Abstract
Background
The presence of breeding sites and distribution of species of Simulium damnosum sensu lato are critical in understanding the epidemiology of onchocerciasis and evaluating the impact of elimination interventions. Reports on breeding sites and species distribution of members of S. damnosum s.l. in Cameroon are scarce and the few ones available date back to more than three decades. The aim of this study is to provide information on S. damnosum breeding sites across the rainy (RS) and dry (DS) seasons and the species composition in three different regions in Cameroon: Southwest (SW), Northwest (NW) and North (N).
Methods
A cross-sectional two-season study was carried out in three regions with different ecological characteristics (SW—rainforest; NW—mixed forest–Guinea savanna; N—Sudan savanna). Pre-control onchocerciasis endemicity, relief maps and historical entomological information were used to identify potential rivers for purposive sampling. Sampled larvae were fixed in Carnoy’s solution and sorted, and S. damnosum s.l. larvae were stored until identification by cytotaxonomy. Geographical coordinates of potential breeding sites were recorded to produce maps using ArcGIS, while Chi-square tests in SPSS were used to test for any differences between black fly seasonal breeding rates.
Results
A total of 237 potential breeding sites were sampled (RS = 81; DS = 156) and 72 were found positive for S. damnosum s.l. The SW had the most positive sites [67 (RS = 24; DS = 43)], with a significant difference in the rate of breeding between the seasons (P < 0.05). Among 68 sites visited in both seasons, 16 (23.5%) were positive in one of the two seasons with more sites positive in DS(11) than RS(05), 14 (20.6%) and 38 (55.9%) respectively positive and negative in both seasons. Simulium damnosum sensu stricto and S. sirbanum were the main species in the N, while S. squamosum and S. mengense were the predominant species in the NW and SW. Simulium soubrense and S. yahense were uniquely recorded in the SW.
Conclusions
A comprehensive mapping of breeding sites requires rainy and dry seasons sampling. This study demonstrates that a breeding site survey of S. damnosum s.l. is achievable in forest as well as savanna zones. Not all potential breeding sites are actual breeding sites. Observation of S. soubrense in the SW indicates changes in species composition over time and could affect onchocerciasis epidemiology in this area.
Graphical abstract

Similar content being viewed by others
Background
Human onchocerciasis, also called river blindness, is a parasitic infection caused by the filarial nematode Onchocerca volvulus and transmitted by female black flies of the genus Simulium (Diptera: Simuliidae) [1]. The most important vectors are members of the Simulium damnosum complex, which have a wide range throughout Africa and the Middle East [2]. The flies breed in fast-flowing stretches of water, where juvenile stages can be found attached to submerged vegetation, rocks or other substrates.
Simulium damnosum sensu lato are the only known vectors of human onchocerciasis in West and Central Africa. The species of this complex vary in their biting cycle and population age structure [3], response to control measures, ecological adaptation and host preference, as well as their ability to transmit the causative agent of human onchocerciasis [4,5,6,7]. Members of the S. damnosum complex have been categorized into forest and savanna species [8] depending on their predominant ecological preferences.
The transition from control to elimination of onchocerciasis gives rise to the need for reassessment of all previously excluded areas from onchocerciasis interventions during the control era, termed ‘elimination mapping’ [9, 10]. Positive findings from breeding site assessments are important in defining the target population for such mapping [9], given that the presence of vector Simulium flies defines the epidemiology of onchocerciasis [11]. In addition, breeding site surveys constitute important first steps in entomological and epidemiological evaluations of interventions in the different stages of onchocerciasis elimination [10].
Among the entomological procedures in the fight against human onchocerciasis, breeding site surveys form the basic foundation, as they are required in most of the phases of onchocerciasis control and elimination. It provides information on where different vector species occur and define communities exposed to different levels of onchocerciasis transmission. However, breeding site surveys are often thought to be impractical or difficult to carry out, especially in forest zones [12]. This has served as a limitation to onchocerciasis control/elimination efforts in forest zones.
