Abstract
Background
Helminthiasis and resistance to commercial anthelmintic compounds are major causes of economic losses for livestock producers, resulting in an urgent need for new drugs and reliable in vitro screening tests capable of detecting potentially active products. Considering this, a series of novel benzimidazole derivatives (5-methylbenzimidazole 1,2-disubstituted, 5-carboxybenzimidazole, 5-methylbenzimidazole 2-one) was screened on exsheathed L3 (xL3) and on the adult stage of Haemonchus contortus (Kirby anthelmintic-susceptible McMaster isolate).
Methods
This work presents the set-up of an automated motility assay on the xL3 stage of H. contortus using an infrared tracking device (WMicrotracker One) together with a larval development test (xL3 to L4) and a motility assay on the adult stage of H. contortus. A comparative study of the sensitivity of these in vitro assays using commercial anthelmintics with different mechanisms of action was carried out, also evaluating anthelmintic activity of a series of novel benzimidazole derivatives.
Results
The automated xL3 assay had the great advantage of being able to analyze many compounds simultaneously, but it showed the limitation of having lower sensitivity, requiring higher concentrations of the commercial anthelmintics tested compared to those needed for the adult motility or development assays. Although none of the novel 1,2,5-tri-substituted benzimidazole derivatives could significantly decrease the motility of xL3s, one of them (1e) significantly affected the development of xL3s to L4, and five new compounds (1b, 1d, 1e, 2a and 2c) reduced the motility of H. contortus adult stage.
Conclusions
The analysis of the results strongly suggests that the in vitro xL3 to L4 development test, particularly for the L4 stage, could be closer to the pharmacological sensitivity of the adult stage of H. contortus (target of interest) for commercial anthelmintic selected, with different mechanisms of action, and for the series of benzimidazole derivatives assayed. Therefore, an automated motility assay on L4 using the infrared tracking device is being set up. Further studies will be conducted to evaluate the in vivo anthelmintic activity of the most active novel benzimidazole derivatives.
Graphical Abstract

Similar content being viewed by others
Background
Parasitic infections, particularly those produced by nematodes, are of interest in both human and veterinary medicine. In small ruminants, Teladorsagia circumcincta, Haemonchus contortus and Trichostrongylus colubriformis have evolved to become the main species of production-limiting parasites that affect sheep, generating significant reductions in live weight gain, wool production and fecundity [1], causing substantial economic losses [2]. For example, the annual cost associated with parasitic diseases in the Australian sheep industry for meat consumption has been estimated at 436 million USD [3], being the second largest sheep-producing country in the world. Chemical control of these parasites has been preferred because of its costs, efficacy and simplicity, but the extensive and inappropriate use of commercialized drugs has led to worldwide resistance [4,5,6]. The discovery and registration of new anthelmintics have been scarce in recent decades [7], with the following being the most recently introduced to the market: derquantel [8], emodepside [9] and monepantel [10]. However, reports of parasite resistance to these new drugs appeared a few years after they were marketed [11,12,13].
The search process for new anthelmintics has been changing in recent decades. Most of the currently commercialized anthelmintic products were discovered from the screening of compounds of natural (e.g. ivermectin [14]) or synthetic (e.g. levamisol [15]) origin, performed in infected animals. Nevertheless, the low throughput and high costs of these studies, together with the need to test a greater number of compounds, led to the development of in vitro methods involving helminths, the so-called physiology-based assays. In these tests, the parasite is cultured in vitro in the presence of the compound to be evaluated, and phenotypic parameters (viability, motility, worm growth) are studied [16,17,18,19]. These physiology-based assays still present limitations as they are not efficient enough for screening large compound libraries, in most cases because of the available subjective and manual methods to evaluate parasite response [20]. Examples of assays that include automated readings of larval motility after short exposures to different compounds are some of the breakthroughs that provide a speedy read of general anthelmintic activity. These new formats are less time-consuming and labor-intensive, although such tests cannot discriminate between death or immobile phenotype [21,22,23].
Haemonchosis, the infection caused by H. contortus, is one of the most important parasitic diseases of livestock species, affecting hundreds of millions of small ruminants and generating substantial economic losses [2]. Haemonchus contortus infections in young sheep and goats can persist for weeks, and each adult female worm can produce thousands of eggs per day, which can be harvested from host feces and cultured in the laboratory to obtain their infective L3 larval stage [20]. The use of the adult stage of H. contortus in anthelmintic activity tests requires the necropsy of the host to extract the parasites from the abomasum and the parasites immediate use for culture (they cannot be preserved). In this regard, this methodology is not feasible for performing high-throughput screenings and is reserved for secondary screening of those compounds that were active in models involving larval stages. Most recently, the use of exsheathed L3 (xL3s), the first parasitic stage of nematodes, was described for in vitro bioassays of anthelmintic activity [23]. This approach presents several advantages: the good conservation of the L3 stage parasitic material (3 months at 10 °C) without affecting phenotypes; the relatively simple exsheathment procedure of L3s; the fact that xL3s are more susceptible than L3s to the commercialized anthelmintics tested (more than 100 times) [23]; and the possibility to evaluate the success of the xL3 development to the L4 stage in vitro. Therefore, the use of xL3 and L4 is presented as an attractive option for primary in vitro screening of new anthelmintics.
