and resembled a shuttle in shape, and measured 10.0–13.5 × 1.5–2.5 μm [21]. However, the two species could not be differentiated at the molecular level at the remaining four loci: 18S rDNA (99.7%), 28S rDNA (99.2%), cox1 (99.7%) and rpoB (99.3%) [19, 27]. Therefore, based on the limited data currently available, confusion remains regarding the relationship of the morphologically similar species of Sarcocystis in Anseriformes birds. In our materials, the 18S rDNA sequences of S. platyrhynchosi shared high similarity (≥ 99.0%) with those of Sarcocystis spp. in different avian species. This indicates that this gene is unsuitable for use as a marker to differentiate the closely related Sarcocystis spp. in avian species, confirming the findings reported in the closely related Sarcocystis spp. in domestic ruminants [6, 28]. However, the 28S rDNA and cox1 sequences of the new species could be unambiguously discriminated from those of Sarcocsytis spp. in different avian species reported in previous studies.
Conclusions
To the best of our knowledge, this report is the first record of sarcocysts in waterfowls in China. To date, only limited data are available for Sarcocystis in wild ducks and geese, and studies of Sarcocystis in domestic ducks are especially rare. In the present study, the microcysts identified in the domestic ducks were named as a new species based on morphology and DNA sequence analyses, but the definitive host of the new species is still unknown. Morphologically similar sarcocysts occur frequently in different avian species of Anseriformes, and the relationships among these species need to be further clarified using more molecular markers in the future.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request. Nucleotide sequences of the 18S rDNA (OP480004), 28S rDNA (OP480005), and mitochondrial cox1 (OP485287) of the new species have been deposited in GenBank.
Abbreviations
- cox1:
-
Cytochrome oxidase subunit 1
- LM:
-
Light microscopy
- mt:
-
Mitochondrial
- 18S rDNA:
-
18S ribosomal DNA
- 28S rDNA:
-
28S ribosomal DNA
- TEM:
-
Transmission electron microscopy
- vps:
-
Villar protrusions
References
Dubey JP, Calero-Bernal R, Rosenthal BM, Speer CA, Fayer R. Sarcocystosis of animals and humans. 2nd ed. Boca Raton: CRC Press; 2016.
Dubey JP, Cawthorn RJ, Speer CA, Wobeser GA. Redescription of the sarcocysts of Sarcocystis rileyi (Apicomplexa: Sarcocystidae). J Eukaryot Microbiol. 2003;50:476–82.
Kutkienė L, Prakas P, Sruoga A, Butkauskas D. The mallard duck (Anas platyrhynchos) as intermediate host for Sarcocystis wobeseri sp. nov. from the barnacle goose (Branta leucopsis). Parasitol Res. 2010;107:879–88.
Kutkienė L, Prakas P, Sruoga A, Butkauskas D. Description of Sarcocystis anasi sp. nov. and Sarcocystis albifronsi sp. nov. in birds of the order Anseriformes. Parasitol Res. 2012;110:1043–6.
Mugridge NB, Morrison DA, Heckeroth AR, Johnson AM, Tenter AM. Phylogenetic analysis based on full–length large subunit ribosomal RNA gene sequence comparison reveals that Neospora caninum is more closely related to Hammondia heydorni than to Toxoplasma gondii. Int J Parasitol. 1999;29:1545–56.
Gjerde B. Phylogenetic relationships among Sarcocystis species in cervids, cattle and sheep inferred from the mitochondrial cytochrome c oxidase subunit I gene. Int J Parasitol. 2013;43:579–91.
Hu J, Sun J, Guo Y, Zeng H, Zhang Y, Tao J. Infection of the Asian gray shrew Crocidura attenuata (Insectivora: Soricidae) with Sarcocystis attenuati n. sp. (Apicomplexa: Sarcocystidae) in China. Parasit Vectors. 2022;15:13.
Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35:1547–9.
International Commission on Zoological Nomenclature (ICZN). Amendment of articles 8, 9, 10, 21 and 78 of the international code of zoological nomenclature to expand and refine methods of publication. Bull Zool Nomencl. 2012;69:161–9.
Kutkienė L, Prakas P, Sruoga A, Butkauskas D. Identification of Sarcocystis rileyi from the mallard duck (Anas platyrhynchos) in Europe: cyst morphology and results of DNA analysis. Parasitol Res. 2011;108:709–14.
Prakas P, Oksanen A, Butkauskas D, Sruoga A, Kutkienė L, Švažas S, et al. Identification and intraspecific genetic diversity of Sarcocystis rileyi from ducks, Anas spp., in Lithuania and Finland. J Parasitol. 2014;100:657–61.