The presence and characteristics of breeding sites of S. damnosum s.l. may change with seasons and over time either due to pressure from human activity such as deforestation [13] and climate change [14], or due to other anthropogenic or natural causes. This might lead to changes in vector species distribution and consequently the transmission pattern of the disease. Therefore, it is important to regularly update the breeding sites and vector species distribution to detect early changes in the epidemiology of onchocerciasis.
Few studies have documented the breeding sites and species distribution of vectors of onchocerciasis in Cameroon. Available studies date from more than three decades ago [15, 16] and/or were restricted to a particular region, locality or river basin of interest [16,17,18,19]. Ten cytoforms of S. damnosum s.l. (S. damnosum sensu stricto Nile and Volta forms, S. sirbanum s.l., S. mengense, S. squamosum A, B, C, D and E2, and S. yahense) have been reported from Cameroon [20], all of which are either confirmed or suspected vectors of human onchocerciasis [21,22,23].
The geographical distribution of S. damnosum s.l. in the country follows the established eco-geography of these vectors, where the savanna cytospecies (S. damnosum s.s. and S. sirbanum) mostly occupy streams and rivers in the Sudan savanna areas, while the forest cytospecies (S. squamosum, S. yahense and S. mengense) are mostly encountered in the forest and Guinea savanna areas [15, 18, 24]. Nevertheless, S. mengense and S. squamosum A are widely distributed throughout the country, and often observed in savanna areas sharing the same habitat as the savanna vectors [15, 18]. Likewise, the savanna species, S. damnosum s.s., has been observed in the forest Southwest region [17, 25]. Around Mount Cameroon, S. squamosum A, C and D occur in sympatry, where hybrids between cytoforms A and C are common [18, 19]. Simulium squamosum B has only been found in the Sanaga River [18] while S. squamosum E2 has recently been described from the Mbam River [26], a tributary of the Sanaga River.
The present study was therefore carried out to provide an updated map of breeding sites and species distribution of black flies in three administrative regions of Cameroon having different vegetation types—North (Sudan savanna), Northwest (mixed forest–savanna) and Southwest (rainforest). The study also demonstrates the feasibility of carrying out breeding site surveys in forest zones similar to savanna zones.
Methods
Study site
This study took place in three administrative regions of Cameroon, namely, the Southwest (SW), Northwest (NW) and North (N) regions (Fig. 1).
The Southwest region has dense evergreen and montane vegetation and a humid tropical rainforest (that is gradually being degraded for lumbering and agricultural activities). It has an equatorial climate with rainfall between 2000 and 4000 mm per year and a dense seasonal and perennial hydrographic network constituting the Manyu (Munaya), Meme, Mungo and Ndian drainage basins. Part of this region falls within the coastal maritime zone with coastal vegetation consisting of mangroves and tropical rain forest. The topography of the region comprises mountains, hills and valleys that generate fast water currents and natural waterfalls. An abandoned dam (formerly used to generate hydroelectric power in the area) over the Yoke River around Muyuka now serves as a small waterfall with several rapids. Coupled with the long rainy seasons (March–October), this ecology favours the establishment of permanent breeding sites required for continuous onchocerciasis transmission.
The Northwest region falls within the mountain range of Cameroon, characterized by relief of massifs and mountains. The once large forest area has been reduced to mostly grassland and bush savanna, partly because of deforestation for timber, firewood and agriculture, with only a few patches of forest left. The region has a tropical climate characterized by two main seasons: the rainy season, which starts in March and ends in October, and the dry season from November to February. Rainfall varies between 1700 and 3000 mm annually. The main rivers are the Menchum, Bui, Katsina, Kumbi, Momo, Noun and Donga rivers, with several seasonal and perennial tributaries.