In this work we present the set-up and verification of an automated motility assay for new anthelmintics discovery on xL3 stage of H. contortus using an infrared tracking device together with a larval development test (xL3 to L4) and a motility assay in the adult stage of H. contortus.
Four commercial anthelmintics, with different mechanisms of action, were evaluated in the different in vitro tests described in an effort to determine which assay has the closest sensitivity to the adult stage test (target of interest).
Finally, the anthelmintic activity of a series of previously synthetized [24], novel benzimidazole derivatives (5-methylbenzimidazole 1,2-disubstituted, 5-carboxybenzimidazole, 5-methylbenzimidazole 2-one) was evaluated.
Methods
Chemicals
Ivermectin (IVM), levamisole (LEV), albendazole (ABZ) and albendazole sulfoxide (ABZ SX) of USP grade were kindly supplied by Laboratorio Uruguay S.A (LUSA). Monepantel (Zolvix™) was purchased from local suppliers. A new series of 1,2,5-tri-substituted benzimidazole derivatives (chemical structures are shown in Fig. 1) was kindly supplied by Dr. Williams Porcal; the purity of the benzimidazole derivatives was ≥ 95% (assessed by 1H-NMR) [24].
Novel benzimidazole derivatives. For chemical details, see [24]
Haemonchus contortus production and maintenance: adult stage
Infective third-stage larvae (L3) from the anthelmintic-susceptible McMaster isolate (Kirby) were kindly provided by Dr. A. Kotze and Dr. M. Knox (CSIRO McMaster Laboratory, Armidale, NSW, Australia). The infection was maintained at the Campo Experimental del Instituto de Higiene de Facultad de Medicina, UdelaR, as we have previously described [25].
The adult worms used in this study were recovered from sheep abomasa by manual picking 25–35 days after infection with L3. The parasitic material was washed first with PBS and then with sterile supplemented RPMI culture medium (RPMI-1640 10.4 g/l—catalog number R6504, Sigma Aldrich, HEPES buffer 2.38 g/l, glucose 8 g/l, 20% of newborn bovine serum, penicillin 100 IU/ml, amphotericin B 2.5 µg/ml and streptomycin 100 µg/ml) both at 37 °C. After washing, the adult worms were incubated at 5% CO2 and 37 °C for 30 min before use in supplemented RPMI culture medium.
Haemonchus contortus production and maintenance: exsheathed third-stage larvae (xL3s)
Third-stage larvae (L3s) were obtained from H. contortus eggs by incubating humidified feces from infected sheep at 27 °C for 1 week according to [26, 27]. Once collected, L3s were maintained in distilled water at 5–9 °C for up to 60 days.
L3s were cultured and exsheathed according to Preston et al. [23], with minor modifications. Briefly, L3s were exsheathed by incubation in a 0.17% w/v active Cl2 solution for 15 min at 40 °C and 10% CO2 and then washed four times in sterile 0.9% NaCl (w/v), centrifuging for 5 min at 500×g each time. The exsheathed L3s (xL3s) were then washed once with Luria Bertani (LB) medium supplemented with 100 IU/ml penicillin, 100 µg/ml streptomycin and 2.5 µg/ml amphotericin. Finally, the xL3s were suspended in this supplemented LB.
In vitro anthelmintic assays: xL3 automated motility assay
The collected xL3s suspended in LB were adjusted to 6000 xL3/ml. The xL3 suspension was placed in a reservoir previously sterilized with UV radiation and homogenized by an air stream using a 0.22 µm filter membrane, and 300 xL3s (50 µl of the suspension) were transferred per well to sterile 96-well flat bottom microplates (CellStar, Greiner), with the exception of the plate edge wells, which were filled with sterile distilled water, using a multichannel pipette. Then, 50 µl of stock solutions of the compounds (prepared in LB and 1% v/v DMSO at a 2× concentration) was added to each well to obtain a final 1× concentration and 0.5% v/v DMSO (six replicates each) (see Table 1 for the drug concentration ranges assayed).
