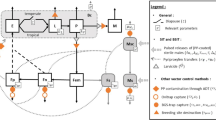
Abstract
Background
The sterile insect technique (SIT), which involves area-wide inundative releases of sterile insects to suppress the reproduction of a target species, has proven to be an effective pest control method. The technique demands the continuous release of sterilized insects in quantities that ensure a high sterile male:wild male ratio for the suppression of the wild population over succeeding generations.
Methods
For these releases, it is important to determine several ecological and biological population parameters, including the longevity of the released males in the field, the dispersal of the released males and the wild pest population size. The Lee County Mosquito Control District initiated a study in a 47-ha portion of Captiva Island (Florida, USA), an island with a total area of 230 ha, to define biological SIT parameters for Aedes aegypti (L.), an invasive disease-vectoring mosquito known to be difficult to control due to a combination of daytime biting activity, use of cryptic breeding habitats that are difficult to target with conventional night-time ultra-low volume methods, and emerging resistance to commonly used insecticides. Another goal was to assess patterns of dispersal and survival for laboratory-reared sterile Ae. aegypti males released over time in the pilot site. These parameters will be used to evaluate the efficacy of a SIT suppression program for Ae. aegypti on Captiva Island.
Results
Over the course of seven mark-release-recapture studies using single- and multiple-point releases, 190,504 sterile marked males were released, for which the recapture rate was 1.5% over a mean period of 12 days. The mean distance traveled by sterile males of the local strain of Ae. aegypti that has colonized Captiva Island was 201.7 m from the release point, with an observed maximum traveled distance of 404.5 m. The released sterile mosquitoes had a probability of daily survival of 0.67 and an average life expectancy of ~ 2.46 days.
Conclusions
These data together with the population size estimate and sterile:wild ratio provide a solid basis for planning the SIT operational phase which is aimed at mosquito population suppression.
Graphical abstract

Similar content being viewed by others
Background
The mosquito Aedes aegypti commonly occurs in and around homes and businesses in urban and suburban locations in many tropical and subtropical areas of the world. It is a major vector of viruses that can cause human disease, including dengue, chikungunya, Zika, and yellow fever viruses [1]. Aedes aegypti is of particular concern in Florida, USA, because of its role in the local transmission of Zika virus in 2016 in Miami, and limited local transmission of dengue in the state on several occasions in the last 20 years [2]. Even in areas where these arboviruses are rare or absent, Ae. aegypti is a serious nuisance because it lives in close proximity to people and exhibits aggressive biting behavior during lengthy periods of the day, which dampens enthusiasm for a broad range of outdoor activities [3].
A number of factors make Ae. aegypti difficult to control in urban and suburban environments. Breeding site removal and the effective application of larvicides can be difficult because Ae. aegypti females lay their eggs in many types of small cryptic oviposition sites that commonly occur in urban and suburban environments [4, 5]. Furthermore, Ae. aegypti adults tend to rest in protected, sheltered habitats that are not easily reached by ultra-low volume adulticides [6]. In addition to difficulties in reaching Ae. aegypti with traditional delivery methods for larvicides and adulticides, the prevalence of insecticide resistance due to the intensive use of insecticides has been increasingly documented in container-breeding mosquitoes worldwide, including Ae. aegypti in Florida [7, 8]. This decreasing efficacy of traditional mosquito control pesticides is also coupled with increasing public demand for the use of environmentally friendly, non-chemical pest control [9, 10].
Several innovative and targeted population suppression methods are under evaluation for use in integrative Ae. aegypti control, including the sterile insect technique (SIT). Although SIT has been used for many decades for several agricultural and livestock pests [11, 12], extensive efforts have recently been made to adapt the technique for mosquito control with the development of an International Atomic Energy Agency (IAEA) thematic plan along with a guidance framework for this subject devised by the World Health Organization and the IAEA [13]. Typically, SIT uses ionizing radiation to induce sterility in massive numbers of laboratory-reared male mosquitoes that, after being released in the field, compete with wild males to mate with wild females. With consistent releases of sterile males, SIT can reduce and even eventually dramatically suppress the wild population [14,15,16].
SIT requires an understanding of the size, spatial patterns of abundance, and dynamics of wild populations, as well as high-quality laboratory-reared sterile males for the successful suppression of mosquito populations [17]. Collecting baseline data on wild populations to characterize population densities in both high and low seasons is vital to estimating how many sterile males should be released to suppress the wild population each season. Indirect measurement of population density over space and time through entomological surveillance using traps to capture adult mosquitoes is the standard method for estimating mosquito population density [18, 19]. Fluctuations in mosquito populations are often tightly correlated with environmental conditions, such as rainfall patterns and temperature [20, 21]. However, documenting mosquito population dynamics alone is insufficient for successful SIT implementation because, ultimately, an understanding is required of how well laboratory-raised sterile males will perform in the field. Mark-release-recapture (MRR) studies are crucial for understanding wild male abundance, as well as for describing ecological parameters of released insects, such as the dispersal and survival dynamics of laboratory-raised sterile males in the field [21,22,23,24].
Lee County Mosquito Control District (LCMCD), located in Florida, USA, is responsible for controlling mosquito populations in an area of ~ 2033 km2 comprising ~ 98% of the county. Established in 1958 as an independent special taxing district, LCMCD operates a robust program aimed at mosquito suppression. Using an area-wide integrated pest management approach, efforts include chemical suppression of both the larval and adult stages, disease surveillance, mosquito population surveillance, and an insecticide-resistance monitoring program [25]. In 2017, LCMCD initiated a SIT pilot program in Captiva Island, Florida, USA, a ~ 230-ha barrier island, with the aim of establishing an operational SIT program for Ae. aegypti control. Following the phased conditional approach [18], LCMCD established a trapping network in 2017 for baseline data collection. Here we describe a series of MRR studies undertaken as preparatory steps for an operational suppression program. The MRR studies were performed using single-point releases [22, 26] to characterize sterile male dispersal patterns, and multiple-point releases [27, 28] were used to provide data on the longevity of released sterile males in the field, along with estimates of wild male population size on Captiva Island.
