Abstract
Background
There is limited information about feline leishmaniosis (FeL) management in clinical practice. Leishmania infantum is the species of Leishmania most frequently reported in both dogs and cats in countries of the Mediterranean region (henceforth ‘Mediterranean countries’), Central and South America, and Iran. This study was conducted to provide veterinary clinicians with an updated overview of evidence-based information on leishmaniosis in cats.
Methods
A review was performed using PubMed, Science Direct, Google Scholar and Web of Science. Case reports of FeL caused by L. infantum were sought for the period 1912 to 1 June 2021.
Results
Sixty-three case reports are included in this review. Fifty-nine out of the 63 cats were from Europe, mostly from Mediterranean countries (88.9%). Most of them were domestic short-haired cats (90%) with a mean age of 7.9 years, and had access to the outdoors (77.3%). Sixty-six percent of the cats had comorbidities, of which feline immunodeficiency virus infection was the most frequent (37.7%). Dermatological lesions (69.8%) was the most frequent clinical sign, and hyperproteinemia (46.3%) the most frequent clinicopathological abnormality. Serology was the most performed diagnostic method (76.2%) and was positive for 93.7% of cats. Medical treatment was applied in 71.4% of cats, and allopurinol was the most used drug (74.4%). Survival time was greater for treated cats (520 days; 71.4% of cats) than non-treated cats (210 days; 25.4%).
Conclusions
The majority of the cats had comorbidities, of which feline immunodeficiency virus was the most frequent. Dermatological lesions were frequently reported, and systemic clinical signs and clinicopathological abnormalities were also common. Serology may be useful for the diagnosis of FeL in clinical practice, and a positive titer of ≥ 1/40 may be a useful cut-off for sick cats. The reported treatments and dosages varied, but there was a good clinical response and longer survival in most of the cats treated with allopurinol monotherapy.
Graphical abstract

Similar content being viewed by others
Background
Leishmaniosis is a zoonotic vector-borne disease with a worldwide distribution. The causal agents of leishmaniosis are intracellular protozoans of the genus Leishmania, which are transmitted by female phlebotomine sand flies. Although dogs are regarded as the main reservoir host, during the last decades feline leishmaniosis (FeL) has gained more attention from veterinary practitioners and researchers in areas endemic for leishmaniosis. Although the number of cats with leishmaniosis is currently considered negligible in endemic areas, a high percentage of cats test positive for the disease [serology, polymerase chain reaction (PCR), or both] [1,2,3,4,5,6,7]. Several Leishmania spp. can infect cats (Leishmania infantum, Leishmania mexicana, Leishmania venezuelensis, Leishmania tropica, Leishmania major, Leishmania amazonensis, and Leishmania braziliensis), and L. infantum is the species most frequently reported in both dogs and cats in countries of the Mediterranean region (henceforth ‘Mediterranean countries’), Central and South America, notably Brazil, and Iran [8,9,10,11,12,13,14].
Although it is likely that the first case of L. infantum infection in a cat was that described in 1912 by Sergent et al. [15], the number of case reports of FeL has been increasing globally, especially in the last 30 years [7, 16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56]. However, compared to canine leishmaniosis (CanL), there is still limited information on the clinical management of FeL. Furthermore, much of the available information on FeL is not specific to L. infantum infection, and is mostly from reports providing little scientific evidence, such as descriptive case series, isolated case reports, extrapolations from CanL studies, or those based on the personal experience of respected experts, whilst few are based on recent research in cats [8,9,10,11, 55, 57,58,59]. Moreover, few of the published research studies describe the clinical management of leishmaniosis in cats, and instead focus on the epidemiology and prevalence of leishmaniosis in cats in regions that are endemic or non-endemic for CanL [1, 4, 5, 13, 57, 60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82].
The following are crucial for the management of FeL, especially within the current context of the lack of clinical guidelines for this disease: understanding how leishmaniosis caused by L. infantum affects cats; identifying the most useful diagnostic tests and most effective treatments; and determining the prognostic factors and expected prognosis. We conducted a review to assess the risk factors, clinical signs and clinicopathological alterations, diagnostic methods, treatment, and outcome of all known published cases of FeL, to provide veterinary clinicians with an updated overview of this disease.
Methods
Search strategy
Independent literature searches were conducted between March and August 2021 by two of the authors (MGT and XR) using the databases and keywords listed in Table 1. When there were potential discrepancies between the selected articles, a third author (MCL) participated in the final decision. Additional studies were identified by contacting the authors of the publications, and by searching the publications’ reference lists. First, the titles and abstracts of all the articles identified in the searches were evaluated, and then the full texts of those considered potentially relevant were examined thoroughly.
Inclusion and exclusion criteria
The inclusion criteria were as follows: feline case reports or case series of FeL caused by L. infantum from 1912 to 1 June 2021, including signalment, a description of clinical signs, diagnostic methods, treatment protocols and outcome for each cat. The exclusion criteria were as follows: duplicate records, case reports of leishmaniosis caused by Leishmania species other than L. infantum, studies that contained information that was confusing or not sufficiently comprehensible for analysis, and reviews or meta-analyses that did not provide specific data on the factors that had been investigated for each cat. Data on treatment and outcome were not available for some of the included cases. The case reports selected in this way were included in a Microsoft Excel database and duplicate data were eliminated. The final collated publications were used for the statistical analysis, for which data from each of the included studies were extracted.
Data extraction
A pre-established protocol was used to extract the following data: when and where the research was carried out (year, country and geographic region), signalment, clinical presentation, breed, sex, age, indoor/outdoor, comorbidities, clinical signs, clinicopathological alterations, diagnostic method, treatment, and outcome.
Statistical analysis
Continuous variables were summarized by mean, range, and SD. For association and risk factor analysis for infection, geographic data were grouped into Mediterranean and non-Mediterranean countries; breed data were grouped into domestic short haired (DSH) and non-DSH (Siamese, Siberian, crossbreed, unknown breeds); lifestyle was divided into indoor or outdoor; and clinical signs were grouped into cutaneous, mucocutaneous, ocular, respiratory and systemic. To group clinical signs, we followed the classification used by the clinicians who authored each case report; lymphadenomegaly, fever, lethargy, poor body condition, pallor, hepato-splenomegaly, weight loss and abdominal distension were considered systemic signs. Comorbidities were defined as diseases other than FeL, other medical conditions, prescribed medication, and pathogens that could modify the immune response
In the statistical evaluation of diagnostic method reliability, cytology and/or histopathology were considered as gold standards for the direct identification of parasites in accordance with experts’ recommendations for FeL. An indirect fluorescent antibody technique (IFAT) had been used in the majority of cases, so to enable the statistical analysis of positive quantitative serological results, all the data were transformed according to the same titer scale based on World Organization for Animal Health recommendations [83] and divided into titer ranges of 1/40 to ≤ 1/80, > 1/80 to ≤ 1/160, > 1/160 to ≤ 1/320, > 1/320 to ≤ 1/640, and > 1/640. For those cases where quantitative serologic data were not supplied, and only a positive or negative result from a test such as western blot, the results were grouped as qualitative.