The North region falls within the Sudano–Sahelian ecological zone of the country, having a dense hydrographic network of streams/rivers (mayos). The streams/rivers are generally seasonal, but for the Vina, Mbere and Benoue rivers. A dam on the Benoue River at Lagdo is used for generating electricity and providing water for irrigation. Aside from the perennial rivers, most of the streams and rivers in the region dry up or are reduced to pools (which later dry up) during the dry season. The region has a short rainy season (July–October) and a long dry season (November–June), with unevenly spatio-temporally distributed mean annual rainfall of about 800–900 mm [27].
Sample collection
Sampling took place in the months of July and September 2012, and December 2012–February 2013 (taking a break from 22 December 2012–10 January 2013). Potential breeding sites were determined from pre-control REMO (rapid epidemiological mapping of onchocerciasis) infection maps and other historical onchocerciasis information, coupled with a detailed relief map (Additional file 1: Fig. S1) of the study regions. Purposive sampling for S. damnosum s.l. larvae was undertaken on the rivers showing potential breeding sites. S. damnosum s.l. larvae were collected from trailing vegetation and other submerged substrates (e.g. grass, leaves and rocks) in fast-running water stretches. The larvae were stored in Carnoy’s solution (absolute ethanol and glacial acetic acid mixture of v/v 3:1) and kept cold in an ice chest containing ice blocks until taken to the lab where they were sorted into various Simulium species and those of the S. damnosum s.l. were stored in the refrigerator for later cytotaxonomic analysis. Larvae of the S. damnosum s.l. were identified by the presence of scales (spines) over the dorsal and lateral surfaces of the body and proleg and their characteristic dorsal tubercles [28].
Cytotaxonomic identification of S. damnosum s.l. larvae
The collected larvae were processed for cytotaxonomic identification as described in [29] and identifications made based on the species diagnostic inversions described in [19, 21, 30]. Briefly, larval abdomens were teased open slightly and stained with orcein. Stained salivary glands were extracted under a dissection microscope. Stained salivary gland cells were then gently compressed between a glass slide and cover slip and the banding pattern of the resulting spread of polytene chromosomes examined for inversions.
Data analysis
SPSS version 25 statistical software was used to perform Chi-square tests to estimate the significance between black fly breeding rate and seasonality.
Results
Breeding site surveys
A total of 81 and 156 potential breeding sites were surveyed during the rainy and dry seasons, respectively, for a period of 37 days (rainy season = 15 days; dry season = 22 days). 72 sites (30.4%) were found to be positive (contain larvae of the S. damnosum complex) in total (a site positive in the two seasons is counted twice). Non-Simulium damnosum s.l. larvae were observed in some positive sites as well as in other sites that were negative for S. damnosum s.l. The number of S. damnosum s.l. breeding sites and the number of survey days, and the positive rates are shown in Table 1.
Breeding rates were higher in the dry season for the Southwest (74.4%) and the Northwest (40.0%), whereas they were higher in the rainy season for the North region (14.3%) (Table 1). A significant difference in breeding rates between the two seasons was observed for the Southwest region (χ2 = 4.07, df = 1, P = 0.04) but not for the Northwest (χ2 = 1.87, df = 1, P = 0.17) and North (X2 = 2.03, df = 1, P = 0.15) regions and not for the three regions combined (χ2 = 1.15, df = 1, P = 0.28).
Out of the 68 sites that were visited during both sampling seasons (once per season), 16 (23.5%) were positive in one of the two seasons, while 14 (20.6%) and 38 (55.9%) sites were respectively positive and negative in both seasons. More of these sites sampled twice, and positive in one of two seasons, were positive in the dry season than in the rainy season for the SW (dry season [DS] = 6; rainy season [RS] = 1) and NW (DS = 02; RS = 00), while in the North region it was almost a tie (DS = 3; RS = 4), Fig. 2.
Species distribution
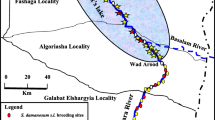
Six different cytospecies were identified in this study. These include the forest species S. squamosum, S. mengense, S. yahense and S. soubrense Beffa form; and the savanna species S. damnosum s.s. and S. sirbanum. The Southwest and Northwest regions had predominantly forest species, while the North region had predominantly savanna species (Fig. 3, Tables 2 and 3).
