Abstract
Background
Leishmaniosis, a vector-borne disease caused by Leishmania infantum, is one of the most important parasitic zoonoses in Europe. The transmission cycle of leishmaniosis is maintained by both domestic and wild animals. However, few data are available on the role of wild mammals in transmitting the parasite in the European Mediterranean basin. As feline leishmaniosis, diagnosis of the infection in ferrets can be a challenge, the use of different serological and molecular methods combined is a recommended approach. Our aim was to investigate the prevalence of infection of L. infantum in apparently healthy domestic ferrets (Mustela putorius furo) in an endemic region of Spain (Community of Valencia), using serological and molecular methods and to evaluate the results comparing the different techniques.
Methods
The prevalence of Leishmania infection was studied in domestic ferrets. Blood was collected from each animal for serology and molecular analysis. Two serological methods, enzyme-linked immunosorbent assay (ELISA) and western blot (WB), were used for the detection of L. infantum antibodies, and real-time polymerase chain reaction (qPCR) was used for the detection of L. infantum DNA.
Results
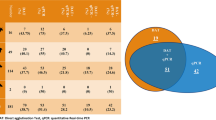
Blood samples from 102 apparently healthy ferrets were analyzed. In the serological study, 25.5% of the animals tested positive by western blot, and 9.0% by enzyme-linked immunosorbent assays. The seroprevalence of L. infantum infection, based on a positive result in any serological test, was 28.4% (95% confidence interval [CI] 20.6–S37.9%). No kinetoplast DNA (kDNA) was detected by qPCR in peripheral blood samples from the ferrets tested.
Conclusions
The immunological response revealed by these tests indicates that the ferrets are exposed to repeated inoculations with the endemic parasite L. infantum. Although the low population of domestic ferrets means their reservoir potential is limited in the absence of a primary host, it would be of interest to carry out further studies using xenodiagnosis to determine whether they are accidental or reservoir host species capable of spreading infection.
Graphical Abstract

Similar content being viewed by others
Background
Leishmaniosis, a vector-borne disease caused by Leishmania infantum, is one of the most important parasitic zoonoses in Europe [1]. In the past decade, several wild animals, including lagomorphs and canids, have been described as playing a significant role in the transmission cycle of L. infantum. Moreover, an increasing number of reports suggest that leishmaniosis is not restricted to dogs (main reservoir) but may also affect other mammalian and avian species [2]. For instance, immunological positivity to L. infantum in cats from European endemic areas suggests they are frequently exposed to the parasite [3,4,5,6] and subjected to repeated inoculations. Additionally, several studies have detected L. infantum kinetoplast DNA (kDNA) in species of the Mustelidae family [7,8,9,10] and anti-L. infantum antibodies in American [11] and European mink in Spain [12].
The most common clinical signs of leishmaniosis described in ferrets (Mustela putorius furo) are peripheral lymphadenomegaly and cutaneous lesions, including papular and ulcerative dermatitis [13]. Frequently observed clinicopathological abnormalities are hyperproteinemia with hyperglobulinemia and polyclonal gammopathy [13]. In ferrets, clinical data for leishmaniosis are still very scarce. Moreover, there is no epidemiological information about the prevalence and seroprevalence of leishmaniosis in domestic ferrets in areas endemic for L. infantum, including the Community of Valencia (Spain), where seropositive clinically sick animals have been reported [14, 15].
Epidemiological surveys are an important step in establishing the presence/absence of a pathogen in a specific region, and the results are of great value to clinicians when infections are potentially underdiagnosed, as in the case of leishmaniosis in domestic ferrets. Epidemiological studies of canine and feline leishmaniosis have combined serological and molecular methods for maximum diagnostic efficiency, an approach that could also be applied to ferrets [3, 16,17,18,19]. However, serological methods need to be adapted and validated for each species to avoid negative results in seropositive animals [13].
Given the absence of studies on leishmaniosis in ferrets in the Community of Valencia, where cases have been recently reported, the present study aimed (1) to provide the first epidemiological data on the role of domestic ferrets in the epidemiology of L. infantum infection in an endemic region; (2) to investigate the prevalence of L. infantum infection in domestic ferrets using serology and quantitative molecular assays (quantitative polymerase chain reaction [qPCR]); and (3) to evaluate the screening results of apparently healthy domestic ferrets living in an endemic region by comparing serological and qPCR data.