All the statistical analyses were performed using IBM SPSS version 20 for Mac. Univariate analyses were performed, and the results are presented as the number of affected cats in relation to the total number of cats for which the finding was described. Data were evaluated for normal distribution using a Kolmogorov–Smirnov test. In the univariate analyses, when only two independent continuous variables were compared, an independent-sample t-test was performed according to the data distribution. For the categorical variables, a chi-square test of association was used, and for paired samples the Wilcoxon test was used. SPSS was used to calculate the expected frequencies. The rule used was that, at most, only 20% of expected frequencies should be less than 5. The Pearson correlation (r) test of association was used for survival and age analyses. A Kaplan–Meier survival analysis with log ranks was used to test for significant differences between survival curves for treatment applied. The treatments were grouped as follows: (i) allopurinol, (ii) allopurinol plus meglumine antimoniate, (iii) allopurinol plus miltefosine, (iv) meglumine antimoniate, (v) other, and (vi) no treatment. When survival data were not exactly defined, the highest known values were used as the survival data for the analyses. P < 0.05 was considered the critical level of significance.
Results
Case selection
The online literature search identified 552 potentially relevant publications. A total of 355 duplicate publications were excluded. After the initial screening of the data, based on title and/or abstract evaluation, another 130 publications were excluded. A further 25 were excluded during a second selection process based on the full-text evaluation of the 67 remaining publications. A total of 42 articles (63 cats) were finally found to be eligible for inclusion in this review and the data subjected to statistical analysis (Fig. 1; Table 2).
Geographic region
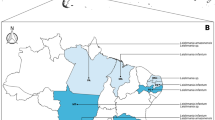
All the cases were of domestic cats living in Europe (59), Brazil (1), Vietnam (1), Reunion Island (1), and Algeria (1). The European cases were from Spain (24), Italy (16), Portugal (9), France (6), Switzerland (3), and Cyprus (1). There was a statistically significant association between location and the number of cases, with a higher prevalence in Mediterranean (56/63, 88.9%) compared to non-Mediterranean (7/63, 11.1%) countries (χ2 = 38.111, df = 1, P < 0.001) (Table 3).
Signalment
The age of the cats at clinical presentation was known in 56/63 cases, and ranged from 2 to 21 years (mean 7.9 ± 4.1 years). Breed was described for 60 out of the 63 cases, and was as follows: 54 DSH (85.7%), four Siamese (6.3%), one Siberian (1.6%) and one crossbreed (1.6%) cat. DSH were more likely to be infected than non-DSH breeds (χ2 = 170.571, df = 4, P < 0.001). Sex was reported in 62 out of 63 cases, and there was a slightly higher prevalence in females (53.2%) than in males (46.7%), although this difference was not statistically significant (χ2 = 0.258, df = 1, P = 0.611). Lifestyle was known for 22 cats, of which 17 were outdoor (77.3%) and five indoor cats (22.7%). There was an association between an outdoor lifestyle and infection (χ2 = 6.545, df = 1, P ≤ 0.011) (Table 3).
Information on comorbidity status was available for 53 cats (Table 4); of these 35 (66.0%) had comorbidities and 18 (34.0%) did not. Of the 35 cats with comorbidities, 22 (62.9%) had only one comorbidity, whereas 13 (37.1%) had two or more. Positive feline immunodeficiency virus (FIV) antibody status was the most prevalent comorbidity, but the association between this and leishmaniosis was not statistically significant (χ2 = 0.277, df = 1, P = 0.599).
Clinical presentation
The clinical signs and lesions that were reported are given in Table 5. The most frequent clinical signs were cutaneous (44/63; 69.8%), followed by systemic (35/63; 55.5%), ocular (22/63; 34.9%), mucocutaneous (18/63; 28.6%) and respiratory (8/63; 12.7%). Many of the cats showed a combination of clinical signs (37/63; 58.7%). There was a statistically significant association between dermatological signs and FIV (χ2 = 7.185, df = 1, P = 0.007), and between ocular signs and the neutered status of females (χ2 = 17.814, df = 3, P < 0.001). However, no other statistically significant associations were found between groups of clinical signs and age, sex, breed, or comorbidities (Table 6). Lymph node size was described for 45/63 cats from physical examination; the percentage of cats with lymph nodes of normal size (27/45; 60.0%) was greater than that of cats with lymphadenomegaly (18/45; 40.0%).
When clinicopathological abnormalities were reported, hyperproteinemia was the most frequent (19/41; 46.3%), followed by anemia (16/48; 33.3%), neutrophilia (9/48, 18%), thrombocytopenia (8/48, 16.6%), proteinuria (7/46; 15.2%) and azotemia (7/47; 14.9%). Hypergammaglobulinemia was the most frequent alteration detected by serum protein electrophoresis (27/38; 71.0%) followed by hypoalbuminemia (9/47; 19.1%). Other reported clinicopathological alterations were neutropenia (4/48, 8.3%), eosinophilia (3/48, 6.25%), pancytopenia (1/48, 2%) and an increase in alanine transaminase level (2/48, 4.1%).
Diagnostic methods
Cytology, for the detection of Leishmania amastigotes, was the most common first line or preferred diagnostic option of practitioners for the diagnosis of FeL (28/63, 44.4%), followed by histopathology (20/63, 31.7%), serology (17/63, 26.9%) and PCR (3/63, 4.7%). There was no statistically significant association (χ2 = 8.980, df = 2, P = 0.062) between results from the PCR and those from cytological and/or histopathological examination (widely used as confirmatory tests for FeL and considered gold standards here). However, a statistically significant association between cytology and/or histopathology and seropositivity (χ2 = 26.913, df = 14, P = 0.020) was found.
Of the complete diagnostic procedures (both first line and additional diagnostic tests), antibody detection techniques were performed most (48/63; 76.2%), and comprised IFAT (28/48), qualitative serology (11/48), enzyme-linked immunosorbent assay (5/48), and direct agglutination (4/48). Antibody tests were positive for 45/48 cats (93.7%) and negative for 3/48 (6.3%); there was a statistically significant association between L. infantum antibody positive status and diagnosis of FeL by cytology and/or histopathology (χ2 = 36.750, df = 2, P < 0.001). In cats seropositive according to quantitative tests (35/45, 77.8%), the titers ranged from 1/40 to ≤ 1/80 in 5/35 (14.3%), from > 1/80 to ≤ 1/160 in 3/35 (8.6%), from > 1/160 to ≤ 1/320 in 5/35 (14.3%), from > 1/320 to ≤ 1/640 in 5/35 (14.3%), and were > 1/640 in 17/35 cats (48.5%). No statistically significant association was found between serological titers and category of clinical signs or clinicopathological abnormalities (P > 0.05). Qualitative positive serology was reported for 10/45 cats (22.2%).