Species of the S. damnosum complex that were identified for both seasons in the Southwest (a), Northwest (b) and North (c) regions. S. da/si is plotted on the map as S. sirbanum. The numbers attached to the symbols correspond to the number (No.) in Table 3
Five of the six species identified in this study occurred in the Southwest region, making it the region with the highest species diversity (S. squamosum, S. mengense, S. soubrense, S. yahense and S. damnosum s.s.), followed by the North region with three species (S. damnosum s.s., S. sirbanum, S. squamosum) and then the Northwest region with two (S. squamosum and S. mengense) (Table 2 and Fig. 3). Simulium soubrense Beffa form was mostly observed in the Yoke/Muyuka and surrounding breeding sites in both seasons.
No marked changes in species distribution were observed in the breeding sites that were sampled in the two seasons. Some species were noted to be absent in one season but present in the other season at the same sites. We did not, however, observe any association between a particular season and the presence/absence of a species, except for the presence of the individual larvae that could not be placed simply as S. sirbanum or S. damnosum s.s. that were only observed in rainy season samples of the North region (Tables 2 and 3) at a frequency of 0.146 (n = 103).
In the Southwest and Northwest regions, S. squamosum was the most prevalent species at nearly constant proportions in both seasons. Simulium mengense was more common during the dry season, but was totally absent in all identified rainy season samples from the Northwest region. Similarly, in the North region, S. damnosum s.s. was the most prevalent species in the two seasons, while the prevalence of S. sirbanum dropped drastically in the rainy season accompanied by observation of the Simulium damnosum/sirbanum cytotype (Table 2).
Discussion
Breeding sites of S. damnosum s.l.
Sampling of the aquatic stages of members of the S. damnosum complex is important in mapping the breeding sites of the vectors. This is necessary for determining the distribution of the vectors, selection of vector collection sites and sentinel communities for entomological and epidemiological evaluations, respectively. Furthermore, it helps in identifying areas of possible onchocerciasis transmission and understanding the local epidemiology of the disease. Hence, the breeding site survey is a key step in the monitoring and evaluation of the different phases of onchocerciasis elimination [10]. Except for the study by Traore-Lamizana et al. [15], the present study has mapped more S. damnosum s.l. breeding sites and species distribution in Cameroon than any previous study. It provides an updated and more comprehensive survey encompassing several regions and vegetation types. This survey did not show any challenges peculiar to any vegetation type, contrary to reports of difficulty in searching for breeding sites in forest areas [12].
Breeding sites of S. damnosum s.l. were more prevalent in the Southwest region than the Northwest and North regions, and more in the Northwest than the North region. This is due to the perennial nature of the rivers in the Southwest region and the dense tropical rainforest in this region, which provides continuous nutrient-rich water for the development of Simulium larvae. Whereas most streams/rivers in the North region are seasonal.
Similar to earlier observations [15, 18] most rivers in the North region, except the few perennial rivers, were reduced to trickles or pools or completely dried out during the dry season. Breeding in the North region occurs such that during the rainy season, it is almost exclusively in the smaller seasonal streams (tributaries) while the perennial and semi-perennial (larger streams/rivers) streams/rivers are overflooded. Breeding gradually begins in the perennial and semi-perennial streams/rivers when the water descends to intermediate levels, during which time the seasonal streams have begun drying out. By the middle of the dry season, breeding occurs exclusively in the perennial rivers as all other streams/rivers would have dried off.
Hence, the number of breeding sites (and therefore, the risk of onchocerciasis transmission) in the North region may be more important during, either the rainy or the dry season, depending on whether the river is seasonal, semi-perennial (larger streams/rivers) or perennial. Variations in seasonal breeding were observed in all three regions, thereby highlighting the importance of proper timing of breeding site surveys and other entomological studies.