Methods
Study areas, ferrets, and sampling
Blood samples were collected from September 2019 to February 2021 from 102 ferrets in different towns of the Province of Valencia (39° 99 28′12.864″ N, 0° 22′36.48″ W), an area on the east coast of the Iberian Peninsula with a high incidence of canine leishmaniosis (50–100/1000 dogs/year) [20, 21]. Based on epidemiological studies, 17.1% of dogs are seropositive in the Community of Valencia in Spain [21, 22]. However, the presence of human cutaneous leishmaniosis outbreak has also been detected in the Valencia region [22]
Before sampling, information was obtained about each animal regarding age, cohabitation with a dog, lifestyle (indoor, outdoor, or mixed) and gender, and a complete physical examination was carried out to establish health status (sick versus healthy). Whenever possible, one ml of blood was collected aseptically by cranial cava venipuncture from each ferret. The collected volume was divided equally between a sterile blood collection tube (to obtain the serum) and a second tube containing ethylenediaminetetraacetic acid (EDTA) anticoagulant (for molecular analysis). EDTA-blood and separated sera were stored at −20 °C until processing. Routine laboratory tests, such as a complete blood count and biochemistry profile, were not performed.
In total, 102 client-owned ferrets were sampled. A single sample was obtained from 94 ferrets. The other eight client-owned ferrets were tested an additional one (n = 5) to two (n = 3) different times during the study period. A total of 113 serum samples were included.
Diagnostic serological tests
Detection of specific antibodies was performed using two in-house serological techniques: enzyme-linked immunosorbent assay (ELISA) and western blot (WB).
Detection of L. infantum antibodies by a quantitative ELISA
The ELISA was performed on all sera as described previously, with some modifications [14]. Briefly, each plate was coated with 20 µg/ml of crude antigen obtained from L. infantum promastigote forms (MHOM/MON-1/LEM 75) in 0.1 M carbonate/bicarbonate buffer (pH 9.6) and incubated overnight at 4 °C. Next, 100 µl of cat sera, diluted 1:200 in phosphate-buffered saline (PBS) containing 0.05% Tween 20 (PBST) and 1% dry skimmed milk (PBST-M), was added to each well. The plates were incubated for 1 h at 37 °C in a moist chamber. Then they were washed, and 100 µl of protein A conjugated to horseradish peroxidase (Thermo Fisher Scientific, Waltham, MA, USA) diluted 1:8000 in PBST-M was added. The plates were incubated for 1 h at 37 °C in the moist chamber and were washed again with PBST and PBS as described above. The substrate solution (ortho-phenylenediamine) and stable peroxide substrate buffer (Thermo Fisher Scientific, Waltham, MA, USA) was added per well and developed for 20 ± 5 min at room temperature in the dark. The reaction was stopped by adding 2.5 M H2SO4 to each well. Absorbance values were read at 492 nm in an automatic microELISA reader (Multiskan ELISA reader, Labsystems, Midland, Canada).
As a positive control (calibrator), each plate included serum from a ferret from Spain diagnosed with leishmaniosis, confirmed by a positive culture, and as a negative control, serum from a healthy, non-infected ferret. The same positive control serum was used for all assays and plates, with a constant inter-assay variation of < 10%. Plates with an inter-assay variation of > 10% were discarded. All samples and controls were run in duplicate. The cut-off was set to 0.180 optical density units (OD) (mean + 3 standard deviations of values from 30 indoor ferrets from northern Spain), and results above this value were considered positive.
Detection of L. infantum antibodies by WB
Anti-Leishmania antibodies were detected by WB using a whole antigen of L. infantum promastigotes (MHOM/FR/78/LEM75 zymodeme MON-1), as described by Alcover et al. [3], with some modifications. The protocol used for WB is based on the technique described by Aisa et al. [23], with sensitivity of 95.8% and specificity of 100% in dogs. Moreover, the specificity has been analyzed including a group of healthy cats (n = 20) from a non-endemic area (Switzerland) with a value of 100%. Antigen electrophoresis on 0.1% sodium dodecyl sulfate (SDS)-15% polyacrylamide gels together with molecular mass protein standards (low-range standard; Bio-Rad, Hercules, CA, USA) was performed on a Mini-Gel AE-6400 Dual Mini Slab Kit (Atto, Bunkyo-ku, Japan).