Polymerase chain reaction (using blood, lymph node, bone marrow, spleen tissue; ocular, lung or skin samples) was performed for 38 of the 63 cats (60.3%), and was positive for 36 of them (94.7%) (Table 7). Cytology was performed for 44/63 cats (69.8%) and histopathology for 35/63 cats (55.5%) using different types of tissue. Cytology and histopathology were positive for Leishmania amastigotes in 39/44 cats (88.6%) and 31/35 (88.6%), respectively (Tables 8, 9). With respect to serological titers, no statistically significant association was found between PCR, cytology or histopathology with any category of clinical sign or clinicopathological abnormality (P > 0.05).
Treatment
Medical treatment was administered in 45/63 cats (71.4%); 16/63 (25.4%) did not receive any treatment, and treatment was not stipulated for 2/63 (3.2%). Allopurinol was used in 37/45 cats (74.4%), followed by meglumine antimoniate in 13/45 (28.9%) and miltefosine in only one cat of the 45 (2.2%). Although the dosages varied, the most frequent were 10 mg/kg twice a day (BID) per os (PO) for at least 6 months for allopurinol (20/37 cats; 54.0%), 50 mg/kg once a day (SID) subcutaneously (SC) for 30 days for meglumine antimoniate (5/13 cats; 38.5%), and 2 mg/kg SID PO for 28 days for miltefosine in the only cat in which it was used. Allopurinol was used as monotherapy in 28/37 cats (75.7%) and in combination with meglumine antimoniate or miltefosine in 8/37 (21.6%) and 1/37 (2.7%) cats, respectively. Meglumine antimoniate was used as monotherapy in 5/13 cats (38.5%) and in combination with allopurinol in 8/13 (61.5%). Adverse effects associated with treatment were reported in 10/45 cats (22.2%), and were mainly associated with allopurinol (7/10), and affected the kidney (4/10), skin (2/10) and liver (1/10).
Outcome
Survival time ranged from 0 to 2700 days, with a mean of 432 days (± 575). Mean survival time was significantly longer for treated cats than non-treated cats (520 days and 210 days, respectively) (χ2 = 15.311, df = 1, P = 0.002) (Fig. 2). However, no significant association was found between survival time and any other variable (Table 10).
Kaplan–Meier survival curves of cats treated with allopurinol, allopurinol plus meglumine antimoniate, allopurinol plus miltefosine, meglumine antimoniate, other, or no treatment. Median survival time of the treated cats was as follows: allopurinol (28/63; 619 days); allopurinol plus meglumine antimoniate (8/63; 614 days); allopurinol plus miltefosine (1/63; 45 days); meglumine antimoniate (5/63; 1372 days); other such as fluconazole, metronidazole, spiramycin or lomidine (3/63; 660 days); no treatment (10/63; 411 days)
Discussion
The results of this study indicate that leishmaniosis should be included in the differential diagnosis of sick cats living in, or with a history of travel to, areas where CanL is endemic [1,2,3, 84]. Prevalences of FeL in endemic areas as shown by positive PCR range from 0 to 100% (mean 21.3%), whilst those indicated by positive serology range from 0 to 70.5% (mean 13.7%) [1, 10, 85]. Prevalences determined by both of these types of tests are lower for cats than dogs (63% and 27% for PCR and serology, respectively) [85]. There are also fewer clinical feline cases than canine cases in the literature [8,9,10,11, 59]. Thus, all this suggests that the prevalence of FeL could be about half that of CanL for the same geographical areas [60, 63, 74, 79,80,81]. Furthermore, as previously reported for CanL [86, 87], increased movement of pet cats between countries, especially inside Europe, could lead to clinical cases of FeL being diagnosed in areas that are not endemic for L. infantum [30, 41]. However, although there have been epidemiological studies on FeL in areas that are also endemic for CanL, such as Brazil [1, 84], only one case from Brazil was included in the current study. Possible reasons for this include the non-detection of other cases from Brazil due to the criteria used in this study, and/or perhaps because feline medicine is less developed there, and/or because most knowledge on leishmaniosis is focused on dogs. Thus, even in areas that are non-endemic for L. infantum, leishmaniosis should be considered as a differential diagnosis in cats with clinical signs or pathological alterations consistent with this disease.
Few epidemiological studies have reported significant associations between L. infantum infection in cats and their access to outdoors, that they are male, or their age when they are adults [60, 64]. Most of the cats affected by leishmaniosis in the present study were DSH with outdoor access, and had a mean age of 7.9 years. In contrast to other publications [60, 65, 66], we found that more female cats were diagnosed with leishmaniosis than male cats, although this difference was not statistically significant.
It is well recognized that susceptibility to progressive Leishmania infection and the development of clinical signs in dogs is mostly linked to an imbalance in the adaptive immune response, and probably associated with a predominant Th2 response and an impaired Th1 immune response [88]. However, in contrast to CanL, to the best of our knowledge, no prospective controlled studies have been published on immune mechanisms involved in the pathogenesis of FeL. Some investigations have suggested that cats may have a better immune response against L. infantum because the Th2 response plays a protective role [81, 89], or because there are other factors in seropositive cats that can control the development of patent leishmaniosis [90], such as the production of higher levels of interferon gamma, which plays a direct role in the regulation of Th1 cell development [91]. Thus, Leishmania spp. infection in cats might be more common than associated disease, and cats might be more resistant to disease development than dogs [9, 59]. Cats that develop leishmaniosis are often suspected of having impaired immunity because of comorbidities [8,9,10,11]. However, to date, a significant association has only been reported between L. infantum and FIV co-infection [67, 69, 70, 82, 92], although the results of other studies contradict this association [71,72,73, 81]. In this review, a high percentage (66.0%) of evaluated cats with leishmaniosis had comorbidities which are associated with potentially impaired immune competence, such as previous corticosteroid treatment, diabetes mellitus, epidermoid carcinoma, squamous cell carcinoma, pemphigus, or co-infections such as FIV, feline leukemia virus, Bartonella henselae, ‘Candidatus Mycoplasma haemominutum,’ feline coronavirus, Toxoplasma spp. and Hepatozoon spp. Furthermore, the current study confirms that FIV, which was detected in 37.7% of cats, is the most frequent comorbidity associated with FeL, although the association was not statistically significant.
Leishmaniosis in dogs has a wide range of clinical signs [8, 93,94,95], but extrapolating these to cats could mean that only the clinical signs of an infected cat that resemble those of CanL would be used in a differential diagnosis for FeL. This could lead to the misdiagnosis of FeL and thus the underestimation of its clinical relevance. The clinical signs of FeL described in this review are mainly dermatological (69.8%), followed by systemic (55.5%), ocular (34.9%), mucocutaneous (28.6%), and respiratory (12.7%). These results agree with those of previous studies, where dermatological lesions and systemic clinical signs, including lymph node enlargement, were reported frequently in cats infected with L. infantum [9, 10, 53]. These findings support the idea that, when caused by L. infantum, CanL and FeL present similarly, and thus FeL should also be included in the differential diagnosis when a wide range of systemic clinical signs of leishmaniosis present alone or in combination in a cat. Less frequent and/or severe, isolated clinical presentations may go unreported or misdiagnosed, and presumably could lead to underestimation of the clinical relevance of FeL [8]. Although multiple factors have been found to be statistically associated with a wide range of clinical manifestations in FeL [66, 96], this review only found a statistically significant association between dermatological signs and FIV positive status. A potential explanation for this is that dermatological lesions associated with leishmaniosis and immunodeficiency are very similar in cats, so they could have been secondary to either condition [55].