Gjerde B. Molecular characterisation of Sarcocystis rileyi from a common eider (Somateria mollissima) in Norway. Parasitol Res. 2014;113:3501–9.
Prakas P, Liaugaudaitėe S, Kutkienė L, Sruoga A, Švažas S. Molecular identification of Sarcocystis rileyi sporocysts in red foxes (Vulpes vulpes) and raccoon dogs (Nyctereutes procyonoides) in Lithuania. Parasitol Res. 2015;114:1671–6.
Wicht RI. Transmission of Sarcocystis rileyi to the striped skunk (Mephitis mephitis). J Wildl Dis. 1981;17:387–8.
Padilla-Aguilar P, Romero-Callejas E, Osorio-Sarabia D, Ramírez-Lezama J, Cigarroa-Toledo N, Machain-Williams C, et al. Detection and molecular identification of Sarcocystis rileyi (Apicomplexa: Sarcocystidae) From a Northern Shoveler (Anas clypeata) in Mexico. J Wildl Dis. 2016;52:931–5.
Szekeres S, Juhász A, Kondor M, Takács N, Sugár L, Hornok S. Sarcocystis rileyi emerging in Hungary: is rice breast disease underreported in the region? Acta Vet Hung. 2019;67:401–6.
Zuo S, Sørensen SR, Kania PW, Buchmann K. Sarcocystis rileyi (Apicomplexa) in Anas platyrhynchos in Europe with a potential for spread. Int J Parasitol Parasites Wildl. 2021;15:270–5.
Kutkienė L, Sruoga A, Butkauskas D. Sarcocystis sp. from the goldeneye (Bucephala clangula) and the mallard (Anas platyrhynchos): cyst morphology and ribosomal DNA analysis. Parasitol Res. 2008;102:691–6.
Prakas P, Kutkienė L, Sruoga A, Butkauskas D. Sarcocystis sp. from the herring gull (Larus argentatus) identity to Sarcocystis wobeseri based on cyst morphology and DNA results. Parasitol Res. 2011;109:1603–8.
Prakas P, Butkauskas D, Juozaitytė-Ngugu E. Molecular identification of four Sarcocystis species in the herring gull, Larus argentatus, from Lithuania. Parasit Vectors. 2020;13:2.
Kutkienė L, Sruoga A, Butkauskas D. Sarcocystis sp. from white–fronted goose (Anser albifrons): cyst morphology and life cycle studies. Parasitol Res. 2006;99:562–5.
Wobeser G, Leighton FA, Cawthorn RJ. Occurrence of Sarcocystis Lankester, 1882, in wild geese in Saskatchewan. Can J Zool. 1981;59:1621–4.
Drouin TE, Mahrt JL. The morphology of cysts of Sarcocystis infecting birds in western Canada. Can J Zool. 1980;58:1477–82.
Costanzo GR. Sarcocystis in American black ducks wintering in New Jersey. J Wildl Dis. 1990;26:387–9.
Moorman TE, Baldassarre GA, Richard DM. The frequency of Sarcocystis spp. and its effect on winter carcass composition of mottled ducks. J Wildl Dis. 1991;27:491–3.
Kutkienė L, Sruoga A. Sarcocystis spp. in birds of the order Anseriformes. Parasitol Res. 2004;92:171–2.
Prakas P, Butkauskas D, Švažas S, Stanevičius V. Morphological and genetic characterisation of Sarcocystis halieti from the great cormorant (Phalacrocorax carbo). Parasitol Res. 2018;117:3663–7.
Hu JJ, Liu TT, Liu Q, Esch GW, Chen JQ, Huang S, et al. Prevalence, morphology, and molecular characteristics of Sarcocystis spp. in domestic goats (Capra hircus) from Kunming China. Parasitol Res. 2016;115:3973–81.
Acknowledgements
Not applicable.
Funding
This study was supported by the Natural Science Foundation of China (Grant 31460557).
Author information
Authors and Affiliations
Contributions
JH conceived the study, analyzed the data and drafted the manuscript. MZ conducted the molecular experiments and the experimental infection. ZW and HZ collected the specimens, calculated the prevalence and carried out the morphological observations. JT analyzed and interpreted some of the results. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The present study was approved by the Animal Ethics Committee of Yunnan University (permission no. ynucae20180002).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hu, J., Zhang, M., Wu, Z. et al. Description of Sarcocystis platyrhynchosi n. sp. (Apicomplexa: Sarcocystidae) from domestic ducks Anas platyrhynchos (Anseriformes: Anatidae) in China. Parasites Vectors 16, 50 (2023). https://doi.org/10.1186/s13071-023-05656-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-023-05656-w