Gels were run at 100 V for 1 h at room temperature. Polypeptides were transblotted onto nitrocellulose sheets (0.45-mm pore size, HAWP 304 FO; Millipore, Bedford, MA, USA), which were blocked with 20 mM Tris, 0.13 mM NaCl, pH 7.6 (TS), and 5% skimmed milk overnight at 4 °C.
The sheets were washed in TS and introduced into a multiscreen apparatus (Mini-PROTEAN II; Bio-Rad, Hercules, CA, USA). Sera were diluted 1:200 in TS-1% skimmed milk and 0.2% Tween 20. Then 500 µl of each sample was introduced into each channel of the multiscreen apparatus and incubated for 2 h at 37 °C. Bound immunoglobulins were developed by incubation with a 1:1000 dilution of protein A peroxidase conjugate (Thermo Fisher Scientific, Waltham, MA, USA) for 1 h. After the sheets were washed three times with TST and a final time with TS, color was developed with 4-chloro-1-naphthol substrate (Thermo Fisher Scientific, Waltham, MA, USA). Based on our experience and the literature, a serum sample was considered positive when immunoreactivity against the L. infantum antigen fraction 14 and /or 16 kDa was observed, and indeterminate when molecular weight bands of 18, 20, 24, 28, 30, 36, 38, and 46 kDa appeared, as reported previously [3].
Detection of L. infantum DNA by qPCR
DNA was extracted from 200 μl of mammalian blood using the High Pure PCR Template Preparation Kit (Roche Applied Science, Mannheim, Germany), which allows genomic DNA to be isolated rapidly and easily from a wide variety of sample materials. All extractions were performed following the manufacturer’s instructions, and multiple PCR templates were obtained in minutes using efficient High Pure spin columns.
The detection and quantification of Leishmania kDNA was carried out by amplification of kinetoplast minicircle DNA sequences by qPCR [13]. Each amplification was performed in triplicate in 10 μl of reaction mixture containing 1× iTaq Supermix with ROX (Bio-Rad, Hercules, CA, USA), 15 pmol of direct primer Leim1 (5′-CTT TTC TGG TCC TCC GGG TAG G-3′), 15 pmol of reverse primer Leim2 (5′- CCA CCC GGC CCT ATT TTA CAC CAA-3′), 50 pmol of the labeled TaqMan probe Leim3 (5′- FAM-TTT TCG CAG AAC GCC CCT ACC CGC TAMRA-3′), and 2.5 μl of sample DNA.
The ABI Prism 7900 HT thermocycler (Applied Biosystems, Waltham, MA, USA) was used at 94 °C and 55 °C cycling over 40 cycles with a FAM detector. A non-template control was used in each run as the qPCR negative control. A 10-fold dilution series of DNA from promastigotes (MHOM/ES/04/BCN-61, L. infantum) was used for calibration (serial dilution from 105 parasites/ml to 10−3 parasites/ml), allowing the plotting of a standard curve. The qPCR was considered positive when the threshold cycle (Ct) was lower than 40 and when the amplification was detected in all the replicates [3, 24, 25].
Statistical analysis
Data collected for the entire population were analyzed using descriptive statistics. Associations between L. infantum and the recorded variables were analyzed. The significance of this difference was assessed using the Chi-square or Fisher’s exact test. A value of P ≤ 0.05 was considered significant. The SPSS v.22 software grogram was used (IBM Corporation, Armonk, NY, USA).
Results
Animals studied
All the tested ferrets (n = 102; 49 females and 53 males) had a mixture of coat colors, and none had been surgically neutered. All ferrets were classified as apparently healthy, with no systemic signs of disease found in the general physical examination. The mean age of the animals was 4 years (ranging from 1 to 8), and they were classified as young (< 2 years), adult (from ≥ 2 years to ≤ 6 years), or senior (> 6 years). None of the ferrets had been treated with a long-acting topical anti-parasitic repellent against sand flies (Table 1).