Methods
Colony rearing and irradiation
A colony of Ae. aegypti developed from field-collected eggs from Captiva Island and nearby Sanibel Island (381371.23 E, 2934234.89 N) was used for all the releases. The lab colony is refreshed annually after it reaches the 10th inbreeding generation by adding material from the field to reduce the side effects of colony inbreeding and to increase the chances of maintaining behavior similar to that of the wild population. A similar blood-feeding method previously described by our group was used for the production of eggs from adult females [29]. A nutrient broth of 200 mg Purina AquaMax Fry Powder in 500 ml water was used for egg hatching. After hatching, larvae were fed a slurry of Purina AquaMax Fry Powder (0.2, 0.3, 0.4, 0.6 and 0.6 mg/larva on days 2, 3, 4, 5 and 6, respectively). Upon pupation, the pupae were collected and separated by Fay-Morlan glass plate separators using pupal size differences to separate males from females [30]. Pupal weights were determined and used to estimate the number of mosquitoes for separate cohorts, which were divided into desired batch sizes for downstream treatment. Females were discarded. At least 30 h after pupation, male pupae were irradiated at a target dose of 52 Gy, which resulted in more than 99% sterility. Irradiation was carried out in a RS 2400 X-ray machine (Rad Source Technologies, Buford, GA), operated at 160 kV and 25 mA, with a dose rate of 13.89 Gy/min, following calibration procedures developed by IAEA [31]. Following sterilization, 2200 pupae were placed in emergence containers (2.37 L; height 14 cm, diameter 17 cm) with a 3-cm horizontal slit cut 1 cm from the bottom and containing 200 ml water. The smooth surface of the interior walls (with the exception of the lowest 2 cm) of the containers was lightly scuffed to provide a vertical area that was easier for mosquitoes to rest on. The lids were modified by replacing the inner 13.5-cm-diameter plastic center with 1-mm by 1-mm mesh screen. Once emergence was completed, the container was pressed just above the slit to create a small gap through which the water was gently poured out, and an absorbent piece of paper was inserted through the slit to soak up and remove all the remaining water. Pupal mortality was assessed from the material in the water. The adults were provided with 10% sucrose solution ad libitum via moist cotton placed on the screen portion of the lid until they were released. The insectary was maintained at a temperature of 27 ± 1 °C, 80% relative humidity, and 12-h light:12-h dark photoperiod.
Study area
The MRR studies were carried out on Captiva Island between 2019 and 2021. Captiva is a ~ 306-ha barrier island off Florida’s southwest coast in Lee County (381371.23 E, 2934234.89 N, ~ 306 ha area). Captiva Island is connected to Sanibel Island to the south by an automotive bridge, and is bordered by the Gulf of Mexico to the west and Pine Island Sound to the east. Captiva has a subtropical climate with a rainy season in the warmest months that usually lasts from late May through September [average rainfall 19.91 cm, average temperature 27.46 °C (National Oceanic and Atmospheric Administration)]. The dry season, during which the temperatures are lower, stretches from October through April [average rainfall 5.44 cm, average temperature 20.3 °C (National Oceanic and Atmospheric Administration)]. There are 1108 housing units on Captiva, of which 188 are occupied and 920 vacant [32]. The island has a total human population of 318, but experiences high seasonal influxes of visitors from December through April [33]. A large resort area with a golf course, privately owned homes, condominiums, and restaurants occupies the north end of the island (~ 94 ha). The middle portion of Captiva Island has the highest density of residences, consisting of condominiums, townhomes and other types of privately owned homes of various sizes. In this area, there is also the greatest concentration of restaurants, many of which are at least partially open air, and other businesses. As Captiva is a narrow island, this area of high occupational density, which is situated in the widest section of the island, was considered the best site in which to perform the MRR studies. The buildings in the narrower southern part of the island (~ 210 ha) are primarily large privately owned homes.
Mark-release-recapture
The MRR studies were carried out across the year in an attempt to account for seasonal differences in the wild Ae. aegypti population. Based on the fluctuations observed in the baseline entomological data (in preparation), the estimated adult population density was categorized as generally low or high for each MRR event, with the additional descriptor of whether we expected the population to be entering a seasonal growth or decline phase. In total, between 2019 and 2021, seven MRR studies were performed at the widest part of the island (~ 47 ha; Fig. 1). Of these seven studies, three involved single-point releases (SP01, SP02, and SP03) and four multiple-point releases (MP01, MP02, MP03, and MP04). SP03 and MP04 correspond to one release because they were carried out on the same day; for these, differently colored fluorescent powder was used to mark the mosquitoes released from the center and those released from the surrounding points (Fig. 1c). Table 1 shows the differences between the MRR studies conducted on Captiva Island. These, minor, differences were due to facilitating and optimizing the studies according to the number of staff available and all the other activities that they needed to undertake.
a Map showing the location of Lee County (in orange) in Florida. Inset shows the location of Florida (in orange) in the USA. b Captiva Island (in orange) and Sanibel Island (in maroon), Lee County. Approximate location of the ca. 47-ha mark–release–recapture (MRR) study site is circled in red. c Areas selected for MRR on Captiva Island were single-point release (pink star) and multiple-point release (green stars) positions. BG-Sentinel 2 trap (BG-Sentinel) positions are shown as blue crosses
Sterile mosquito marking
Three different approaches to marking sterile males were employed over the course of the study. Marking methods were modified and refined over time to ensure that males were marked sufficiently while minimizing an excess of fluorescent powder and handling of males.
Males marked through contact with fluorescent powder in conditioned pots
Approximately 0.25 g of fluorescent powder (Techno Glow, Ennis, TX) per emergence pot was manually applied in an even coat to the interior walls (excluding the lowest, unscuffed 2 cm) prior to the addition of sterile males. After applying the powder, 200 ml of water was carefully added to the containers to avoid splashing and contamination of the water by the powder. Post-irradiation, sterile male pupae were placed in the marked containers and allowed to eclose. The males marked themselves through contact with the fluorescent powder on the walls of the pots. Water was removed after eclosion was completed, as described previously. Males were provided with sucrose solution until the time of release, at ~ 48 h after eclosion.