The new benzimidazole derivatives were tested at 25 µM; the screening concentration was defined based on previous reports of xL3 motility assay [23, 28] as well as by the limitation in the solubility observed for this series of compounds in the incubation conditions. Culture medium control (50 µl LB medium), negative control (50 µl of 1% v/v DMSO LB, giving a final concentration of 0.5% v/v DMSO in the well) and positive control (50 µl of albendazole 40 µM in 1% v/v DMSO LB, giving a final concentration of albendazole 20 µM and 0.5% v/v DMSO in the well) were also placed in each plate. The plates were incubated at 40 °C in a humidified incubator with a 10% CO2 atmosphere for 72 h. The motility assessment of the xL3 stage was performed using an automated tracking apparatus: WMicrotracker One (Phylumtech, Argentina). Briefly, worm motility measurements of each well were carried out at 24, 48 and 72 h for 30 min at 20–22 °C. Before analyzing, the plates were agitated in an orbital shaker at 180 rpm for 6 min to stimulate the worms, corroborating the normal sinusoidal movement of the xL3s in the culture medium control wells under the microscope. The WMicrotracker One uses an infrared tracking device which detects animal movement through infrared microbeam light scattering where each well is crossed by two infrared microbeams that scan more than ten times per second and are interrupted when worms pass by. The detected signal is digitally processed to calculate the amount of animal movement in a fixed period of time (http://www.phylumtech.com/home/es/soporte) [22, 29]. The raw motility data were normalized against the motility measurements obtained for the DMSO control group to remove plate-to-plate variation by calculating the percentage of motility of each treatment as follows.
For assay verification, the data from the concentration-response curves of the different commercial anthelmintics (used as reference drugs) were transformed to log10 to determine the concentration that leads to 50% maximal response (IC50) and 10% maximal response (IC90) from three independent trials. The data were adjusted using a variable slope and a four-parameter logarithmic equation (Eq. 2), where A1 is the bottom asymptote, A2 is the top asymptote, log x0 is the center of the curve and represents the log IC50, and p is the Hill variable slope of the logarithmic dose-response curve.
The IC50 (Eq. 3) and IC90 (Eq. 4) were determined using GraphPad Prism version 9.0.2, 2021, for Windows (GraphPad software, San Diego, CA, USA).
To study whether the vehicle used, DMSO, affects the motility of the larvae at 24, 48 and 72 h of incubation, we compared the motility of the medium control with the DMSO control for each experiment. We applied an unpaired t-test with Welch correction and a Holm-Šídák multiple comparisons test, using GraphPad Prism version 9.0.2, 2021, for Windows (GraphPad software, San Diego, CA, USA). A probability of P < 0.05 was considered statistically significant.
In vitro anthelmintic assays: xL3 to L4 development assay
For the xL3 to L4 development assay, we proceeded according to bibliography [23] with minor modifications. In brief, the plates tested for xL3 motility were re-incubated for 4 more days at 40 °C and 10% v/v CO2, completing a total of 7 days of incubation. Then, worms were fixed with 50 µl of a 1% iodine solution, and 20 µl of each well was examined at 10× magnification (Nikon TS100, inverted microscope, Tokyo, Japan) to assess their development, based on the presence or absence of a well-developed pharynx characteristic of H. contortus L4s (see Additional file 1: Fig. S1). The number of L4s was expressed as a percentage of the total worm number present in the 20 µl larval suspension examined under the microscope. Each concentration of anthelmintics or the new benzimidazole derivatives was tested in triplicate, at least in three different trials. Also, to study whether the vehicle used, DMSO, affects the development of the xL3 to L4 larvae at 7 days of incubation, we compared the development of the medium control with the DMSO control for each experiment. We applied an unpaired t-test with Welch correction and a Holm-Šídák multiple comparisons test, using GraphPad Prism version 9.0.2, 2021, for Windows (GraphPad software, San Diego, CA, USA). A probability of P < 0.05 was considered statistically significant.
In vitro anthelmintic assays: adult stage motility assay
This assay was carried out according to O’Grady and Kotze [19], with minor modifications. Briefly, ten adult worms were placed into glass tubes containing 2 ml of sterile supplemented RPMI culture medium. Stock solutions of the compounds in 100% DMSO were prepared at a 200× concentration to obtain final drug concentrations of 1× and 0.5% DMSO in each tube when 10 µl of stock solutions was incorporated (six replicates at each drug concentration tested, see Table 1). Culture medium and 0.5% DMSO in culture medium controls were also included in each assay. The new benzimidazole derivatives were tested at a final concentration of 25 µM (six replicates). The worms in the tubes were incubated at 37 °C and 5% CO2 for 72 h, and the motility score was determined at 0, 24, 48 and 72 h according to the following scoring system: 0 = no movement, 1 = very limited movement and not in a normal sinusoidal form; 2 = at least one individual is able to move in a normal sinusoidal form; 3 ≥ 50% of the individuals present smooth sinusoidal motion. A compound with a motility score ≤ 1.5 at 72 h was considered active. Each drug assay was carried on three separate occasions.