In comparison to CanL, there is limited information about clinicopathological abnormalities associated with L. infantum infection in cats [41, 57, 97]. Of the cases analyzed here, hyperproteinemia (46.3%) was the most frequent laboratory abnormality, and hypergammaglobulinemia (71.0%) followed by hypoalbuminemia (19.1%) were the most frequent alterations seen on serum protein electrophoresis, in agreement with the results of two studies [41, 57], but in contrast with those of another study [58]. The percentages of cats presenting with neutrophilia, thrombocytopenia, proteinuria, and azotemia (18%, 16.6%, 15.2% and 14.9%, respectively) reported in the present study suggest that leishmaniosis should be a differential diagnosis for hypergammaglobulinemic cats with any of these conditions. However, although gamma globulin levels were significantly elevated in FeL, they could not be used to differentiate FeL from other inflammatory, neoplastic or vector-borne diseases in cats [97]. Furthermore, we report here the important finding that, in FeL, unlike in CanL, azotemia and proteinuria are frequently concurrent [98,99,100]. Although in dogs leishmaniosis is an important cause of proteinuria, this is not thought to be the case in cats [57, 58]. However, the present study shows that proteinuria is not that infrequent in cats with FeL caused by L. infantum, which suggests that it is important to perform urinalysis and measure the urinary protein to creatinine ratio to help in the early detection, and subsequent management, of chronic kidney disease in cats with leishmaniosis [101].
In contrast to CanL, there are fewer clearly recommended diagnostic tests for FeL [8,9,10,11]. Previous reviews and expert opinions suggest that, in clinical practice, the best means of confirming FeL is the detection of Leishmania amastigotes by cytology and/or histopathology, or the detection of Leishmania DNA by PCR; all of these tests can be performed using a sample of any type of affected tissue, including lymph node tissue, bone marrow or blood [8,9,10, 102, 103]. In agreement with published recommendations, cytology was found to be the preferred first line diagnostic technique for FeL, followed by histopathology, serology, and PCR. Furthermore, Leishmania DNA was easily detected by PCR, as were amastigotes by cytology and histopathology (i.e. in 94.7%, 88.6%, and 88.6% of cats with clinical leishmaniosis, respectively).
Conversely, serology has been suggested as being less useful for cats than for dogs for the diagnosis of leishmaniosis in clinical practice, and thus it may be more useful as an additional test to support the diagnosis of leishmaniosis and the follow up of sick cats with the disease [8,9,10,11]. Anti-Leishmania antibody detection techniques such as IFAT, enzyme-linked immunosorbent assay, direct agglutination and western blot have been extensively used in a wide range of studies on cats [1, 4, 5, 10, 57, 60, 62,63,64, 66,67,68, 70,71,72,73,74,75,76,77,78, 80,81,82]. However, the low levels of antibodies produced in cats due to their differing immune responses [74, 90] as compared to dogs, and/or the fact that few laboratories offer validated serological tests for FeL (compared to serological tests for CanL) may explain why serology has previously been overlooked as a diagnostic test for FeL in clinical practice [5, 57, 60, 62,63,64, 68,69,70,71,72]. For these reasons, it has been recommended that serologic results should be interpreted with caution and in combination with other diagnostic test results and the assessment of clinical signs for the diagnosis of FeL [8,9,10,11, 58]. Although a cut-off titer of 1:80 or above is considered adequate to discriminate between infected and non-infected cats [57, 64, 70, 74], the level of this titer is still controversial. In the current study, among first line and additional diagnostic tests, serology (73%) was the diagnostic technique most used by practitioners (positive serology in 93.5% of the sick cats), and positive serological results showed a statistically significant association with cytology and/or histopathology results. Furthermore, the positive seroreactivity titer range was wide (1/40 to > 1/640) in sick cats. However, no statistically significant association was found between serological titers and any type of clinical sign or clinicopathological abnormality, showing that there is no apparent direct relationship between clinical alterations and serological titers. Thus, we suggest that serology may be more useful as an initial diagnostic test than previously thought when cats that are suspected of having leishmaniosis are evaluated in clinical practice, and that a serological titer of ≥ 1/40 could be a suitable cut-off for the diagnosis of leishmaniosis caused by L. infantum in cats with clinical signs or clinicopathological alterations compatible with this disease. However, further studies are needed to confirm the best serological positive cut-off titer for the diagnosis of patent FeL.
An empirical therapeutic approach to FeL usually involves the extrapolation of recommendations for CanL [94, 104] as no studies have been conducted to evaluate the efficacy and safety of treatments in cats [8,9,10,11]. In line with the recommendations for CanL, the long-term administration of allopurinol, used as monotherapy (75.7%), or in combination with meglumine antimoniate (21.6%) or miltefosine (2.7%), was the drug of choice used for the treatment of 74.4% of the cats, followed by meglumine antimoniate and miltefosine for 28.9% and 2.2%, respectively. Adverse effects associated with treatment were reported in 22.2% of the cats [30, 48, 51, 53,54,55]. Although recent reports indicate that allopurinol can have different adverse effects in cats [48, 49] compared to those observed in dogs [94, 104], it should be noted that both spontaneous [105] and allopurinol-induced [51, 54] xanthinuria and urolithiasis have also been described in cats, hence it is recommended that urinalysis be performed during the treatment of FeL with allopurinol, as is done for CanL. Finally, propylene glycol is one of the excipients in the oral formulation of miltefosine that is licensed for the treatment of CanL, and has been reported to potentially cause a decrease in the lifespan of feline red blood cells due to the formation of Heinz bodies [8, 106]. Thus, the use of miltefosine should possibly be avoided in cats, or at least only used with caution until more scientific evidence on its effects have been published.
Survival time ranged from 0 to 2,700 days, with a mean of 432 days. Mean survival time was statistically greater for treated cats than non-treated cats (520 and 210 days, respectively), which supports previous recommendations that cats with clinical signs of leishmaniosis should be treated [8,9,10]. No statistically significant difference in survival time was found between treatment with allopurinol as monotherapy and allopurinol in combination with other drugs. This suggests that monotherapy with allopurinol could be used as a first line treatment in cats for at least for 6 months, and for those cats not responding to treatment with allopurinol alone, meglumine antimoniate could be added to try and further improve the clinical signs of leishmaniosis. However, caution is warranted because the effects of specific leishmaniosis treatments on survival could not be assessed here due to the great variability in the dosages of the different drugs used, and because the type, quality and intensity of supportive treatments used for each of the cats included in this study were unknown. No other statistically significant association was found between survival time and any other variable such as age, breed, sex, indoor/outdoor access, comorbidities, clinical signs or clinicopathological abnormalities, diagnostic test results and type of treatment, and thus no potential predictive factors for the prognosis of sick cats diagnosed with leishmaniosis were identified.