Males marked through rolling in fluorescent powder
After eclosion, water was removed as described above, ~ 16 h prior to marking. On the day of release, emergence containers (containing 2200 males ~ 48 h after emergence) were placed in a refrigerator at ~ 1 °C for 1 h. After removal of the containers from the refrigerator, the males were transferred to similar containers with the interior surface pre-marked with ~ 0.25 g of fluorescent powder. Groups of sterile males were then gently rolled in these containers for 30 s to apply the marking dust. All 2200 males underwent this treatment prior to placement in a single screen cage (60 cm × 60 cm × 60 cm) for release. The males were provided with 10% sucrose solution until release.
Insufflation
Sterile males were released ~ 48 h after eclosion. On the day of release, the emergence containers (containing 2200 males) were placed in a refrigerator at ~ 1 °C for 7–10 min for brief immobilization of the males that allowed for their consistent marking. SP01 was the only release in which the males did not undergo this treatment. Once the containers had been removed from the refrigerator, adults hanging from the sides were gently dislodged by tapping. Sterile males were dusted with fluorescent powder (~ 1.6 g/15 containers) using a nasal aspirator which blew the powder inside the container to create a cloud that evenly settled on the immobilized mosquitoes, similarly to Balestrino et al. [34]. To ensure that large clusters of dye did not adhere to the males, the aspirator bulb was inserted into a fine mesh bag so that the powder was aerosolized. Once the pigment had been applied, the males in the containers were checked with ultraviolet light to confirm that they had been sufficiently marked, and the sucrose solution was replenished on the screened portion of the container lids until the males were released.
Sterile marked mosquito releases
The number of release points depended on the type of release that was conducted, i.e. single-point releases at a single central point, and multi-point releases at 15 points (Fig. 1c). Releases SP01, SP02, MP01, and MP02 were conducted in the morning (0900–1100 hours), while releases SP03, MP03, and MP04 were performed in the afternoon (1200–1400 hours). For releases, lids were removed from the containers at the designated locations, and the mosquitoes were allowed to fly out. Gentle agitation was applied to encourage any remaining males to leave the container after the initial bout of dispersal. The container lids were replaced after the mosquitoes had been allowed to disperse from the container for 5–10 min. Any remaining mosquitoes, which included dead mosquitoes and mosquitoes that were alive but did not fly out of the containers after gentle agitation, were retained to determine mortality. All mortalities (pupal and adult) were recorded and subtracted from the initial estimated release number to improve the accuracy of subsequent calculations.
Sterile marked mosquito recapture
All MRR events took place at the exact locations shown in Fig. 1 for trapping mosquitoes, for the duration of these studies. Forty BG-Sentinel 2 traps with lure (Biogents, Regensburg, Germany) were set outdoors in a radial pattern from a central location (Fig. 1c) with the traps arrayed in nine concentric annuli at 50-m intervals from the central point, with a maximum trap distance of 420 m [35, 36]. Trap servicing began 24 h after release and was carried out daily, with catch bags and batteries replaced approximately every 24 h. For all the studies, trap servicing was conducted until no marked mosquitoes were caught for two consecutive trap checks (a 48-h period with no captures). Thus, the total number of days over which trapping occurred varied among the releases, with the longest duration a 12-day recapture period. Collected samples were identified to species and sex. After identification, Ae. aegypti were examined under ultraviolet light to detect fluorescent dust, and marking status was recorded. The recapture rate was defined as the proportion of recaptured marked males in relation to the total number of marked males released for each collection day.
Population parameters
Sterile male survival
Survival of released sterile males was estimated by the recapture rate per collection day with respect to the total number of marked sterile males released. The probability of daily survival (PDS) was estimated as the antilog of the regression slope of log(recaptured mosquitoes + 1), using date as a fixed parameter [23, 36]. The average life expectancy (ALE), defined as 1/− loge (PDS), was used as our metric of longevity [23, 28, 37,38,39].
Sterile male dispersal
To generate estimates of dispersal for the SIT operational phase, three single-point MRRs (SP01, SP02, and SP03) were performed across the seasons. The minimum and maximum distances flown from the release point and positive trap location were defined using Vincenty’s formula for geodesic distance with an accurate ellipsoidal model of the earth [40]. For the mean distance traveled (MDT), the circular area was divided into nine concentric annuli, with a maximum radius of 450 m (Fig. 1c). The formula used to calculate MDT was MDT = ∑(ER × AD)/(total ER), where ER is the estimated recapture, and AD is the annulus distance. ER was estimated as the number of marked mosquitoes recaptured in each annulus divided by the number of traps in that annulus and multiplied by a factor used to correct the total number of traps. This correction factor was the area of the annulus divided by the total area in which the traps were set, multiplied by the total number of traps. AD was defined as the sum of the inner and outer annulus radius (from the release point) divided by 2 [41, 42]. The MDT value was used to extrapolate the distance estimates from our single-point releases to create a potential distribution (MDT-cloud) that allows one to visualize how far mosquitoes are likely to travel based on our MDT estimates, an estimated “MDT cloud.” By applying this approach to each multiple release point, the limits of the MDT-cloud were defined as the outermost line that the released mosquitoes were likely to have flown to. The flight range (FR) at 50% (FR50) and 90% (FR90) ER was determined as the antilog slope from the linear regression log10(AD + 1) ~ ER [37, 43].
Wild population size estimation
The Fisher-Ford index was used to estimate the wild population size for each MRR study performed for the first recapture day by considering the number of recaptured marked males and captured unmarked males [44]. The Fisher-Ford index was defined as (S × n × M/m) − M, where n is the number of captured unmarked (fertile) males, M is the total number of marked (sterile) males released, m is the number of recaptured marked (sterile) males, and S is the survival probability. The sterile:wild male ratio was determined by the number of marked recaptured males divided by the total number of captured unmarked wild males from the first day of trap capture after release.
Statistical analysis
Statistical analyses were performed in R using RStudio environment [45, 46]. The outcome of the R script analyses is available as additional material (Additional file 1: Text S1). Relevant R packages and functions can also be found in the scripts provided in Additional file 1. Kernel density estimation interpolations (heat maps) were developed in QGIS version 3.16.9 Hannover [47], with a background map obtained from OpenStreetMap (Creative Commons Attribution-ShareAlike 2.0 license). Generalized linear models (GLMs) were used to evaluate male recapture parameters (recapture percentage or the number of recaptured males with distance, date, and MRR cycles as explanatory factors, using a binomial distribution for percentages and Poisson distribution for counts).