No significant association (P > 0.05) was detected between Leishmania positivity and gender (male/female), age (young, adult, senior), cohabitation with a dog, or lifestyle (indoor, outdoor, or mixed). All statistical analysis can be found in Additional file 1: Table S1.
For the serological study, a total of 113 serum samples were analyzed, 11 of which were obtained from the eight seropositive animals that were followed up. Molecular tests were applied to 62 peripheral blood samples, seven of which belonged to five seropositive animals that were followed up. EDTA-blood could not be obtained from some of the domestic ferrets (n = 47) due to their small size and the absence of an anesthetic procedure during blood extraction.
Serology and qPCR for L. infantum
In the serological study, 100% of the animals were analyzed by both ELISA and WB. By ELISA, nine positive animals were detected, the seropositive rate being 8.8% (95% CI 4.5–16.1%). Using WB, 26 were positive, which constitutes a seropositivity of 25.5% (95% CI 18.0–34.8%), and nine ferrets (8.8%) gave indeterminate results (i.e., no bands were observed at 14 and/or 16 kDa). The WB data for sensitivity to the L. infantum antigen are provided in Fig. 1. Among the 26 positive ferrets, bands were observed at both 14 and 16 kDa for nine animals, at 16 kDa and other molecular weights for 12, and only at 16 kDa for five ferrets.
Of the nine animals diagnosed as indeterminate by WB, eight were negative by other techniques, and one with a band at 46 kDa tested positive by ELISA. Twenty-nine ferrets (28.4%) tested positive by at least one of the three techniques (95% CI 20.6–37.9%). Twenty of these were diagnosed as positive only by WB, and three only by ELISA (including one of the three WB indeterminate sera), whereas six ferrets tested positive by both serological techniques. Eight of the 29 positive animals were followed up, four of them testing negative in the second sample; two of the four were analyzed only by serological techniques and two by ELISA, WB, and PCR. The WB result for one of these four animals was indeterminate, yielding a band only at 20 kDa.
During the follow-up of eight animals originally found positive by WB, three remained positive by WB in the second analysis, and only one (M5706) tested positive in a third analysis (Table 2). One of the three ferrets (M5931) was also positive by ELISA, and again tested positive by both serological techniques in the second analysis (ELISA titer of 0.241 OD and WB band at 16 kDa); however, in the subsequent follow-up, both tests were negative (Table 2). The third of the three ferrets (M6188) tested negative in the second analysis. This animal was classified as apparently healthy ferret without evidence clinical signs detected during physical examination. In this sense, it is possible to detect seasonal variation in anti-Leishmania antibodies in dogs [26] and ferrets [27] in endemic areas of canine leishmaniosis. Finally, all the asymptomatic ferrets with full blood samples, previously screened for Leishmania DNA, tested negative.
Discussion
In this study, 102 domestic ferrets in the Community of Valencia (Spain) were tested for L. infantum infection using serological and molecular techniques. The rate of seropositivity was high by both ELISA and WB, although the number of positive cases detected by WB was statistically significantly higher (P < 0.008). WB is known to be a more sensitive method than ELISA, which is more specific [28]. Generally used as a confirmatory test of ELISA results, WB is more difficult to perform and requires higher laboratory skills. However, the results of the present study do not reflect these differences in sensitivity and specificity.
The detection of L. infantum kDNA by qPCR has been previously evaluated in a range of matrices [3, 24, 25]. In veterinary medicine, it is known, especially in dogs, that blood is not the best matrix for Leishmania qPCR [29]. Noninvasive samples including oral and conjunctival swabs, hair, or saliva have been evaluated, but there is a lack of studies that support the usefulness of such samples applied in clinical practice as follow-up and clinical prognostic parameters [30,31,32]. In Spain, blood samples from stray cats have been tested by qPCR to detect the parasite, and variations in prevalence between studies and endemic regions have been reported [3]. Parasite detection using qPCR assays based on highly repetitive gene loci or extrachromosomal kDNA sequences is restricted to the genus level. In the present study, we used extremely sensitive genus-specific primers to be able to detect asymptomatic animals. Even so, the qPCR results of peripheral blood samples were all negative, possibly because the parasite load in the circulatory system was insufficient for detection by this technique or was nonexistent. Leishmania protozoa mainly multiply in macrophages of the skin and spleen, and therefore in mild infections their levels in blood are low [33, 34]. Thus, although peripheral blood samples are easy to obtain and are minimally invasive, they proved unsuitable for detecting kDNA of Leishmania spp. in asymptomatic ferrets, which may have low or unstable parasitemia over time. A study analyzing Leishmania infection in mustelids using tissue macerates reported high percentages of kDNA detection by qPCR [7], suggesting that this type of sample is more suitable for genetic analysis, although it is also more invasive. In ferrets, we suspect that L. infantum would be more prevalent in organ tissues than in blood. This may imply that ferrets are not efficient Leishmania reservoirs and cannot sustain the parasite transmission cycle alone. For further clarification of the nature of Leishmania infection in ferrets, xenodiagnostic studies would be useful [3, 35,36,37].