This study had several limitations, including (i) the risk of bias associated with the lack of inclusion of more cases due to the search criteria used, (ii) difficulties associated with the statistical analysis due to the fact that most of the included publications described a single clinical case, (iii) the heterogeneity of data due to different clinical management among years, (iv) imprecision regarding treatment effects due to the variability in dosages amongst the included cases, and (v) the lack of a comprehensive data set due to the retrospective nature of this study.
Conclusions
The case reports of FeL caused by L. infantum included in the present study showed that the cats often had a comorbidity, with FIV infection being the most frequent of these. Dermatological alterations were the most frequently reported clinical sign in FeL caused by L. infantum, although systemic clinical signs and clinicopathological abnormalities, alone or in combination, were also common. Leishmaniosis should be included as a differential diagnosis for sick cats who live in, or have traveled from, areas endemic for CanL. The results of this study also indicate that serology could be useful as a first line diagnostic test for FeL, and that a positive titer of ≥ 1/40 could be a suitable cut-off for the diagnosis of leishmaniosis in sick cats that are suspected of having the disease. Finally, despite the limitations of this study, we found that there was good clinical response and prolonged survival in many cats given allopurinol as a monotherapy at 10 mg/kg PO BID for at least 6 months.
Availability of data and materials
Not applicable.
Abbreviations
- CanL:
-
Canine leishmaniosis
- DSH:
-
Domestic short haired
- FeL:
-
Feline leishmaniosis
- FIV:
-
Feline immunodeficiency virus
- IFAT:
-
Indirect fluorescent antibody technique
- PCR:
-
Polymerase chain reaction
References
Asfaram S, Fakhar M, Teshnizi SH. Is the cat an important reservoir host for visceral leishmaniasis? a systematic review with meta-analysis. J Venom Anim Toxins Incl Trop Dis. 2019;25:e20190012.
Cardoso L, Schallig H, Persichetti MF, Pennisi MG. New epidemiological aspects of animal leishmaniosis in Europe: the role of vertebrate hosts other than dogs. Pathogens. 2021;10:307.
Gramiccia M. Recent advances in leishmaniosis in pet animals: epidemiology, diagnostics and anti-vectorial prophylaxis. Vet Parasitol. 2011;181:23–30.
Dalvi APR, De CTDG, Werneck GL. Is there an association between exposure to cats and occurrence of visceral leishmaniasis in humans and dogs? Vector-Borne Zoonotic Dis. 2018;18:335–42.
Bezerra JAB, de IVPOliveira M, Yamakawa AC, Nilsson MG, Tomaz KLR, de Oliveira KDS, et al. Serological and molecular investigation of Leishmania spp. infection in cats from an area endemic for canine and human leishmaniasis in northeast Brazil. Rev Bras Parasitol Vet. 2019;28:790–6.
Pereira A, Parreira R, Cristóvão JM, Vitale F, Bastien P, Campino L, et al. Leishmania infantum strains from cats are similar in biological properties to canine and human strains. Vet Parasitol. 2021;298:109531.
Maroli M, Pennisi MG, Di Muccio T, Khoury C, Gradoni L, Gramiccia M. Infection of sandflies by a cat naturally infected with Leishmania infantum. Vet Parasitol. 2007;145:357–60.
Pennisi MG, Persichetti MF. Feline leishmaniosis: is the cat a small dog? Vet Parasitol. 2018;251:131–7.
Pennisi MG, Hartmann K, Lloret A, Addie D, Belák S, Boucraut-Baralon C, et al. Leishmaniosis in cats: ABCD guidelines on prevention and management. J Feline Med Surg. 2013;15:638–42.
Pennisi MG, Cardoso L, Baneth G, Bourdeau P, Koutinas A, Miró G, et al. LeishVet update and recommendations on feline leishmaniosis. Parasit Vectors. 2015;8:302.
Soares CSA, Duarte SC, Sousa SR. What do we know about feline leishmaniosis? J Feline Med Surg. 2016;18:435–42.
da Silva SM, Rabelo PFB, de Gontijo N, F, Ribeiro RR, Melo MN, Ribeiro VM, et al. First report of infection of Lutzomyia longipalpis by Leishmania (Leishmania) infantum from a naturally infected cat of Brazil. Vet Parasitol. 2010;174:150–4.
Reza Hatam G, Jafar Adnani S, Asgari Q, Fallah E, Hossein Motazedian M, Mahmoud Sadjjadi S, et al. First report of natural infection in cats with Leishmania infantum in Iran. Vector Borne Zoonotic Dis. 2010;10:313–6.
Madruga G, Ribeiro AP, Ruiz T, Sousa VRF, Campos CG, Almeida ABPF, et al. Ocular manifestations of leishmaniasis in a cat: first case report from Brazil. Arq Bras Med Vet Zootec. 2018;70:1514–20.
Sergent E, Lombard J, Quilichini M. Infection simultanée d’un enfant, d’un chien et d’un chat dans la meme habitation. Bull Soc Pathol Exot. 1912;5:93–8.
Bergeon M. Un case de leishmaniose chez le chat. Bull Soc Vet Lyon. 1927;30:52–3.
Bosselut H. Un cas de leishmaniose générale du chat. Arch Inst Pasteur Alger. 1948;26:14.
Denuzière C. Un chat leishmanien. Sem Vet. 1977;32:1–2
Dunan N, Mary C, Garbe L, Breton Y, Olivon B, Ferrey P. A propos d’un cas de leishmaniose chez un chat de la région marseillaise. Bull Soc Fr Parasitol. 1989;7:17–20.
Costa Durao J, Rebelo E, Peleteiro M, Cooreia J. Primeiro caso de leishmaniose em gato doméstico (Felis catus) detectado em Portugal (Concelho de Sesimbra). Nota Prelim Rev Port Cienc Vet. 1994;89:140–4.
Laruelle-Magalon C, Toga I. Un cas de leishmaniose féline. Prat Med Chir Anim Comp. 1996;31:255–61.
Ozon C, Marty P, Pratlong F, Breton C, Blein M, Lelievre A. Dissseminated feline leishmaniosis due to Leishmania infantum in southern France. Vet Parasitol. 1998;75:273–7.
Hervás J, De Chacón-M Lara F, Sánchez-lsarria MA, Pellicer S, Carrasco L, Castillo JA, et al. Two cases of feline visceral and cutaneous leishmaniosis in Spain. J Feline Med Surg. 1999;1:101–5.
Hervás J, Chacón M, de Lara F, Moreno A, López J. Leishmaniasis cutánea nodular en gato: diagnóstico y tratamiento. Clin Vet Peq Anim. 2001;21:199.