Results
Recapture success
Overall, we released 190,504 marked sterile male mosquitoes over our ~ 47-ha study area across the seven release cycles, with 3165 marked males recaptured, i.e. a mean recapture of ~ 1.5%. Table 2 summarizes each MRR study, presenting the number of marked sterile mosquitoes released, the recapture period [previously described as the number of days before a 2-day (48-h) period without recapture of marked males], the total number of marked (sterile) and unmarked (wild) male mosquitoes caught, and the male recapture rate. The proportion of sterile males recaptured varied from 1.73 to 4.10% for the single-point releases (average 2.70%), while in studies with multiple release points the proportion of sterile males recaptured ranged from 0.94 to 2.24% (average 1.39%). No significant difference was detected in the overall recapture proportions among the MRRs for the single-point releases (binomial GLM, deviance 0.462, df = 2, P = 0.638) or the multi-point releases (binomial GLM, deviance 1.371, df = 3, P = 0.269).
Survival
Recapture rates were similar between single- and multiple-point releases (Fig. 2). The maximum time to recapture sterile males varied from 9 to 12 days among the studies, with the greatest number of recaptures occurring within the first 3 days after release (7.46% recapture/3 days) for both single- and multi-point releases. While the majority of marked, sterile males were recaptured within the first 3 days after release (Fig. 2), captures of the wild population fluctuated among days of trapping and across seasons. The longevity of released sterile males in the field, here estimated as ALE within each release, ranged from 1.45 in SP01 to 3.91 in MP03. The PDS ranged from 0.5 in SP01 to 0.77 in MP03 across all the releases in the study. Estimates of each parameter for every release are shown in Table 2.
Daily mean proportion of marked males recaptured. Generalized linear model curves showing a binomial distribution of the recapture proportion of males as a the function of the day of recapture. a MRRs of single-point releases (SP01, SP02, SP03). b MRRs of multiple-point releases (MP01, MP02, MP03, and MP04). The dashed grey line represents the average life expectancy (ALE) for all the MRRs
Dispersal from single-point releases
We divided the circular area of our recapture zone into nine concentric annuli, each 50 m apart, from the most central point (Fig. 1), to calculate the MDT and FR. Across our three single-point releases (SP01, SP02, and SP03), we determined that sterile marked males had an MDT of 201.7 m, with a minimum DT and maximum DT of 36.2 and 404.5 m, respectively (Table 3; Fig. 3). The majority of sterile marked males recaptured were concentrated within the first 200-m radius from the release point (Fig. 3). The overall FR50 and FR90 estimates for sterile marked males were 132.97 and 188.4 m, respectively (Table 3).
Spatial distribution of recaptured marked sterile males for each BG-Sentinel trap combined throughout the collection period. a SP01, b SP02, c SP03. This configuration was used to estimate dispersal parameters from a single-release point (the central pink star on each map). Annuli are 50 m from each other. Blue crosses represent the positions of the BG-Sentinel traps
There was a marginally significant difference in marked sterile male dispersal (AD) among the three single-point releases (Poisson GLM, deviance − 2.055, df = 2, P = 0.04), but pairwise differences in dispersal distance among the three single-point releases were not statistically significant. Similarly, while there was a statistically significant difference in the proportion of sterile males recaptured per trap among releases (binomial GLM, deviance − 28.6, df = 2, P < 0.001), there were no significant pairwise differences among the three single-point releases (log odds ratio of recapture probability among the traps, SP01 vs SP02, SP01 vs SP03, and SP02 vs SP03; for further details, see Additional file 2: Fig. S1).
Dispersal from multiple-point releases
The MDT data from SP01, SP02, and SP03 were extrapolated to represent a potential dispersal cloud when taking into consideration the 14 additional release points of each MRR-multi-point release (MP01, MP02, MP03, and MP04). For this calculation, the MDT was used as a reference distance for each release point to give a final release perimeter of potential flight around the release points. This perimeter was defined as the MDT-cloud. Figure 4 shows each MRR-multi-point release, with the marked male distribution within the MDT-cloud. The multiple-point releases showed that, among our studies (according to the time of year), the most central area had the greatest recapture frequencies. However, recapture frequencies changed among traps during the MRR-multi-point releases, which resulted in captures outside the predicted MDT-cloud (Fig. 4C).
Spatial distribution of recaptured marked sterile males showing clusters within the release area observed in the multiple-point MRR studies (a MP01, b MP02, c MP03, d MP04). The MDT was based on the cumulative estimated recapture, the trap density in the annuli area, and male capture from the single-point releases. The heat maps represent kernel density estimations, which took into consideration the BG-Sentinel capture radius of 50 m and the number of mosquitoes captured. Blue crosses represent BG-Sentinel positions, while green stars indicate release points
Estimates of wild population size
Population size estimates for wild Ae. aegypti fluctuated through time as conditions in the field changed from favorable to hostile for mosquito growth and activity, which reflected periods of population increase and decline, respectively (Table 4). The first multiple-point release (MP01) was conducted in May during the time that wild Ae. aegypti activity was ramping up toward that of the typical high population period on Captiva, which occurs in July, and the estimated population size recorded using the Fisher-Ford index was ~ 13,000 wild (fertile) males in our 47-ha MRR area. The second release (MP02) took place in August, not long after the peak of seasonal activity, as populations were still high but beginning to decline (~ 27,000 wild males estimated via the Fisher-Ford index). MP03 took place in December, the low-density season (~ 653 wild males estimated via the Fisher-Ford index), and finally, MP04 took place in April, a time during which the wild Ae. aegypti population had begun to increase rapidly again (~ 6000 males estimated via the Fisher-Ford index). From MP01 to MP02 (12 weeks apart, May–August), we observed that the population more than doubled, followed by a drastic reduction of ~ 98.2% from MP02 to MP03 (16 weeks apart, August–December). The overall sterile:wild male ratio induced by our releases was low due to the wild population sizes; for MP01, the ratio was 0.059 in May; for MP02, the ratio was 0.16 in August; and for MP04, the ratio was 0.98 in April; meanwhile for MP03 the ratio was 21 in December when the wild population was at its lowest (Table 4).