In general, seroprevalence studies on specific pathogens in animals produce variable results depending on factors such as the geographical location, lifestyle, age, or analytical methodology. The data obtained in the present study provide an estimation of L. infantum seroprevalence in domestic ferrets in Spain. Although we obtained data demonstrating ferret exposure to L. infantum inoculations, in accord with a previous report [27], the mere presence of antibodies or parasite DNA is not sufficient evidence of reservoir host status [38]. To confirm whether an animal is an accidental or reservoir (primary or secondary) host of L. infantum requires xenodiagnosis. Also, in areas with a high density of sand fly vectors and biting rates, if a suitable vertebrate reservoir host species for Leishmania is absent or rare, the transmission cycle of the parasite is not sustained in the long term. Such a case has been reported in a peri-urban zoological park in southern Spain, where most sand flies feed on large herbivores that do not act as Leishmania reservoirs [37].
Although ferrets may not play a crucial role as reservoir hosts, their involvement in the parasite cycle may still have a significant impact. More studies are needed to elucidate the epidemiological role of domestic ferrets in the spread of leishmaniosis in L. infantum endemic areas such as the Community of Valencia in Spain.
Conclusions
Our serological results revealed that domestic ferrets in the Mediterranean basin are exposed to the endemic parasite L. infantum. To our knowledge, this study demonstrates for the first time the seroprevalence and prevalence rates of L. infantum in domestic ferrets in an endemic region of Spain. Further prevalence surveys in other endemic regions, using other diagnostic methods and more suitable matrices for molecular analysis, would be useful to clarify the role of this popular pet in the epidemiology of L. infantum transmission.
Availability of data and materials
The datasets supporting the findings of this article are included within the article and its additional files.
Abbreviations
- Ct:
-
Threshold cycle
- EDTA:
-
Ethylenediaminetetraacetic acid
- ELISA:
-
Enzyme-linked immunosorbent assay
- qPCR:
-
Quantitative real-time PCR
- PCR:
-
Polymerase chain reaction
- WB:
-
Western blot
References
Baneth G, Thamsborg SM, Otranto D, Guillot J, Blaga R, Deplazes P, et al. Major parasitic zoonoses associated with dogs and cats in Europe. J Comp Pathol. 2016;155:S54-74. https://doi.org/10.1016/j.jcpa.2015.10.1792.
Cardoso L, Schallig H, Persichetti MF, Pennisi MG. New epidemiological aspects of animal leishmaniosis in Europe: the role of vertebrate hosts other than dogs. Pathogens. 2021;10:307. https://doi.org/10.3390/pathogens10030307.
Alcover MM, Basurco A, Fernández A, Riera C, Fisa R, González A, et al. A cross-sectional study of Leishmania infantum infection in stray cats in the city of Zaragoza (Spain) using serology and PCR. Parasit Vectors. 2021;14:178. https://doi.org/10.1186/s13071-021-04682-w.
Gramiccia M. Recent advances in leishmaniosis in pet animals: epidemiology, diagnostics and anti-vectorial prophylaxis. Vet Parasitol. 2011;181:23–30. https://doi.org/10.1016/j.vetpar.2011.04.019.
Spada E, Canzi I, Baggiani L, Perego R, Vitale F, Migliazzo A, et al. Prevalence of Leishmania infantum and co-infections in stray cats in northern Italy. Comp Immunol Microbiol Infect Dis. 2016;45:53–8. https://doi.org/10.1016/j.cimid.2016.03.001.