Statistical analysis
An ANOVA test was conducted in each assay (once the normal distribution of data was confirmed) followed by Dunnett’s multiple comparison test against the DMSO control group, with 95% confidence using GraphPad Prism version 9.0.2, 2021, for Windows (GraphPad software, San Diego, CA, USA). A probability of P < 0.05 was considered statistically significant. The Dunnett’s multiple comparison test is restricted and recommended for its use comparing several experimental groups against a single control group [30], as the DMSO control.
Results
Set-up and verification of anthelmintic in vitro assays using H. contortus (xL3s, xL3 to L4 development and adults)
The xL3 automated motility assay and xL3 to L4 development assay were verified against four commercial anthelmintics with different mechanisms of action: albendazole sulfoxide, monepantel, ivermectin and levamisole [31], at the range of concentrations indicated in Table 1. The IC50 and IC90 values were determined for these compounds at 24, 48 and 72 h of incubation for the xL3 motility assay and at day 7 of incubation for the xL3 to L4 development assay (Table 2 for IC50 values and Additional file 1: Table S1 for IC90 values). The log10 concentration-response curves are provided as supplementary data (Additional file 1: Figs. S2–S8). Also, the influence of DMSO on the motility and development of the xL3 larvae was studied, showing no significant differences (unpaired t-test with Welch correction and a multiple comparison Holm-Šídák post test, P < 0.05), with the motility or development of xL3s of the medium control group, in each experiment carried out (Additional file 1: Tables S2–S5).
The adult stage motility assay was optimized and verified against albendazole sulfoxide, monepantel, ivermectin and levamisole. The motility of worms in culture medium (culture medium control) or vehicle control (negative control) showed a mean motility score of 2.8 ± 0.3 and 2.6 ± 0.4, respectively, at 72 h, thus showing a normal sinusoidal movement during the cultivation time. The mean motility scores achieved at 72 h of incubation with anthelmintic drugs, for four different concentrations, are presented in Table 3. The effects of these anthelmintics on the motility of H. contortus adult stage at 24, 48 and 72 h are presented in the Additional file 1: Figs. S9–S12.
To analyze the differences in the range of concentrations at which anthelmintics are active in the three assays against H. contortus, the results obtained for four different concentrations of anthelmintic drugs in the xL3 motility assay, the xL3 to L4 development assay and the adult stage motility test are presented in Table 3. Additionally, Table S6 of the supplementary data section (Additional file 1) shows these results informed with their corresponding coefficient of variation.
Screening of new benzimidazole derivatives against different stages of H. contortus
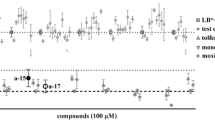
A series of new benzimidazole derivatives were tested at 25 µM against xL3s (xL3 automated motility assay and xL3 to L4 development assay) and the adult stage (adult motility assay). The results of these three assays are shown in Table 4 (Additional file 1: Table S7 shows these results informed with their corresponding coefficient of variation). Albendazole was tested at 20 µM as a positive control.
Discussion
Parasitic helminth infections are a serious health problem which, added to the resistance phenomena to most commercial anthelmintics, results in great economic losses for livestock production worldwide [32, 33]. In this context, it is necessary to invest in the search for new anthelmintics and to have validated screening bioassays for their evaluation.
Several physiology-based screening strategies using larval stages of parasitic nematodes have been developed, where the phenotypes of motility (larval motility assay, LMA) [34, 35] and development (larval development assay, LDA) [36, 37] were the most studied. The LDA is an assay that facilitates the detection of molecules that could present fast or slow mechanisms of action, since activity is read out after 7 days of incubation. For instance, the LDA allows to detect active molecules such as benzimidazole anthelmintics (with a slow mechanism of action, involving the inhibition of microtubule polymerization) but also macrocyclic lactones (that present a faster mechanism by affecting the neuromuscular system of the parasite). On the other hand, the LMA is often more sensitive to compounds with fast mechanisms of action, since the activity readings are performed at 24 h of incubation. In both tests, LDA and LMA, the phenotypes are measured by visual inspection under the microscope, and although they have been very useful for the discovery of new hit compounds for anthelmintic development, they are time-consuming and subjective and involve the use of free-living parasite stages instead of the parasitic stages of greatest interest as the pharmacological target. To deal with these problems, automatic motility reading methods have been developed, such as the micromotility meter [21, 38], designed to measure the motility of nematodes in culture tubes. More recently, automated reading methods were designed for multiwell plates, measuring motility of nematodes through electrical interference [39] or video acquisition and analysis [23, 40], allowing screening of a greater number of compounds. The measurement of the motility phenotype is a rapid detection method in order to study the affectation of the normal physiology of the parasite, which generally ends up affecting their viability [41]. On the other hand, bioassays where the viability or death of the parasite is measured as a phenotype require the use of chemical reactions that indicate an affectation of mitochondrial metabolism, making it difficult to process big libraries of chemical compounds [42, 43].