Hervás J, De Chacón-Manrique Lara F, López J, Gómez-Villamandos JC, Guerrero MJ, Moreno A. Granulomatous (pseudotumoral) iridociclitis associated with leishmaniasis in a cat. Vet Rec. 2001;149:624–5.
Poli A, Abramo F, Barsotti P, Leva S, Gramiccia M, Ludovisi A, et al. Feline leishmaniosis due to Leishmania infantum in Italy. Vet Parasitol. 2002;106:181–91.
Pennisi MG, Venza M, Reale S, Vitale F, Lo GS. Case report of leishmaniasis in four cats. Vet Res Commun. 2004;28:363–6.
Savani ESMM, de Oliveira Camargo MCG, de Carvalho MR, Zampieri RA, dos Santos MG, D’Áuria SRN, et al. The first record in the Americas of an autochthonous case of Leishmania (Leishmania) infantum chagasi in a domestic cat (Felix catus) from Cotia County, São Paulo State. Brazil Vet Parasitol. 2004;120:229–33.
Grevot A, Jaussaud Hugues P, Marty P, Pratlong F, Ozon C, Haas P, et al. Leishmaniosis due to Leishmania infantum in a FIV and FelV positive cat with a squamous cell carcinoma diagnosed with histological, serological and isoenzymatic methods. Parasite. 2005;12:271–5.
Rüfenacht S, Schaerer V, Roosje PJ, Sager H, Heier A, Welle MM, et al. Two cases of feline leishmaniosis in Switzerland. Vet Rec. 2005;156:542–5.
Leiva M, Lloret A, Peña T, Roura X. Therapy of ocular and visceral leishmaniasis in a cat. Vet Ophthalmol. 2005;8:71–5.
Britti D, Vita S, Aste A, Williams D, Boari A. Sindrome da malassorbimento in un gatto con leishmaniosi. Atti Soc Ital Sci Vet. 2005;59:281–2.
Saló F, Gonzalez F, Altimira J, Vilafranca M. Dermatitis granulomatosa de la membrana nictitante causada por Leishmania infantum en un gato doméstico. Clin Vet Peq Anim. 2007;27:173–7.
Dalmau A, Ossó M, Oliva A, Anglada L, Sarobé X, Vives E. Leishmaniosis felina a propósito de un caso clínico. ¿nos olvidamos de que existe? Clin Vet Peq Anim. 2008;8:233–7.
Marcos R, Santos M, Malhaõ F, Pereira R, Fernandes AC, Montenegro L, et al. Pancytopenia in a cat with visceral leishmaniasis. Vet Clin Pathol. 2009;38:201–5.
Ibba F. Un caso di rinite cronica in corso di leishmaniosi felina. Proc 62nd Int SCIVAC Congr Rimini Soc Cult Ital Vet Anim Comp. 2009. 29–31.
Ortuñez A, Gomez P, Verde M, Al. E. Lesiones granulomatosas en la mucosa oral y lengua y multiples nódulos cutáneos en un gato causado por Leishmania infantum. V South Eur Vet. 30 September to 3 October 2010.
Soler M, Medina N, Repiso M, Roca A, Peña Giménez T. Leishmaniosis mucocutánea y ocular en un gato. VI South Eur Vet Conf. 29 September to 2 October 2011.
Pocholle E, Reyes-Gomez E, Giacomo A, Delaunay P, Hasseine L, Marty P. Un cas de leishmaniose féline disséminée dans le sud de la France. Parasite. 2012;19:77–80.
Verneuil M. Leishmaniose oculaire féline: à propos d’un cas. J Fr Ophtalmol. 2013;36:e67-72.
Richter M, Schaarschmidt-Kiener D, Krudewig C. Ocular signs, diagnosis and long-term treatment with allopurinol in a cat with leishmaniasis. Schweiz Arch Tierheilkd. 2014;156:289–94.
Pimenta P, Alves-Pimenta S, Barros J, Barbosa P, Rodrigues A, Pereira MJ, et al. Feline leishmaniosis in Portugal: 3 cases (year 2014). Vet Parasitol Reg Stud Rep. 2015;1–2:65–9.
Maia C, Sousa C, Ramos C, Cristóvão JM, Faísca P, Campino L. First case of feline leishmaniosis caused by Leishmania infantum genotype E in a cat with a concurrent nasal squamous cell carcinoma. J Feline Med Surg Open Rep. 2015;1:205511691559396.
Migliazzo A, Vitale F, Calderone S, Puleio R, Binanti D, Abramo F. Feline leishmaniosis: a case with a high parasitic burden. Vet Dermatol. 2015;26:69–70.
Basso MA, Marques C, Santos M, Duarte A, Pissarra H, Carreira LM, et al. Successful treatment of feline leishmaniosis using a combination of allopurinol and N-methyl-glucamine antimoniate. J Feline Med Surg Open Rep. 2016;2:2055116916630002.
Fernandez Gallego A, Pertegaz i Folch J, Feo Bernabé LF. Fallo renal agudo en un gato con leishmaniosis visceral. X South Eur Vet Conf. 20 to 22 October 2016.
Attipa C, Neofytou K, Yiapanis C, Martínez-Orellana P, Baneth G, Nachum-Biala Y, et al. Follow-up monitoring in a cat with leishmaniosis and coinfections with Hepatozoon felis and ‘Candidatus Mycoplasma haemominutum.’ J Feline Med Surg Open Report. 2017;3:205511691774045.
Leal RO, Pereira H, Cartaxeiro C, Delgado E, da Peleteiro MC, Pereira da Fonseca I. Granulomatous rhinitis secondary to feline leishmaniosis: report of an unusual presentation and therapeutic complications. J Feline Med Surg Open Rep. 2018;4:2055116918811374 (Sage UK: London, England).
Brianti E, Celi N, Napoli E, Abbate JM, Arfuso F, Gaglio G, et al. Treatment and long-term follow-up of a cat with leishmaniosis. Parasit Vectors. 2019;12:121.
Pereira A, Valente J, Parreira R, Cristovão JM, Azinheira S, Campino L, et al. An unusual case of feline leishmaniosis with involvement of the mammary glands. Top Companion Anim Med. 2019;37:100356.
Domínguez Ruiz M, Hernández Rodríguez J, Tabar Rodríguez MDM. Leishmaniosis en un gato con pancitopenia. XIII South Eur Vet Conf. 7 to 9 November 2019.
Altuzarra R, Movilla R, Roura X, Espada Y, Majo N, Novellas R. Computed tomographic features of destructive granulomatous rhinitis with intracranial extension secondary to leishmaniasis in a cat. Vet Radiol Ultrasound. 2020;61:E64–8.
Fernandez-Gallego A, Feo Bernabe L, Dalmau A, Esteban-Saltiveri D, Font A, Leiva M, et al. Feline leishmaniosis: diagnosis, treatment and outcome in 16 cats. J Feline Med Surg. 2020;22:993–1007.