Discussion
Effective mosquito control using SIT requires the collection of baseline data on local wild population dynamics which makes it possible to efficiently plan the suppression phase for that specific location, and includes population size estimates and seasonal patterns of abundance, as these will dictate when sterile male releases should occur [18]. In addition to estimates of local pest population sizes and dynamics, knowledge of sterile male dispersal and survival in the field is used to determine the numbers of sterile males that must be released to suppress the local population, as well as the frequency of releases. These data can be obtained through sequential MRR studies conducted across the seasons to track the local pest mosquito population in the target area [48,49,50]. While the marking methodologies differed across the MRR studies presented here, the goals of these studies were not to compare different marking techniques but rather to best estimate wild Ae. aegypti population size, along with sterile male dispersal, FR, and longevity. Alterations in the marking protocols reflect operational improvements that were determined through experience accumulated when marking sterile males for release.
The results presented here provide essential information for SIT planning and implementation for Ae. aegypti on Captiva Island. Typically, the aim of SIT programs is to release enough sterile males to overflood the population of wild males during low wild population density, to quickly reach ratios of 10–100 sterile males for each wild male, thereby reducing opportunities for wild females to mate with fertile wild males [49, 50]. Thus, it is important to estimate the wild male mosquito abundance to plan how many sterile males must be reared and released to affect the wild population. The ratio of marked sterile males to unmarked wild males taken from consecutive MRR studies provides a robust general idea about the release densities of sterile males required based on the seasonal dynamics [49, 50]. Some studies suggest that Fisher-Ford index estimations may overestimate wild populations due to a lack of knowledge about some population parameters, such as birth rate, mortality, age structure, immigration and emigration, and competition, etc. [51,52,53].
Additionally, population modeling is demanding due to the number of parameters needed. For effective SIT, the level of accuracy of population estimation at the initial stages is critical and quite challenging [51, 54]. However, practitioners can also use the sterile:wild (fertile) ratio as a viable alternative to population estimation to estimate the number of sterile males needed for release in a site to achieve a minimum sterile:wild male ratio of 10:1, which have been shown to generally cause wild population reduction. However, if production of sterile males allows overflooding ratios that are even greater than 10:1, the SIT may be even more effective; indeed, in some programs, as many as 100 sterile males are released for each wild male [51]. In our study there were, on average, 3.64 sterile males for each wild (fertile) one, which indicates that sterile male production needs to be increased in our operational releases, i.e. to reach the numbers necessary to have at least 10 sterile males for each wild male. Furthermore, releases for population suppression should ideally start early in the season when wild populations naturally have lower abundances so that the initial overflooding ratios are very high, which in our case is possible given the production capacity at our facility [17, 49]. Our current results form a basis for planning and implementing releases. We will also implement consistent monitoring of wild females with periodic releases of marked males to evaluate sterile:wild male ratios while the suppression phase is implemented, allowing future adjustments to the numbers of sterile males released to achieve successful suppression [55, 56].
Previous trapping studies on Captiva Island have shown substantial seasonal variation in Ae. aegypti adult densities (R. Morreale, unpublished data), with populations low over the cooler dry season months (October through April) and population expansion during the warmer, rainy season months (May through September). As expected, our estimates of wild male population size fluctuated across the MRR studies, from relatively low in December (~ 650 in the study area for MP03) to growing in April (~ 6000 in the study area for MP04) to very high in August (~ 26,800 in the study area for MP02) [50, 57, 58]. For the most effective population suppression over this ~ 47-ha urban site, we should aim to release ~ 500,000 sterile males per week at the beginning of spring before wild populations build up, to prevent early population growth.
The number of males needed to drive successful population control is based on the size of the wild population and the performance of the released sterile males in the field [18, 51]. The daily male recapture rate, PDS, and ALE are fundamental parameters for assessing the quality of the males reared and released. These parameters are helpful for deciding how often sterile males should be released in an operational program. Despite the fact that our MRR insects were submitted to sterilization through irradiation, our results were close to those found by Maciel-de-Freitas et al. [59] in a Brazilian study, in which PDS varied from 0.7 to 0.9, while in our study PDS was 0.67. The large range in PDS in the former study was reflected in the range in ALE, from 3 to 12 days, while in our study ALE was 2.46 days (or approximately 3 days). This supports the need to release high-quality males into the field to increase the chances of wild females mating with sterile males [60,61,62]. This ALE, which we consider a minimum, agrees with that of similar, established SIT pilot trials (not only for mosquitoes), and indicates that the release of sterile males, especially in our area, should occur two times per week with 2–3 days between releases. For example, the success of a program used to suppress the codling moth (Cydia pomonella), which employed a release frequency of two times per week, is considered an excellent example of successful SIT application [63]. The interval is of fundamental importance since the age of males can also impact their recapture rate, as described by Harrington et al. [64], who found that recapture rate decreases as mosquitoes age, with a small difference during dry and wet seasons, with a recaptured proportion of less than 5% in both periods.
Information on dispersal and distribution are also fundamental to determining release patterns of sterile males for population suppression programs, to ensure that releases occur where needed and to minimize releases in sites where they are not as important for mosquito control [18, 28]. Furthermore, dispersal studies may identify barriers specific to a particular location that may affect sterile male distribution or the migration of wild insects into the study area. The MDT of 195 m reported here is comparable to the long MDT, 240 m, reported in 2019 from another American study, for non-irradiated Ae. aegypti males [65], despite the differences between our site, on a Florida barrier island, and the former’s, which was in central California. Specifically, southwest Florida has a humid subtropical climate, while the study in California took place in an area with a cold semi-arid climate, yet male Ae. aegypti appeared to disperse over roughly the same distance at both locations [66]. Another study from the US reported similar results, with an overall MDT of 241.82 m and a maximum of 428.45 m for non-irradiated insects [67]. This indicates that, despite the fact our males were irradiated, they were fit enough to perform similarly to non-irradiated insects. The FR50 of 133.5 m reported here suggests that, for population suppression, we should develop a release plan based on a distance of ~ 100 m between release points to ensure even coverage of sterile males throughout our population suppression site. While a maximum radius of 133 m between release points could be considered, we feel that having a release radius of ~ 100 m will provide an excellent overlap of released mosquitoes to improve the probability of sterile male mating success; this distance is also close to the average MDT, 105.69, estimated by Moore and Brown [68] from 27 experiments.