Generally, it is considered that the highest transmission period occurs during the rainy season, when water flows in all the riverbeds. However, this is not always the case as we observed in the Southwest and Northwest regions. Our data showed that in these regions, the proportion of breeding sites increased during the dry season, compared with the rainy season. In fact, comparing the potential breeding sites that were visited in both seasons in these two regions, it was observed that all the rainy season positive breeding sites were again positive during the dry season, while several sites that were negative during the rainy season became positive during the dry season. Achukwi et al. [24] also observed higher biting during the dry season in the Vina du Sud in Cameroon, indicating increased breeding during the dry season.
Higher breeding in the dry season compared with the rainy season could be explained by the flooding of some of these rivers during the rainy season that leads to the sweeping away of potential larval supports (e.g. vegetation, trapped fallen leaves, fallen tree branches, etc.) by the high water current, and/or the submergence of breeding sites by the overflooding turbid dirty waters [31].
On the other hand, during the dry season (or early dry season for the large but seasonal rivers in the North region), the water levels drop, exposing submerged rocky beds and creating rapids, and the waters are less turbid, therefore favouring breeding. Hence, seasonal changes in water level [1, 31], turbidity [31] and availability of rapids and submerged vegetation are important factors in the breeding and distribution of black fly vectors.
Distribution of S. damnosum s.l.
The distribution of S. damnosum s.l. species was according to the bio-climate of the regions, with the North (Sudan savanna) region harboring the savanna species (S. damnosum s.s. and S. sirbanum) and the Southwest (rainforest—S. squamosum, S. mengense, S. soubrense Beffa form, S. yahense, S. damnosum s.s.) and Northwest (mixed forest–savanna—S. squamosum and S. mengense) regions harbouring the forest species. The high diversity of species found in the Southwest region could be due to the abundant breeding sites that provide a wide range of favourable microhabitat conditions for the adaptation of the diverse black fly species of different ecological preferences. This area has been suggested to be one of the centres of greatest diversity of the S. damnosum complex in Africa, where Mount Fako and the associated forests may play an important role in their evolution [32].
Based on its predominance in the Southwest and Northwest regions, S. squamosum could be playing a major role in onchocerciasis transmission in these regions, assisted by S. mengense. Similarly, the main vector in the North region is S. damnosum s.s., followed by a small S. sirbanum proportion which increases during the dry season [16] and may play a minor role in transmission in this area. The species and vectorial status of the S. damnosum/sirbanum cytoform is yet to be elucidated.
Various species of the S. damnosum complex present different transmission efficiencies [5] hence, increased diversity may lead to complex epidemiology of onchocerciasis. For example, S. squamosum and S. mengense (the major onchocerciasis vectors in the Southwest and Northwest regions) have been shown to present bilateral blindness similar to what has previously been established for savanna vectors [33]. Generally, there were no seasonal changes in species composition in the different regions. However, at some breeding sites, different species were observed in different seasons. This could be due to seasonal dispersal of species or seasonal variation in physiochemical composition of the rivers that do not favour survival of certain species.
This study has shown how widespread S. mengense is, since its first description from the Menge River in the Southwest region [34]. Furthermore, contrary to previous studies [17, 19, 35] which only recorded S. squamosum in Yoke/Muyuka and surrounding breeding sites, the present study observed a predominance of S. soubrense Beffa form in the area. This is the first report of the S. soubrense Beffa form in Cameroon. Longitudinal studies would be required to determine whether these changes in cytospecies composition in the Yoke/Muyuka breeding site and its environs are permanent or temporary.
The observation of S. soubrense in the Southwest region is probably due to the recent spread of this cytospecies from neighbouring Nigeria where it has previously been reported [21], especially as all previous studies in this area did not record this species [17, 19, 35]. This finding has implications for the epidemiology of onchocerciasis in this region given that S. soubrense Beffa form seems a closer relative to the savanna than the forest cytospecies [36], and it is a more efficient vector than most forest and savanna vectors, including S. squamosum [5], which has been the main cytospecies in this area [17, 19, 35].