Morganti G, Veronesi F, Stefanetti V, Di Muccio T, Fiorentino E, Diaferia M, et al. Emerging feline vector-borne pathogens in Italy. Parasit Vectors. 2019;12:193. https://doi.org/10.1186/s13071-019-3409-8.
Del Rio L, Chitimia L, Cubas A, Victoriano I, De la Rúa P, Gerrikagoitia X, et al. Evidence for widespread Leishmania infantum infection among wild carninvores in L. infantum periendemic northern Spain. Prev Vet Med. 2014;113:430–5. https://doi.org/10.1016/j.prevetmed.2013.12.001.
Oleaga A, Zanet S, Espí A, Macedo MRP, Gortázar C, Ferroglo E. Leishmania in wolves in northern Spain: a spreading zoonosis evidenced by wildlife sanitary surveillance. Vet Parasitol. 2018;255:26–31. https://doi.org/10.1016/j.vetpar.2018.03.015.
Risueño J, Ortuño M, Pérez-Cutillas P, Goyena E, Maia C, Cortes S, et al. Epidemiological and genetic studies suggest a common Leishmania infantum transmission cycle in wildlife, dogs and humans associated to vector abundance in Southeast Spain. Vet Parasitol. 2018;259:61–7. https://doi.org/10.1016/j.vetpar.2018.05.012.
Cantos-Barreda A, Navarro R, Pardo-Marín L, Martínez-Subiela S, Ortega E, Cerón JJ, et al. Clinical leishmaniosis in a captive Eurasian otter (Lutra lutra) in Spain: a case report. Vet Parasitol. 2018;251:131–7. https://doi.org/10.1186/s12917-020-02509-x.
Azami-Conesa I, Sansano-Maestre J, Martínez-Díaz RA, Gómez-Muñoz MT. Invasive species as hosts of zoonotic infections: the case of American Mink (Neovison vison) and Leishmania infantum. Microorganisms. 2021;9:1531. https://doi.org/10.3390/microorganisms9071531.
Giner J, Villanueva-Saz S, Fernández A, Gómez MA, Podra M, Lizarraga P, et al. Detection of Anti-Leishmania infantum antibodies in wild European and American Mink (Mustela lutreola and Neovison vison) from Northern Spain, 2014–20. J Wildl Dis. 2022;58:198–204. https://doi.org/10.7589/JWD-D-21-00027.
Villanueva-Saz S, Giner J, Marteles D, Verde M, Yzuel A, Riera C, et al. Leishmaniosis caused by Leishmania infantum in ferrets: update review. Vet Anim Sci. 2021;15:100229. https://doi.org/10.1016/j.vas.2021.100229.
Giner J, Basurco A, Alcover MM, Riera C, Fisa R, López RA, et al. First report on natural infection with Leishmania infantum in a domestic ferret (Mustela putorius furo) in Spain. Vet Parasitol Reg Stud Reports. 2020;19:100369. https://doi.org/10.1016/j.vprsr.2020.100369.
Giner J, Villanueva-Saz S, Alcover MM, Riera C, Fisa R, Verde M, et al. Clincial leishmaniosis in a domestic ferret (Mustela putorius furo) treated with miltefosine plus allopurinol: serological and clinical follow-up. Vet Parasitol Reg Stud Reports. 2021;25:100607. https://doi.org/10.1016/j.vprsr.2021.100607.
Najafi L, Omidian M, Rezaei Z, Shahabi S, Ghorbani F, Arefkhah N, et al. Molecular and serological evaluation of zoonotic visceral leishmaniasis in dogs in a rural area of Fars province, southern Iran, as a source of Leishmania infantum infection. Vet Med Sci. 2021;7:1082–9. https://doi.org/10.1002/vms3.432.
Urbani L, Tirolo A, Salvatore D, Tumbarello M, Segatore S, Battilani M, et al. Serological, molecular and clinicopathological findings associated with Leishmania infantum infection in cats in Northern Italy. J Feline Med Surg. 2020;22:935–43. https://doi.org/10.1177/1098612X19895067.