In this work, an automated motility assay using the first parasitic stage of H. contortus, the xL3s, was successfully set up with the WMicrotracker One equipment. In fact, the xL3 automated motility assay was verified using four commercial anthelmintics with different mechanisms of action (monepantel, albendazole sulfoxide, levamisole and ivermectin).
The IC50 value obtained for monepantel was of the same order of concentration as that previously reported for motility assays in xL3s at 72 h of incubation [28, 44, 45]. Interestingly, the IC50 values obtained for levamisole and ivermectin at 72 h of incubation were significantly higher than the IC50 values reached after 24 h of incubation (unpaired t test with Welch correction, for levamisol t = 4.962, df = 3.876, P = 0.0083 and for ivermectin t = 10.630, df = 3.114, P = 0.0015), showing a decrease in activity with incubation time without total reversion (Table 2). In this sense, some studies have shown that the nematode ATP binding cassette (ABC) transporter proteins such as P-glycoproteins (P-gp) or multidrug resistance proteins (MDR) are involved in avermectin and levamisole detoxification and in resistance development [31, 46]. In addition, studies of the transcription patterns of ABC transporters in susceptible (Kirby) and resistant (Wallangra) isolates of H. contortus L3 stage, after 3 h of in vitro exposure to ivermectin or levamisole, only showed an increase of the transcription of multiple ABC transporter genes in the resistant isolate [47]. It would be possible that the transcriptional response observed at 3 h in resistant L3 is delayed in susceptible L3 [47]; therefore, longer incubation times with these drugs could lead to a decrease in sensitivity, such as that we observed at 72 h of incubation in the xL3 motility assay. Contrarily, moxidectin, a derivative of milbemycin with low affinity for P-gp transporters [48, 49], did not show a decrease in its activity with incubation time in an automated xL3 motility test [28].
In addition to the automated xL3 motility assay, an xL3 to L4 development assay was set up and verified as well as a motility test against the adult stage of H. contortus. In the adult stage motility assay, motility scores obtained in this work for albendazole, ivermectin and levamisole were the same as those reported by O’Grady et al. [19], at the same concentrations and incubation times tested.
The use of the adult stage for drug screening is of great interest since it ensures that the drug is effective against the parasite life stage to be targeted in vivo. The IC50 values obtained in the xL3 motility assay were 1.4, 4.5 and 10 times greater than the IC50 values obtained in the xL3 to L4 development assay for monepantel, albendazole sulfoxide and ivermectin, respectively (Table 2). These results were consistent with the sensitivity observed for the LDA versus the LMA. Although the LDAs had relatively prolonged times to read out, they proved to be much more sensitive to standard anthelmintics than a 24-h test that directly measured the effects of anthelmintics on L3 motility [7].
The results obtained with the adult motility assay showed that lower concentrations of anthelmintics were necessary to affect adult motility compared to those needed to affect xL3 motility (Table 3). This observation was particularly notable for ivermectin, which affected adult motility over the entire range of concentrations tested (Table 3).
These differences in the pharmacological sensitivity to drugs observed for the different stages of H. contortus could be explained in terms of distinct parasitic drug bioavailabilities (parasitic diffusion and biotransformation of xenobiotics) [50] or dissimilar drug target expressions [51]. In particular, it has been reported that the expression of most cytochrome P-450 enzymes (CYP450, phase I biotransformation) in H. contortus was higher in the larval stages L1 and L3 than in the adult stage [52]; therefore, the differential expression of these enzymes could lead to differences in drug sensitivity between larval and adult stages [53].