Torrano A, Juarez C, Bermudez E, Arenas C, Castro J. Anemia en una gata con múltiples patologías infecciosas y desarrollo de xantinuria probablemente secundaria al tratamiento con alopurinol. XXI Congr Virtual Espec Vet GTA-AVEPA. 11 to 13 May 2021.
Abramo F, Albanese F, Gattuso S, Randone A, Fileccia I, Dedola C, et al. Skin lesions in feline leishmaniosis: a systematic review. Pathogens. 2021;10:472.
Garcia M, Blasi C, Dueso M, Roura X. Rinitis piogranulomatosa por Leishmania infantum en un gato con estornudos inversos. XXI Congr Virtual Espec Vet GTA-AVEPA. 11 to 13 May 2021.
Urbani L, Tirolo A, Salvatore D, Tumbarello M, Segatore S, Battilani M, et al. Serological, molecular and clinicopathological findings associated with Leishmania infantum infection in cats in northern Italy. J Feline Med Surg. 2020;22:935–43.
Chatzis MK, Xenoulis PG, Leontides L, Kasabalis D, Mylonakis ME, Andreadou M, et al. Evaluation of clinicopathological abnormalities in sick cats naturally infected by Leishmania infantum. Heliyon. 2020;6:e05177.
Pennisi MG. Leishmaniosis of companion animals in Europe: an update. Vet Parasitol. 2015;208:35–47.
Cardoso L, Lopes AP, Sherry K, Schallig H, Solano-Gallego L. Low seroprevalence of Leishmania infantum infection in cats from northern Portugal based on DAT and ELISA. Vet Parasitol. 2010;174:37–42.
Maia C, Nunes M, Campino L. Importance of cats in zoonotic leishmaniasis in Portugal. Vector-Borne Zoonotic Dis. 2008;8:555–9.
Aksulu A, Bilgiç HB, Karagenç T, Bakırcı S. Seroprevalence and molecular detection of Leishmania spp. in cats of West Aegean region Turkey. Vet Parasitol Reg Stud Rep. 2021;24:100573.
Sarkari B, Hatam GR, Adnani SJ, Asgari Q. Seroprevalence of feline leishmaniasis in areas of Iran where Leishtnania infantum is endemic. Ann Trop Med Parasitol. 2009;103:275–7.
Pennisi M, Lupo T, Malara D, Masucci M, Migliazzo A, Lombardo F, et al. Serological and molecular prevalence of Leishmania infantum infection in cats from southern Italy. J Feline Med Surg. 2012;14:80.
Pennisi M, Maxia L, Vitale F, Masucci M, Borruto G, Caracappa S. Studio dell’infezione da Leishmania mediante PCR in gatti che vivono in zona endemica. Convegno Naz SISVET. 2000;54:215–6.
Ayllón T, Diniz PPVP, Breitschwerdt EB, Villaescusa A, Rodríguez-Franco F, Sainz A. Vector-borne diseases in client-owned and stray cats from Madrid Spain. Vector Borne Zoonotic Dis. 2012;12:143–50.
Sobrinho LSV, Rossi CN, Vides JP, Braga ET, Gomes AAD, de Lima VMF, et al. Coinfection of Leishmania chagasi with Toxoplasma gondii, feline immunodeficiency virus (FIV) and feline leukemia virus (FeLV) in cats from an endemic area of zoonotic visceral leishmaniasis. Vet Parasitol. 2012;187:302–6.
Sherry K, Miró G, Trotta M, Miranda C, Montoya A, Espinosa C, et al. A serological and molecular study of Leishmania infantum infection in cats from the island of Ibiza (Spain). Vector Borne Zoonotic Dis. 2011;11:239–45.
Pennisi M, Masucchi M, Catarsini O. Presenza di anticorpi anti-Leishmania in gatti FIV+ che vivono in zona endemica. Convegno Naz SISVET. 1998;51:265–6.
Spada E, Proverbio D, Migliazzo A, Della Pepa A, Perego R, Bagnagatti De Giorgi G. Serological and molecular evaluation of Leishmania infantum infection in stray cats in a nonendemic area in northern Italy. ISRN Parasitol. 2013;2013:916376.
Vita S, Santori D, Aguzzi I, Petrotta E, Luciani A. Feline leishmaniasis and ehrlichiosis: serological investigation in Abruzzo region. Vet Res Commun Vet Res Commun. 2005;29:319–21.
Solano-Gallego L, Rodríguez-Cortés A, Iniesta L, Quintana J, Pastor J, Espada Y, et al. Cross-sectional serosurvey of feline leishmaniasis in ecoregions around the northwestern Mediterranean. Am J Trop Med Hyg. 2007;76:676–80.
Coelho WMD, Do Amarante AFT, De Carvalho Apolinário J, Coelho NMD, De Lima VMF, Perri SHV, et al. Seroepidemiology of Toxoplasma gondii, Neospora caninum, and Leishmania spp. infections and risk factors for cats from Brazil. Parasitol Res. 2011;109:1009–13.
Maia C, Gomes J, Cristóvão J, Nunes M, Martins A, Rebêlo E, et al. Feline Leishmania infection in a canine leishmaniasis endemic region. Portugal Vet Parasitol. 2010;174:336–40.
Otranto D, Napoli E, Latrofa MS, Annoscia G, Tarallo VD, Greco G, et al. Feline and canine leishmaniosis and other vector-borne diseases in the Aeolian Islands: Pathogen and vector circulation in a confined environment. Vet Parasitol. 2017;236:144–51.
Braga AR, Langoni H, Lucheis S. Evaluation of canine and feline leishmaniasis by the association of blood culture, immunofluorescent antibody test and polymerase chain reaction. J Venom Anim Toxins Incl Trop Dis. 2014;20:5.
Can H, Döşkaya M, Özdemir HG, Şahar EA, Karakavuk M, Pektaş B, et al. Seroprevalence of Leishmania infection and molecular detection of Leishmania tropica and Leishmania infantum in stray cats of Izmir. Turkey Exp Parasitol. 2016;167:109–14.
Attipa C, Papasouliotis K, Solano-Gallego L, Baneth G, Nachum-Biala Y, Sarvani E, et al. Prevalence study and risk factor analysis of selected bacterial, protozoal and viral, including vector-borne, pathogens in cats from Cyprus. Parasit Vectors. 2017;10:130.
Metzdorf IP, da Costa Lima MS, de Fatima Cepa Matos M, de Souza Filho AF, de Souza Tsujisaki RA, Franco KG, et al. Molecular characterization of Leishmania infantum in domestic cats in a region of Brazil endemic for human and canine visceral leishmaniasis. Acta Trop. 2017;166:121–5.
Silaghi C, Knaus M, Rapti D, Kusi I, Shukullari E, Hamel D, et al. Survey of Toxoplasma gondii and Neospora caninum, haemotropic mycoplasmas and other arthropod-borne pathogens in cats from Albania. Parasit Vectors. 2014;7:62.