Pennisi MG, Cardoso L, Baneth G, Bourdeau P, Koutinas A, Miró G, et al. LeishVet update and recommendations on feline leishmaniosis. Parasite Vectors. 2015;8:302. https://doi.org/10.1186/s13071-015-0909-z.
Rivas AK, Alcover MM, Martínez-Orellana P, Montserrat-Sangrà S, Nachum-Biala Y, Fisa R, et al. Serological and molecular survey of Leishmania infection in dogs from Venezuela. Vet Parasitol Reg Stud Reports. 2020;21:100420. https://doi.org/10.1016/j.vprsr.2020.100420.
Le Rutte EA, van der Wilt LS, Bulstra CA, Nieboer D, Kontoroupis P, de Vlas SJ, et al. Incidence and geographical distribution of canine leishmaniosis in 2016–2017 in Spain and France. Vet Parasitol Reg Stud Reports. 2021;25:100613. https://doi.org/10.1016/j.vprsr.2021.100613.
Montoya-Alonso JA, Morchón R, Costa-Rodríguez N, Matos JI, Falcón-Cordón Y, Carretón E. Current distribution of selected vector-borne diseases in dogs in Spain. Front Vet Sci. 2020;7:564429. https://doi.org/10.3389/fvets.2020.564429.
Roth-Damas P, Sempere-Manuel M, Mialaret-Lahiguera A, Fernández-García C, Gil-Tomás JJ, Colomina-Rodríguez J, et al. Community outbreak of cutaneous leishmaniasis in La Ribera region of Valencia, Spain: public Health measures. Enferm Infecc Microbiol Clin. 2017;35:338–43. https://doi.org/10.1016/j.eimc.2016.04.006.
Aisa MJ, Castillejo S, Gallego M, Fisa R, Riera MC, de Colmenares M, et al. Diagnostic potential of Western blot analysis of sera from dogs with leishmaniasis in endemic areas and significance of the pattern. Am J Trop Med Hyg. 1998;58:154–9. https://doi.org/10.4269/ajtmh.1998.58.154.
Martín-Ezquerra G, Fisa R, Riera C, Rocamora V, Fernández-Casado A, Barranco G, et al. Role of Leishmania spp. infestation in nondiagnostic cutaneous granulomatous lesions: report of a series of patients from a Western Mediterranean area. Brit J Dermatol. 2009;161:320–5. https://doi.org/10.1111/j.1365-2133.2009.09282.x.
Tomás-Pérez M, Khaldi M, Riera C, Mozo-León D, Ribas A, Hide M, et al. First report of natural infection in hedgehogs with Leishmania major, a possible reservoir of zoonotic cutaneous leishmaniasis in Algeria. Acta Trop. 2014;135:44–9. https://doi.org/10.1016/j.actatropica.2014.03.018.
Cavalera MA, Iatta R, Panarese R, Mendoza-Roldan JA, Gernone F, Otranto D, et al. Seasonal variation in canine anti-Leishmania infantum antibody titres. Vet J. 2021;271:105638. https://doi.org/10.1016/j.tvjl.2021.105638.
Villanueva-Saz S, Giner J, Verde M, Yzuel A, Ruiz H, Lacasta D, et al. Antibodies to Leishmania in naturally exposed domestic ferrets (Mustela putorius furo) in Spain. Vet Parasitol. 2021;296:1094. https://doi.org/10.1016/j.vetpar.2021.109492.
Riera C, Fisa R, Udina M, Gállego M, Portus M. Detection of Leishmania infantum cryptic infection in asymptomatic blood donors living in an endemic area (Eivissa, Balearic Islands, Spain) by different diagnostic methods. T Roy Society Trop Med Hyg. 2004;98:102–10. https://doi.org/10.1016/s0035-9203(03)00015-4.
Solano-Gallego L, Miró G, Koutinas A, Cardoso L, Pennisi MG, Ferrer L, et al. LeishVet guidelines for the practical management of canine leishmaniosis. Parasit Vectors. 2011;4:86. https://doi.org/10.1186/1756-3305-4-86.