Conclusion
Although not exhaustive, this study provides comprehensive, updated and representative data on breeding sites and species distribution in the Southwest, Northwest and North regions of Cameroon. The difference in positive breeding sites with respect to seasons emphasizes the importance of carrying out breeding site surveys in both rainy and dry seasons. This study has also demonstrated the feasibility of breeding site surveys, in both rainy and dry seasons, in a rainforest zone. Also, this study has shown that not all potential breeding sites are actual breeding sites. The report of S. soubrense in the Southwest region indicates changes in species composition over time and could affect the epidemiology of onchocerciasis in this area.
Availability of data and materials
The datasets analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- DS:
-
Dry season
- RS:
-
Rainy season
- REMO:
-
Rapid epidemiological mapping of onchocerciasis
- SW:
-
Southwest region
- NW:
-
Northwest region
- N:
-
North region
References
Boakye DA, Back C, Fiasorgbor GK, Sib APP, Coulibaly Y. Sibling species distributions of the Simulium damnosum complex in the West African Onchocerciasis Control Programme area during the decade 1984–93, following intensive larviciding since 1974. Med Vet Entomol. 1998;12:345–58.
Amazigo U, Noma M, Bump J, Brenton B, Bernhard L, Yaméogo L, et al. Onchocerciasis. In: Jamison DT, Feachem RG, Makgoba MW, Bos ER, Baingana FK, Hofman KJ, et al., editors. Disease and mortality in sub-Saharan Africa. 2nd ed. Washington (DC): World Bank; 2006. p. 215–22.
Garms R, Walsh JF. The migration and dispersal of blackflies: Simulium damnosum s.l., the main vector of human onchocerciasis. In: Kim KC, Merritt RW, editors. Blackflies ecology, population management, and annotated world list. London: The Pennsylvania State University; 1987. p. 201–14.
Lamberton PHL, Cheke RA, Winskill P, Tirados I, Walker M, Osei-Atweneboana MY, et al. Onchocerciasis transmission in Ghana: persistence under different control strategies and the role of the simuliid vectors. PLoS Negl Trop Dis. 2015;9:e0003688.
Cheke RA, Garms R. Indices of onchocerciasis transmission by different members of the Simulium damnosum complex conflict with the paradigm of forest and savanna parasite strains. Acta Trop. 2013;125:43–52.
Adler PH, Crosskey RW. World Blackflies (Diptera: Simuliidae): A comprehensive revision of the taxonomic and geographical inventory. 2009. http://blackflies.info/sites/blackflies.info /files/u13/SIMULIIDAE_INVENTORY__NE_ VE RSION_2009.pdf. Accessed 16 Mar 2021.
Post RJ, Mustapha M, Krueger A. Taxonomy and inventory of the cytospecies and cytotypes of the Simulium damnosum complex (Diptera: Simuliidae) in relation to onchocerciasis. Trop Med Int Health. 2007;12:1342–53.
Cheke RA, Young S, Garms R. Ecological characteristics of Simulium breeding sites in West Africa. Acta Trop. 2017;167:148–56.
Rebollo MP, Zoure H, Ogoussan K, Sodahlon Y, Ottesen EA, Cantey PT. Onchocerciasis: shifting the target from control to elimination requires a new first-step—elimination mapping. Int Health. 2018;10:i14–9.
Cantey PT, Sharon LR, Boakye D, Mwingirad U, Ottesen EA, Hopkins AD, et al. Transitioning from river blindness control to elimination: steps toward stopping treatment. Int Health. 2018;10:i7–13.
WHO. Guidelines for stopping mass drug administration and verifying elimination of human onchocerciasis, criteria and procedures. Geneva: WHO; 2016.
Zouré HGM, Noma M, Tekle AH, Amazigo UV, Diggle PJ, Giorgi E, et al. The geographic distribution of onchocerciasis in the 20 participating countries of the African programme for Onchocerciasis Control: (2) pre-control endemicity levels and estimated number infected. Parasit Vectors. 2014;7:326.