Finally, assays using different stages of H. contortus were utilized to evaluate the anthelmintic activity of a new series of benzimidazole derivatives synthesized [24]. The benzimidazole ring is a very important and versatile pharmacophore in drug discovery, and its derivatives are an important class of bioactive molecules in the pharmaceutical field [54, 55]. The differences observed for the measured activity of these compounds in the three assays follow the same behavior observed for the commercial drugs: the sensitivity of the adult stage motility assay is higher than those of the development to L4 and xL3 motility assays, the xL3 motility assay being the least sensitive. This observation could also be related to the different drug bioavailability levels reaching each parasite stage. Although none of these new compounds could significantly decrease the motility of xL3s, one of them (1e) significantly affected the development of xL3 to L4 (ANOVA, F(11,24) = 7.399 and P < 0.0001, followed by Dunnett’s multiple comparison test, P = 0.0025), and five new compounds (1b, 1d, 1e, 2a and 2c) affected the motility of H. contortus adult stage. The most remarkable results regarding these anthelmintic activity screening were obtained with compounds 1d and 1e, which affected adult motility even more than the positive control albendazole.
The effects of structural modifications to benzimidazole-like molecules on microtubule inhibitory activity have been reviewed by Lacey [56]. This author has postulated that the presence of a carbamate group in the 2 position is essential for potent microtubule inhibitory activity and alkylation of the -N- at the 1 position of the benzimidazole ring reduces activity 10- to 20-fold, regardless of the size of the substituent in the 5 position. Another important observation is that the pharmacological effect depends heavily on the nature of the molecule adjacent to the benzimidazole ring system [57, 58]. The new compounds evaluated in the present work are 1,2,5-tri-substituted benzimidazole derivatives, which would not meet the structure–activity relationships conditions necessary to bind to tubulins at the anthelmintic benzimidazole binding site. The antifungal benomyl, which is a 1,2-di-substituted benzimidazole, binds with low affinity to nematode β-tubulin [59] and mammalian tubulin [60] to a different site from the well-characterized colchicine and vinblastine binding site [61]. These new benzimidazole derivatives, like benomyl, could bind tubulins at a different site from 2-methyl-benzimidazole carbamate, or present a different drug target, so further studies should be conducted to identify the pharmacological target of these potential anthelmintic compounds.
Conclusions
We successfully set-up an automated motility assay for xL3s of H. contortus using the WMicrotracker One equipment, complemented with an xL3 to L4 development assay and a motility test in adult worms. The automated assay presented the advantage of being able to analyze a large number of compounds, but has the limitation of having lower sensitivity to the commercial anthelmintics tested since lower concentrations of anthelmintics were necessary to affect adult motility or xL3 to L4 development compared to those needed to affect xL3 motility. The analysis of the results led to interest in the set-up and verification of an automated motility assay with the WMicrotracker using the L4 stage of H. contortus. In addition, we could explore the influence of inhibitors of the xenobiotic metabolism enzymes and efflux transporter proteins on the sensitivity of the xL3 motility assay. A deeper knowledge of parasite pharmacokinetics can lead to a better understanding of the different drug sensitivities observed among the parasitic stages. This information could guide the design of more reliable in vitro models to test anthelmintic activity using a larval stage that closely resembles adult worms, as could be the case in the L4 stage. Finally, one new 1,2,5-tri-substituted benzimidazole derivative (1e) significantly (ANOVA followed by Dunnett’s multiple comparison test, F(11,24) = 7.399, P = 0.0025) affected the development of xL3 to L4, and five derivatives (1b, 1d, 1e, 2a and 2c) presented a motility score ≤ 1.5 in the motility test against the adult stage, classifying them as active. Particularly, compounds 1d and 1e affected adult motility even more than the positive control albendazole. These results encourage us to go deeper into the biological characterization of these active compounds and evaluate their in vivo activity in sheep infected with H. contortus.
Availability of data and materials
The datasets during and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Sargison ND. Pharmaceutical treatments of gastrointestinal nematode infections of sheep-Future of anthelmintic drugs. Vet Parasitol. 2012;189:79–84.
Waller PJ. From discovery to development: current industry perspectives for the development of novel methods of helminth control in livestock. Vet Parasitol. 2006;139:1–14.
Lane J, Jubb T, Shephard RW, Webb-Ware J, Fordyce G. Priority list of endemic diseases for the red meat industries. 2015.
Kaplan RM. Drug resistance in nematodes of veterinary importance: a status report. Trends Parasitol. 2004;20:477–81.
Jabbar A, Iqbal Z, Kerboeuf D, Muhammad G, Khan MN, Afaq M. Anthelmintic resistance: the state of play revisited. Life Sci. 2006;79:2413–31.
Scott I, Pomroy W, Kenyon P, Smith G, Adlington B, Moss A. Lack of efficacy of monepantel against Teladorsagia circumcincta and Trichostrongylus colubriformis. Vet Parasitol. 2013;198:166–71.
Geary T. Haemonchus contortus: applications in drug discovery. Adv Parasitol. 2016;93:429–63.
Lee BH, Clothier MF, Johnson SS. Semi-synthesis of 2-deoxo-and 3-epi-paraherquamide A. Bioorganic Med Chem Lett. 2001;11:553–4.