Martín-Sánchez J, Acedo C, Muñoz-Pérez M, Pesson B, Marchal O, Morillas-Márquez F. Infection by Leishmania infantum in cats: epidemiological study in Spain. Vet Parasitol. 2007;145:267–73.
Costa TAC, Rossi CN, Laurenti MD, Gomes AAD, Vides JP, Vicente Sobrinho LS, et al. Occurrence of leishmaniasis in cats from endemic area for visceral leishmaniasis. Braz J Vet Res Anim Sci. 2010;47:213–7.
OIE (World Organization for Animal Health). Terrestrial manual on diseases. 2021. https://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.01.11_LEISHMANIOSIS.pdf. Accessed Feb 28, 2022
Nascimento LFJ, Cirilo TM, Gomes DS, Gomes ACA, Lima VFS, Scher R, et al. Epidemiological and diagnostic aspects of feline leishmaniasis with emphasis on Brazil: a narrative review. Parasitol Res. 2022;121:21–34.
Solano-Gallego L, Morell P, Arboix M, Alberola J, Ferrer L. Prevalence of Leishmania infantum infection in dogs living in an area of canine leishmaniasis endemicity using PCR on several tissues and serology. J Clin Microbiol J Clin Microbiol. 2001;39:560–3.
Traversa D. Vector-borne disease distributions and risks to the UK. Companion Anim. 2020;25:1–6.
Wright I, Jongejan F, Marcondes M, Peregrine A, Baneth G, Bourdeau P, et al. Parasites and vector-borne diseases disseminated by rehomed dogs. Parasit Vectors. 2020;13:546.
Hosein S, Blake DP, Solano-Gallego L. Insights on adaptive and innate immunity in canine leishmaniosis. Parasitology. 2017;144:95.
Simões-Mattos L, Mattos MRF, Teixeira MJ, Oliveira-Lima JW, Bevilaqua CML, Prata-Júnior RC, et al. The susceptibility of domestic cats (Felis catus) to experimental infection with Leishmania braziliensis. Vet Parasitol. 2005;127:199–208.
Akhtardanesh B, Kheirandish R, Sharifi I, Mohammadi A, Mostafavi A, Mahmoodi T, et al. Low susceptibility of domestic cats to experimental Leishmania infantum infection. J Vector Borne Dis. 2018;55:230–4.
Priolo V, Martínez-Orellana P, Pennisi MG, Masucci M, Prandi D, Ippolito D, et al. Leishmania infantum-specific IFN-γ production in stimulated blood from cats living in areas where canine leishmaniosis is endemic. Parasit Vectors. 2019;12:133.
Akhtardanesh B, Moeini E, Sharifi I, Saberi M, Sadeghi B, Ebrahimi M, et al. Leishmania infection in cats positive for immunodeficiency virus and feline leukemia virus in an endemic region of Iran. Vet Parasitol Reg Stud Rep. 2020;20:100387.
Solano-Gallego L, Koutinas A, Miró G, Cardoso L, Pennisi MG, Ferrer L, et al. Directions for the diagnosis, clinical staging, treatment and prevention of canine leishmaniosis. Vet Parasitol. 2009;165:1–18.
Solano-Gallego L, Miró G, Koutinas A, Cardoso L, Pennisi MG, Ferrer L, et al. LeishVet guidelines for the practical management of canine leishmaniosis. Parasit Vectors. 2011;4:86.
Paltrinieri S, Solano-Gallego L, Fondati A, Lubas G, Gradoni L, Castagnaro M, et al. Guidelines for diagnosis and clinical classification of leishmaniasis in dogs. J Am Vet Med Assoc. 2010;236:1184–91.
Iatta R, Furlanello T, Colella V, Tarallo VD, Latrofa MS, Brianti E, et al. A nationwide survey of Leishmania infantum infection in cats and associated risk factors in Italy. PLoS Negl Trop Dis. 2019;13:e0007594.
Savioli G, Archer J, Brianti E, Benelli G, Schnyder M, Iatta R, et al. Serum amyloid a levels and alpha 2 and gamma globulins on serum protein electrophoresis in cats exposed to and infected with Leishmania infantum. Parasit Vectors. 2021;14:217.
Paltrinieri S, Gradoni L, Roura X, Zatelli A, Zini E. Laboratory tests for diagnosing and monitoring canine leishmaniasis. Vet Clin Pathol. 2016;45:552–78.
Roura X, Cortadellas O, Day MJ, Benali SL, D’Anna N, Fondati A, et al. Canine leishmaniosis and kidney disease: Q&A for an overall management in clinical practice. J Small Anim Pract. 2021;62:1–19.
Meléndez-Lazo A, Ordeix L, Planellas M, Pastor J, Solano-Gallego L. Clinicopathological findings in sick dogs naturally infected with Leishmania infantum: comparison of five different clinical classification systems. Res Vet Sci. 2018;117:18–27.
Lopez M, Aybar V, Zatelli A, Vila A, Vega J, Hernando E, et al. Is proteinuria a rare condition in apparently healthy and sick cats? a feline practice experience (2007–2018). Open Vet J. 2021;11:508–16.
Brianti E, Falsone L, Napoli E, Gaglio G, Giannetto S, Pennisi MG, et al. Prevention of feline leishmaniosis with an imidacloprid 10%/flumethrin 4.5% polymer matrix collar. Parasit Vectors. 2017;10:334.
Benassi JC, Benvenga GU, Ferreira HL, Pereira VF, Keid LB, Soares R, et al. Detection of Leishmania infantum DNA in conjunctival swabs of cats by quantitative real-time PCR. Exp Parasitol. 2017;177:93–7.
Oliva G, Roura X, Crotti A, Maroli M, Castagnaro M, Gradoni L, et al. Guidelines for treatment of leishmaniasis in dogs. J Am Vet Med Assoc. 2010;236:1192–8.
Furman E, Hooijberg EH, Leidinger J, Zedinger C, Leidinger E, Giger U. Hereditary xanthinuria and urolithiasis in a domestic shorthair cat. Comp Clin Path. 2015;24:1325–9.
Christopher MM, White JG, Eaton JW. Erythrocyte pathology and mechanisms of Heinz body-mediated hemolysis in cats. Vet Pathol. 1990;27:299–310.
Acknowledgements
This paper is sponsored by Elanco Animal Health in the framework of the 16 Companion Animal Vector-Borne Diseases (CVBD) World Forum Symposium.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
MGT, MCL, CB and XR designed and developed the study. MGT and MCL collected and processed the database data. MGT and XR wrote the manuscript. ST, MCL, MRL and CB revised the manuscript. All the authors read and approved the final manuscript.
Author information
ST, MRL and XR are members of the CVBD World Forum, which is supported by Elanco Animal Health.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Garcia-Torres, M., López, M., Tasker, S. et al. Review and statistical analysis of clinical management of feline leishmaniosis caused by Leishmania infantum. Parasites Vectors 15, 253 (2022). https://doi.org/10.1186/s13071-022-05369-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-022-05369-6