Wilson MD, Cheke RA, Flasse SPJ, Grist S, Osei-Ateweneboana MY, Tetteh-Kumah A, et al. Deforestation and the spatio-temporal distribution of savannah and forest members of the Simulium damnosum complex in southern Ghana and southwestern Togo. Trans R Soc Trop Med Hyg. 2002;96:632–9.
Cheke RA, Basanez M-G, Perry M, White MT, Garms R, Obuobie E, et al. Potential effects of warmer worms and vectors on onchocerciasis transmission in West Africa. Philos Trans R Soc B. 2015;370:20130559.
Traoré-Lamizana M, Lemasson J-J. Participation à une étude de faisabilité d’une campagne de lutte contre l’onchocercose dans la région du bassin du Logone. Répartition des espèces du complexe Simulium damnosum dans la zone camerounaise du projet. Cahier O R S T O M Série Entomologie Médical et Parasitologie. 1987;25:171–86.
Renz A, Wenk P. Studies on the dynamics of transmission of onchocerciasis in a Sudan-savanna area of North Cameroon I: prevailing Simulium vectors, their biting rates and age-composition at different distances from their breeding sites. Ann trop med parasitol. 1987;81:215–28.
Post RJ, Flook PK, Milles AL, Cheke RA, McCall PJ, Wilson MD, et al. Cytotaxonomy, morphology and molecular systematics of the Bioko form of Simulium yahense (Diptera: Simuliidae). Bull Entomol Res. 2003;93:145–57.
Traore-Lamizana M, Somiari S, Mafuyai HB, Vajime CG, Post RJ. Sex chromosome variation and cytotaxonomy of the onchocerciasis vector Simulium squamosum in Cameroon and Nigeria. Med Vet Entomol. 2001;15:219–23.
Boakye DA. A pictorial guide to the chromosomal identification of members of the Simulium damnosum Theobald complex in West Africa with particular reference to the Onchocerciasis Control Programme Area. Trop Med Parasitol. 1993;44:223–44.
Adler PH. World blackflies (Diptera: Simuliidae): A comprehensive revision of the taxonomic and geographical inventory [2022]. 2022. http://biomia.sites.clemson.edu/pdfs/blackflyinventory.pdf. Accessed 22 Jun 2022
Post RJ, Onyenwe E, Somiari SAE, Mafuyai HB, Crainey JL, Ubachukwu PO. A guide to the Simulium damnosum complex (Diptera: Simuliidae) in Nigeria, with a cytotaxonomic key for the identification of the sibling species. Ann Trop Med Parasitol. 2011;105:277–97.
Renz A, Barthelmess C, Eisenbeiß W. Vectorial capacity of Simulium damnosum s.l. populations in Cameroon. Trop Med Parasitol. 1987;38:344–5.
Demanou M, Enyong P, Pion SD, Basanez MG, Boussinesq M. Experimental studies on the transmission of Onchocerca volvulus by its vector in the Sanaga valley (Cameroon): Simulium squamosum B. Intake of microfilariae and their migration to the haemocoel of the vector. Ann Trop Med Parasitol. 2003;97:381–402.
Achukwi M, Harnett W, Renz A. Onchocerca ochengi transmission dynamics and the correlation of O. ochengi microfilaria density in cattle with the transmission potential. BMC Vet. 2000;31:611–21.
Crosskey RZ. A taxa summary for the Simulium damnosum complex, with special reference to distribution outside the control areas of West Africa. Ann Trop Med Parasitol. 1987;81:181–92.
Hendy A, Krit M, Pfarr K, Laemmer, De Witte J, Nwane P, et al. Onchocerca volvulus transmission in the Mbam valley of Cameroon following 16 years of annual community-directed treatment with ivermectin, and the description of a new cytotype of Simulium squamosum. Parasit Vectors. 2021;14:1–14.