As Captiva is an offshore island, the population of mosquitoes there is considered mostly closed, which increases the potential for successful suppression, and even eradication, of Ae. aegypti from the island. Our aim of releasing ~ 500,000 sterile males per week in our ~ 47-ha focal area in the most densely populated northern part of the island may need to be revised if immigration of fertile Ae. aegypti from the contiguous southern end of the island into our initial suppression area occurs. Ultimately, our goal is to eliminate Ae. aegypti across the entire ~ 240-ha island, but this will require substantially increasing the production of sterile males to cover the additional area.
Conclusions
Knowledge of the spatial distribution of trap captures yields essential information about a mosquito population in real time, and helps us identify areas of interest and hot spots that may need additional efforts or higher numbers of sterile males released [61, 68]. We used single- and multiple-release points across our studies to take advantage of the unique type of information acquired using these different approaches. We also showed that it is possible to perform single- and multi-point releases simultaneously, which helps to maximize the impact of sterile male releases on population density and dispersal. Finally, we argue that collecting data from the field through a baseline data collection system in parallel with sequential MRR studies is fundamental to the planning of an operational SIT phase. The information gained through both efforts generates a solid and stable basis from which to guide release and monitoring measures to achieve mosquito population suppression on Captiva Island.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
World Health Organization. Global vector control response: progress in planning and implementation. Geneva: World Health Organization; 2020.
Centers for Disease Control and Prevention. Statistics and maps-2020|Dengue|CDC. CDC dengue. 2021. https://www.cdc.gov/dengue/statistics-maps/2020.html. Accessed 24 Aug 2021.
Ponlawat A, Harrington LC. Blood feeding patterns of Aedes aegypti and Aedes albopictus in Thailand. J Med Entomol. 2005;42:844–9.
Kittayapong P, Strickman D. Distribution of container-inhabiting Aedes larvae (Diptera: Culicidae) at a dengue focus in Thailand. J Med Entomol. 1993;30:601–6.
Reiter P. Oviposition, dispersal, and survival in Aedes aegypti: implications for the efficacy of control strategies. Vector-Borne Zoonotic Dis. 2007;7:261–73.
Perich MJ, Davila G, Turner A, Garcia A, Nelson M. Behavior of resting Aedes aegypti (Culicidae: Diptera) and its relation to ultra-low volume adulticide efficacy in Panama City. Panama J Med Entomol. 2000;37:541–6.
Moyes CL, Vontas J, Martins AJ, Ng LC, Koou SY, Dusfour I, et al. Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PLoS Negl Trop Dis. 2017;11:e0005625.
Campos KB, Martins AJ, de Rodovalho CM, Bellinato DF, dos Dias LS, de da Macoris MLG, et al. Assessment of the susceptibility status of Aedes aegypti (Diptera: Culicidae) populations to pyriproxyfen and malathion in a nation-wide monitoring of insecticide resistance performed in Brazil from 2017 to 2018. Parasit Vectors. 2020;13:531.
Suarez GP, Udiani O, Allan BF, Price C, Ryan SJ, Lofgren E, et al. A generic arboviral model framework for exploring trade-offs between vector control and environmental concerns. J Theor Biol. 2020;490:110161.
Schoch-Spana M, Watson C, Ravi S, Meyer D, Pechta LE, Rose DA, et al. Vector control in Zika-affected communities: local views on community engagement and public health ethics during outbreaks. Prev Med Rep. 2020;18:101059.
Enkerlin WR, Gutiérrez Ruelas JM, Pantaleon R, Soto Litera C, Villaseñor Cortés A, Zavala López JL, et al. The Moscamed Regional Programme: review of a success story of area-wide sterile insect technique application. Entomol Exp Appl. 2017;164:188–203.
Klassen W, Curtis CF. History of the sterile insect technique. In: Hendrichs J, Dyck VA, editors. Sterile insect technique. Boca Raton: CRC Press; 2021. p. 1–44.
FAO/IAEA. Thematic plan for the development and application of the sterile insect technique (SIT) and related genetic and biological control methods for disease transmitting mosquitoes. IAEA; 2019. https://www.iaea.org/resources/thematic-plan/thematic-plan-for-the-development-and-application-of-the-sterile-insect-technique-sit-and-related-genetic-and-biological-control-methods-for-disease-transmitting-mosquitoes.
Gato R, Menéndez Z, Prieto E, Argilés R, Rodríguez M, Baldoquín W, et al. Sterile insect technique: successful suppression of an Aedes aegypti field population in Cuba. Insects. 2021;12:469.
Bellini R, Medici A, Puggioli A, Balestrino F, Carrieri M. Pilot field trials with Aedes albopictus irradiated sterile males in Italian urban areas. J Med Entomol. 2013;50:317–25.
Tur C, Almenar D, Benlloch-Navarro S, Argilés-Herrero R, Zacarés M, Dalmau V, et al. Sterile insect technique in an integrated vector management program against tiger mosquito Aedes albopictus in the Valencia region (Spain): operating procedures and quality control parameters. Insects. 2021;12:272.
Dyck VA, Hendrichs J, Robinson AS. Sterile insect technique: principles and practice in area-wide integrated pest management. Oxfordshire: Taylor and Francis; 2021.
Bouyer J, Yamada H, Pereira R, Bourtzis K, Vreysen MJB. Phased conditional approach for mosquito management using sterile insect technique. Trends Parasitol. 2020;36:325–36.
Paternina LE, Rodas JD. Sampling design and mosquito trapping for surveillance of arboviral activity. In: Salvato MS, editor. Hemorrhagic fever viruses. New York: Springer; 2018. p. 89–100.
Bisht B, Kumari R, Nagpal BN, Singh H, Kumar S, Gupta AK, et al. Influence of environmental factors on dengue fever in Delhi. Int J Mosq Res. 2019;6:11–8.
Barrera R, Amador M, MacKay AJ. Population dynamics of Aedes aegypti and dengue as influenced by weather and human behavior in San Juan, Puerto Rico. PLoS Negl Trop Dis. 2011;5:e1378.