Harder A, Samson-Himmelstjerna G. Activity of the cyclic depsipeptide emodepside (BAY 44–4400) against larval and adult stages of nematodes in rodents and the influence on worm survival. Parasitol Res. 2001;87:924–8.
Kaminsky R, Gauvry N, Weber SS, Skripsky T, Bouvier J, Wenger A, et al. Identification of the amino-acetonitrile derivative monepantel (AAD 1566) as a new anthelmintic drug development candidate. Parasitol Res. 2008;103:931–9.
Mederos AE, Ramos Z, Banchero GE. First report of monepantel Haemonchus contortus resistance on sheep farms in Uruguay. Parasites Vectors. 2014;7:598.
Van den Brom R, Moll L, Kappert C, Vellema P. Haemonchus contortus resistance to monepantel in sheep. Vet Parasitol. 2015;209:278–80.
Niciura SCM, Cruvinel GG, Moraes CV, Chagas ACS, Esteves SN, Benavides MV, et al. In vivo selection for Haemonchus contortus resistance to monepantel. J Helminthol. 2019;94:e46.
Campbell WC. History of avermectin and ivermectin, with notes on the history of other macrocyclic lactone antiparasitic agents. Curr Pharm Biotechnol. 2012;13:853–65.
Thienpont D, Vanparijs OFJ, Raeymaekers AHM, Vandenberk J, Demoen PJA, Allewijn FTN, et al. Tetramisole (R 8299), a new, potent broad spectrum anthelmintic. Nature. 1966;209:1084–6.
Kotze A, Coleman G, Mai A, McCarthy J. Field evaluation of anthelmintic drug sensitivity using in vitro egg hatch and larval motility assays with Necator americanus recovered from human clinical isolates. Int J Parasitol. 2005;35:445–53.
Kotze A, Le Jambre L, O’Grady J. A modified larval migration assay for detection of resistance to macrocyclic lactones in Haemonchus contortus, and drug screening with Trichostrongylidae parasites. Vet Parasitol. 2006;137:294–305.
Rohwer A, Lutz J, Chassaing C, Uphoff M, Heckeroth AR, Selzer PM. Identification and profiling of nematicidal compounds in veterinary parasitology. In: Parasitic helminths. Wiley-VCH Verlag GmbH & Co KGaA: Germany; 2012. p. 135–57.
O’Grady J, Kotze AC. Haemonchus contortus: in vitro drug screening assays with the adult life stage. Exp Parasitol. 2004;106:164–72.
Geary TG, Chibale K, Abegaz B, Andrae-Marobela K, Ubalijoro E. A new approach for anthelmintic discovery for humans. Trends Parasitol. 2012;28:176–81.
Bennett J, Pax R. Micromotility meter: an instrument designed to evaluate the action of drugs on motility of larval and adult nematodes. Parasitology. 1986;93:341–6.
Liu M, Landuyt B, Klaassen H, Geldhof P, Luyten W. Screening of a drug repurposing library with a nematode motility assay identifies promising anthelmintic hits against Cooperia oncophora and other ruminant parasites. Vet Parasitol. 2019;265:15–8.
Preston S, Jabbar A, Nowell C, Joachim A, Ruttkowski B, Baell J, et al. Low cost whole-organism screening of compounds for anthelmintic activity. Int J Parasitol. 2015;45:333–43.
Ríos N, Varela J, Birriel E, González M, Cerecetto H, Merlino A, et al. Identification of novel benzimidazole derivatives as anti-Trypanosoma cruzi agents: solid-phase synthesis, structure–activity relationships and molecular docking studies. Future Med Chem. 2013;5:1719–32.
Munguía B, Michelena M, Melian E, Saldaña J, Ures X, Manta E, et al. Development of novel valerolactam-benzimidazole hybrids anthelmintic derivatives: diffusion and biotransformation studies in helminth parasites. Exp Parasitol. 2015;153:75–80.
Mes TH, Eysker M, Ploeger HW. A simple, robust and semi-automated parasite egg isolation protocol. Nat Protoc. 2007;2:486–9.
Nikolaou S, Hartman D, Presidente PJ, Newton SE, Gasser RB. HcSTK, a Caenorhabditis elegans PAR-1 homologue from the parasitic nematode, Haemonchus contortus. Int J Parasitol. 2002;32:749–58.
Dilrukshi Herath HMP, Preston S, Hofmann A, Davis RA, Koehler AV, Chang BCH, et al. Screening of a small, well-curated natural product-based library identifies two rotenoids with potent nematocidal activity against Haemonchus contortus. Vet Parasitol. 2017;244:172–5.