Molua E, Lambi C. Climate, hydrology and water resources in Cameroon. 2006. http://www.researchgate.net/publication/266448446. Accessed 23 Jun 2020.
Crosskey RW. A taxonomic study of the larvae of West African Simuliidae (Diptera: Nematocera) with comments on the morphology of the larval blackfly head. Bull br Mus nat Hist Entomol. 1960;10:1–74.
Boakye DA, Post RJ, Mosha FW, Surtees DP, Baker RHA. Cytotaxonomic revision of the Simulium sanctipauli subcomplex (Diptera: Simuliidae) in Guinea and the adjacent countries including descriptions of two new species. Bull Entomol Res. 1993;83:171–86.
Mustapha M, Post RJ, Enyong P, Lines J. A new cytotype of Simulium squamosum (Diptera: Simuliidae) from southwest Cameroon. Med Vet Entomol. 2004;18:296–300.
Zarroug IMA, Hashim K, Elaagip AH, Samy AM, Frah EA, ElMubarak WA, et al. Seasonal variation in biting rates of Simulium damnosum sensu lato, vector of Onchocerca volvulus, in two Sudanese foci. PLoS ONE. 2016;11:e0150309.
Krueger A, Hennings IC. Molecular phylogenetics of blackflies of the Simulium damnosum complex and cytophylogenetic implications. Mol Phyl Evol. 2006;39:83–90.
Cheke RA, Little KE, Young S, Walker M, Basáñez MG. Taking the strain out of onchocerciasis? A reanalysis of blindness and transmission data does not support the existence of a savannah blinding strain of onchocerciasis in West Africa. Adv Parasitol. 2021;112:1–50.
Vajime CG, Dunbar RW. Chromosomal identification of Simulium (Edwardsellum) mengense new species (Diptera: Simuliidae). Parassitologia. 1977;19:95–108.
Bassey SAE. Studies on the cytospecies and distribution of Simulium damnosum complex (Diptera: simuliidae) in parts of Nigeria, Cameroon and Equatorial Guinea (Doctoral dissertation, Ahmadu Bello University Zaria, Kaduna State, Nigeria). 1998. http://kubanni.abu.edu.ng/jspui/handle/123456789/2392. Accessed 12 Jan 2021.
Adeleke MA, Mafiana CF, Sam-Wobo SO, Olatunde GO, Ekpo UF, Akinwale OP, et al. Biting behaviour of Simulium damnosum complex and Onchocerca volvulus infection along the Osun River Southwest Nigeria. Parasit Vectors. 2010;3:93.
Acknowledgements
The authors are indebted to the Ministry of Health, Cameroon, heads of the National Onchocerciasis Control Programme—Dr. Fanné Mahamat and Dr. Didier Biholong—and the Regional Focal persons Mr. Akiunbeni Montesquieu Ataubo and Mr. Jean Baldiagaï. The authors thank Mr. Nlem Paul and Mr. Kolo Zoa Armands for providing logistic and transport support, and the community members for providing useful information leading to potential breeding sites. We are grateful to the former African Programme for Onchocerciasis Control (WHO-APOC) who funded this work before it was dissolved at the end of 2015. We are indebted to Mr. Bismark Akurugu, who graciously volunteered to produce all the maps used in this publication.
Funding
This study was funded by the World Health Organization’s African Programme for Onchocerciasis Control (WHO–APOC).
Author information
Authors and Affiliations
Contributions
DAB was involved in all aspects of the research. FA contributed to study design, acquisition of data, and writing of the manuscript. NS contributed to study design and acquisition of data. SKD, FY, FT, BB, and CEN contributed to acquisition of data. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Fig. S1.
Relief map of the North region.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ayisi, F., Sedou, N., Dieunang, S.K. et al. A cross-sectional study of Simulium damnosum sensu lato breeding sites and species distribution in Sudan savanna, mixed savanna–forest and rainforest regions in Cameroon. Parasites Vectors 15, 382 (2022). https://doi.org/10.1186/s13071-022-05462-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-022-05462-w