Russell RC, Webb CE, Williams CR, Ritchie SA. Mark–release–recapture study to measure dispersal of the mosquito Aedes aegypti in Cairns, Queensland, Australia. Med Vet Entomol. 2005;19:451–7.
Buonaccorsi JP, Harrington LC, Edman JD. Estimation and comparison of mosquito survival rates with release–recapture–removal data. J Med Entomol. 2003;40:6–17.
Villela DAM, de Garcia GA, Maciel-de-Freitas R. Novel inference models for estimation of abundance, survivorship and recruitment in mosquito populations using mark–release–recapture data. PLoS Negl Trop Dis. 2017;11:e0005682.
Foley EW, Morreale RL, Hoel DF, Lloyd AM. Area-wide mosquito management in Lee County, Florida, USA. In: Hendrichs J, Pereira R, Vreysen MJB, editors. Area-wide integrated pest management. Boca Raton: CRC Press; 2021. p. 319–38.
Winskill P, Carvalho DO, Capurro ML, Alphey L, Donnelly CA, McKemey AR. Dispersal of engineered male Aedes aegypti mosquitoes. PLoS Negl Trop Dis. 2015;9:e0004156.
Neira M, Lacroix R, Cáceres L, Kaiser PE, Young J, Pineda L, et al. Estimation of Aedes aegypti (Diptera: Culicidae) population size and adult male survival in an urban area in Panama. Mem Inst Oswaldo Cruz. 2014;109:879–86.
Trewin BJ, Pagendam DE, Johnson BJ, Paton C, Snoad N, Ritchie SA, et al. Mark–release–recapture of male Aedes aegypti (Diptera: Culicidae): use of rhodamine B to estimate movement, mating and population parameters in preparation for an incompatible male program. PLoS Negl Trop Dis. 2021;15:e0009357.
Tyler-Julian K, Darrisaw C, Lloyd A, Hoel D. The use of frozen, food-grade blood to successfully maintain colonies of four species of mosquitoes (Diptera: Culicidae). J Insect Sci. 2021;21:1.
Fay RW, Morlan HB. A mechanical device for separating the developmental stages, sexes and species of mosquitoes. Mosq News. 1959;19:144–7.
Mehta K, Parker A. Characterization and dosimetry of a practical X-ray alternative to self-shielded gamma irradiators. Radiat Phys Chem. 2011;80:107–13.
USA. Captiva CDP, Florida-Census Bureau profile, https://data.census.gov/cedsci/profile?g=1600000US1210425/. Accessed 5 Aug 2022.
Sanibel and Captiva Chamber of Commerce. The best time to visit Sanibel Island. Sanibel Isl Chamb Commer. 2019. https://sanibel-captiva.org/the-best-time-to-visit-sanibel-island/. Accessed 5 Aug 2022.
Balestrino F, Puggioli A, Malfacini M, Albieri A, Carrieri M, Bouyer J, et al. Field performance assessment of irradiated Aedes albopictus males through mark–release–recapture trials with multiple release points. Front Bioeng Biotechnol. 2022. https://doi.org/10.3389/fbioe.2022.876677.
Barrera R. Spatial stability of adult Aedes aegypti populations. Am J Trop Med Hyg. 2011;85:1087–92.
Gorsich EE, Beechler BR, van Bodegom PM, Govender D, Guarido MM, Venter M, et al. A comparative assessment of adult mosquito trapping methods to estimate spatial patterns of abundance and community composition in southern Africa. Parasit Vectors. 2019;12:462.
Gillies MT. Studies on the dispersion and survival of Anopheles gambiae Giles in East Africa, by means of marking and release experiments. Bull Entomol Res. 1961;52:99–127.
de Garcia GA, dos Santos LMB, Villela DAM, Maciel-de-Freitas R. Using Wolbachia releases to estimate Aedes aegypti (Diptera: Culicidae) population size and survival. PLoS ONE. 2016;11:e0160196.
Lacroix R, Delatte H, Hue T, Reiter P. Dispersal and survival of male and female Aedes albopictus (Diptera: Culicidae) on Reunion Island. J Med Entomol. 2009;46:1117–24.
Vincenty T. Direct and inverse solutions of geodesics on the ellipsoid with a application of nested equations. Surv Rev. 1975;23:88–93.
Marini F, Caputo B, Pombi M, Tarsitani G, Della TA. Study of Aedes albopictus dispersal in Rome, Italy, using sticky traps in mark-release-recapture experiments. Med Vet Entomol. 2010;24:361–8.
Morris CD, Larson VL, Lounibos LP. Measuring mosquito dispersal for control programs. J Am Mosq Control Assoc. 1991;7:608–15.
White DJ, Morris CD. Bionomics of anthropophilic Simuliidae (Diptera) from the Adirondack mountains of New York State, USA. 1. Adult dispersal and longevity. J Med Entomol. 1985;22:190–9.
Bailey NT. On estimating the size of mobile populations from recapture data. Biometrika. 1951;38:293–306.
R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing; 2021.
RStudio Team. RStudio: integrated development environment for R. Boston: RStudio, PBC; 2021.
QGIS Development Team. QGIS geographic information system. QGIS Association; 2021.
Vavassori L, Saddler A, Müller P. Active dispersal of Aedes albopictus: a mark-release-recapture study using self-marking units. Parasit Vectors. 2019;12:583.
Le Goff G, Damiens D, Ruttee A-H, Payet L, Lebon C, Dehecq J-S, et al. Field evaluation of seasonal trends in relative population sizes and dispersal pattern of Aedes albopictus males in support of the design of a sterile male release strategy. Parasit Vectors. 2019;12:81.
Epopa PS, Millogo AA, Collins CM, North A, Tripet F, Benedict MQ, et al. The use of sequential mark-release-recapture experiments to estimate population size, survival and dispersal of male mosquitoes of the Anopheles gambiae complex in Bana, a West African humid savannah village. Parasit Vectors. 2017;10:376.
Barclay HJ. Mathematical models for using sterile insects. In: Dyck VA, Hendrichs J, Robinson AS, editors. Sterile insect technique. Boca Raton: CRC Press; 2021. p. 201–44.
Silver JB. Mosquito ecology: field sampling methods. Berlin: Springer Science and Business Media; 2007.