Lombardo G, Pennisi MG, Lupo T, Migliazzo A, Caprì A, Solano-Gallego L. Detection of Leishmania infantum DNA by real-time PCR in canine oral and conjunctival swabs and comparison with other diagnostic techniques. Vet Parasitol. 2012;184:10–7. https://doi.org/10.1016/j.vetpar.2011.08.010.
Belinchón-Lorenzo S, Iniesta V, Parejo JC, Fernández-Cotrina J, Muñoz-Madrid R, Soto M, et al. Detection of Leishmania infantum kinetoplast minicircle DNA by Real Time PCR in hair of dogs with leishmaniosis. Vet Parasitol. 2013;192:43–50. https://doi.org/10.1016/j.vetpar.2012.11.007.
Cantos-Barreda A, Escribano D, Siriyasatien P, Cerón JJ, Thomas MC, Afonso-Lehmann RN, et al. Detection of Leishmania infantum DNA by real-time PCR in saliva of dogs. Comp Immunol Microbiol Infect Dis. 2020;73:101542. https://doi.org/10.1016/j.cimid.2020.101542.
Alcover MM, Ribas A, Guillén MC, Berenguer D, Tomás-Pérez M, Riera C, et al. Wild mammals as potential silent reservoirs of Leishmania infantum in a Mediterranean area. Prev Vet Med. 2020;175:104874. https://doi.org/10.1016/j.prevetmed.2019.104874.
Di Pietro S, Crinò C, Falcone A, Crupi R, Francaviglia F, Vitale F, et al. Parasitemia and its daily variation in canine leishmaniasis. Parasitol Res. 2020;119:3541–8. https://doi.org/10.1007/s00436-020-06845-7.
García N, Moreno I, Alvarez J, de la Cruz ML, Navarro A, Pérez-Sancho M, et al. Evidence of Leishmania infantum infection in rabbits (Oryctolagus cuniculus) in a natural area in Madrid Spain. Biomed Res Int. 2014;2014:318254. https://doi.org/10.1155/2014/318254.
Ben Othman S, Ghawar W, Chaouch M, Ayari C, Chemkhi J, Cancino-Faure B, et al. First detection of Leishmania DNA in Psammomys obesus and Psammomys vexillaris: their potential involvement in the epidemiology of leishmaniasis in Tunisia. Infect Genet Evol. 2018;59:7–15. https://doi.org/10.1016/j.meegid.2018.01.013.
Muñoz C, de la Puente JM, Figuerola J, Pérez-Cutillas P, Navarro P, Ortuño M, et al. Molecular xenomonitoring and host identification of Leishmania sand fly vectors in a Mediterranean periurban wildlife park. Transboun Emerg Dis. 2019;66:2546–61. https://doi.org/10.1111/tbed.13319.
WHO. World Health Organization. 2010, WHO Technical Report Series, 949. https://apps.who.int/iris/bitstream/handle/10665/44412/WHO_TRS_949_eng.pdf?sequence=1&isAllowed=y. Accessed 14 September 2022.
Acknowledgements
The authors would like to acknowledge Carmen Guillén for technical support in serological testing. The authors thank Lucy Brzoska for her help with the English.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
SVS, CR, MA and RF designed the survey. CR, MA, RF and SVS supervised technical work. MA, JG, JR, XR, MV, AF, CR, RF and SVS contributed with data analysis. MA, JG, JR, CR, RF and SVS and wrote the manuscript. JG and SVS performed physical examination and collected samples from ferrets. MA, JR, MV and SVS performed serological testing and JR and XR performed the molecular work of this study. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This survey was included under Project Licence PI25/20 approved by the Ethics Committee for Animal Experiments for the University of Zaragoza. The care and use of animals were performed in accordance with the Spanish Policy for Animal Protection RD 53/2013, which meets the European Union Directive 2010/63 on the protection of animals used for experimental and other scientific purposes.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Statistical analysis of the positivity to IgG antibodies against L. infantum in ferrets in Spain, in relation to sex, age, housing shelter, and cohabitation with a dog.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Alcover, M.M., Giner, J., Rabasedas, J. et al. First epidemiological survey of Leishmania infantum in the domestic ferret (Mustela putorius furo) in a canine leishmaniosis endemic area using serology and PCR. Parasites Vectors 15, 372 (2022). https://doi.org/10.1186/s13071-022-05517-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-022-05517-y