Silver JB. Estimating the size of the adult population. In: Silver JB, editor. Mosquito ecology field sample methods. Berlin: Springer; 2008. p. 1273–376.
Itô Y, Yamamura K, Manoukis NC. Role of population and behavioural ecology in the sterile insect technique. In: Dyck VA, Hendrichs J, Robinson AS, editors. Sterile insect technique. 2nd ed. Boca Raton: CRC Press; 2021.
Vreysen MJB. Monitoring sterile and wild insects in area-wide integrated pest management programmes. In: Dyck VA, Hendrichs J, Robinson AS, editors. Sterile insect technique: principles and practice in area-wide integrated pest management. 2nd ed. Boca Raton: CRC Press; 2021. p. 487–528.
Parker AG, Mamai W, Maiga H. Mass-rearing for the sterile insect technique. In: Dyck VA, Hendrichs J, Robinson AS, editors. Sterile insect technique. Boca Raton: CRC Press; 2021. p. 283–316.
Gouagna LC, Dehecq J-S, Fontenille D, Dumont Y, Boyer S. Seasonal variation in size estimates of Aedes albopictus population based on standard mark–release–recapture experiments in an urban area on Reunion Island. Acta Trop. 2015;143:89–96.
Heinisch MRS, Diaz-Quijano FA, Chiaravalloti-Neto F, Menezes Pancetti FG, Rocha Coelho R, dos Santos AP, et al. Seasonal and spatial distribution of Aedes aegypti and Aedes albopictus in a municipal urban park in São Paulo, SP, Brazil. Acta Trop. 2019;189:104–13.
Maciel-de-Freitas R, Codeço CT, Lourenço-de-Oliveira R. Daily survival rates and dispersal of Aedes aegypti females in Rio de Janeiro, Brazil. Am J Trop Med Hyg. 2007;76:659–65.
Bakri A, Mehta K, Lance DR. Sterilizing insects with ionizing radiation. In: Dyck VA, Hendrichs J, Robinson AS, editors. Sterile insect technique: principles and practice in area-wide integrated pest management. Boca Raton: CRC Press; 2021. p. 355–98.
Oliva CF, Benedict MQ, Collins C, Baldet T, Bellini R, Bossin H, et al. Sterile insect technique (SIT) against Aedes species mosquitoes: a roadmap and good practice framework for designing, implementing and evaluating pilot field trials. Insects. Multidisciplinary Digital Publishing Institute; 2021;12:191.
Balestrino F, Puggioli A, Carrieri M, Bouyer J, Bellini R. Quality control methods for Aedes albopictus sterile male production. PLoS Negl Trop Dis. 2017;11:e0005881.
Dyck VA, Graham SH, Bloem KA. Implementation of the sterile insect release programme to eradicate the codling moth, Cydia pomonella (L.) (Lepidoptera: Olethreutidae), in British Columbia, Canada. Manag Insect Pests Nucl Relat Mol Genet Tech. 1993.
Harrington LC, Vermeylen F, Jones JJ, Kitthawee S, Sithiprasasna R, Edman JD, et al. Age-dependent survival of the dengue vector Aedes aegypti (Diptera: Culicidae) demonstrated by simultaneous release-recapture of different age cohorts. J Med Entomol. 2008;45:307–13.
Marcantonio M, Reyes T, Barker CM. Quantifying Aedes aegypti dispersal in space and time: a modeling approach. Ecosphere [Internet]. 2019 [cited 2021 Dec 4];10. Available from: https://onlinelibrary.wiley.com/doi/https://doi.org/10.1002/ecs2.2977
Chen D, Chen HW. Using the Köppen classification to quantify climate variation and change: an example for 1901–2010. Environ Dev. 2013;6:69–79.
Juarez JG, Garcia-Luna S, Chaves LF, Carbajal E, Valdez E, Avila C, et al. Dispersal of female and male Aedes aegypti from discarded container habitats using a stable isotope mark-capture study design in South Texas. Sci Rep. 2020;10:1–12.
Moore TC, Brown HE. Estimating Aedes aegypti (Diptera: Culicidae) Flight distance: meta-data analysis. J Med Entomol. 2022;59:1164–70.
Acknowledgements
We would like to thank Dr. Laura Vavassori for her assistance in the dispersal calculations, and Travis Edwards, Kylee Flint, Kyrsten Gage, Alexis Lizotte, Inspirion Small, and Judy Thomas for their assistance in rearing the mosquitoes, releasing the sterile males, and trapping for the duration of these studies.
Funding
Participation in this study by DAH was supported in part by the National Institute of Food and Agriculture, US Department of Agriculture, the Hatch project FLA-ENY-005943, the Florida Department of Agriculture and Consumer Services Projects 25367 and 20220, and the Florida Department of Health CDC-FDOH Hurricane Co-Agreement grant CODRE. LCMCD’s program was partly supported by another CDC-FDOH Hurricane Co-Agreement grant, CODRF.
Author information
Authors and Affiliations
Contributions
Conceptualization was performed by DOC, RM, and SS. Data curation was done by DOC and RM. The formal analysis was conducted by DOC and DAH. The funding acquisition was obtained through RM and DH. The investigation was conducted by SS and RM. The definition of the methodology was agreed upon by RM, DOC, and SS. The project administration was done by RM, while resources were obtained through AL, DAH, and DH. Supervision was conducted by AL. Writing and preparation of the original draft was the role of DOC, RM, DAH, MGP, AL, and DH, while the final review and editing was conducted by DOC, RM, DAH, MGP, AL, and DH. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
: Text S1. Statistical analysis and R code.
Additional file 2
: Figure S1. BG-Sentinel male collections (marked and unmarked) across our seven MRR studies including single-point releases (a SP01, SP02, SP03) and multiple-point releases (b MP01, MP02, MP03, MP04) covering seasonal periods of relatively low and high mosquito densities across 2019 and 2020 on Captiva Island.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Carvalho, D.O., Morreale, R., Stenhouse, S. et al. A sterile insect technique pilot trial on Captiva Island: defining mosquito population parameters for sterile male releases using mark–release–recapture. Parasites Vectors 15, 402 (2022). https://doi.org/10.1186/s13071-022-05512-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-022-05512-3