Risi G, Aguilera E, Lados E, Suarez G, Carrera I, Alvarez G, et al. Caenorhabditis elegans infrared-based motility assay identified new hits for nematicide drug development. Vet Sci. 2019;6:29.
Salkind NJ. Encyclopedia of research design. 2010;1:394–7.
Kotze AC, Hunt PW, Skuce P, von Samson-Himmelstjerna G, Martin RJ, Sager H, et al. Recent advances in candidate-gene and whole-genome approaches to the discovery of anthelmintic resistance markers and the description of drug/receptor interactions. Int J Parasitol: Drugs Drug Resist. 2014;4:164–84.
Miller CM, Waghorn TS, Leathwick DM, Candy PM, Oliver AMB, Watson TG. The production cost of anthelmintic resistance in lambs. Vet Parasitol. 2012;186:376–81.
Rose Vineer H, Morgan ER, Hertzberg H, Bartley DJ, Bosco A, Charlier J, et al. Increasing importance of anthelmintic resistance in European livestock: creation and meta-analysis of an open database. Parasite. 2020;27:69.
Boisvenue RJ, Brandt MC, Galloway RB, Hendrix JC. In vitro activity of various anthelmintic compounds against Haemonchus contortus larvae. Vet Parasitol. 1983;13:341–7.
Lorimer SD, Perry NB, Foster LM, Burgess EJ, Douch PGC, Hamilton MC, et al. A nematode larval motility inhibition assay for screening plant extracts and natural products. J Agric Food Chem. 1996;44:2842–5.
Ibarra OF, Jenkings DC. The relevance of in vitro anthelmintic screening tests employing the free-living stages of trichostrongylid nematodes. J Helminthol. 1984;58:107–12.
Taylor MA. A larval development test for the detection of anthelmintic resistance in nematodes of sheep. Res Vet Sci. 1990;49:198–202.
Folz SD, Pax RA, Thomas EM, Bennett JL, Lee BL, Conder GA. Detecting in vitro anthelmintic effects with a micromotility meter. Vet Parasitol. 1987;24:241–50.
Smout MJ, Kotze AC, McCarthy JS, Loukas A. A novel high throughput assay for anthelmintic drug screening and resistance diagnosis by real-time monitoring of parasite motility. PLoS Negl Trop Dis. 2010;4:e885.
Storey B, Marcellino C, Miller M, Maclean M, Mostafa E, Howell S, et al. Utilization of computer processed high definition video imaging for measuring motility of microscopic nematode stages on a quantitative scale: “The Worminator.” Int J Parasitol Drugs Drug Resist. 2014;4:233–43.
Abatan MO. Efficacy of some commercial formulations of anthelmintics on Haemonchus contortus and Trichostrongylus columbianum. J Chemother. 1990;2:377–9.
Nguyen LT, Zajíčková M, Mašátová E, Matoušková P, Skálová L. The ATP bioluminescence assay: a new application and optimization for viability testing in the parasitic nematode Haemonchus contortus. Vet Res. 2021;52:124.
Hördegen P, Cabaret J, Hertzberg H, Langhans W, Maurer V. In vitro screening of six anthelmintic plant products against larval Haemonchus contortus with a modified methyl-thiazolyl-tetrazolium reduction assay. J Ethnopharmacol. 2006;108:85–9.
Preston S, Jiao Y, Jabbar A, McGee SL, Laleu B, Willis P, et al. Screening of the ‘Pathogen Box’ identifies an approved pesticide with major anthelmintic activity against the barber’s pole worm. Int J Parasitol Drugs Drug Resist. 2016;6:329–34.
Jiao Y, Preston S, Song H, Jabbar A, Liu Y, Baell J, et al. Assessing the anthelmintic activity of pyrazole-5-carboxamide derivatives against Haemonchus contortus. Parasites Vectors. 2017;10:272.
Sarai RS, Kopp SR, Coleman GT, Kotze AC. Drug-efflux and target-site gene expression patterns in Haemonchus contortus larvae able to survive increasing concentrations of levamisole in vitro. Int J Parasitol Drugs Drug Resist. 2014;4:77–84.
Raza A, Kopp SR, Bagnall NH, Jabbar A, Kotze AC. Effects of in vitro exposure to ivermectin and levamisole on the expression patterns of ABC transporters in Haemonchus contortus larvae. Int J Parasitol Drugs Drug Resist. 2016;6:103–15.
Lespine A, Martin S, Dupuy J, Roulet A, Pineau T, Orlowski S, et al. Interaction of macrocyclic lactones with P-glycoprotein: structure–affinity relationship. Eur J Pharm Sci. 2007;30:84–94.
Godoy P, Che H, Beech RN, Prichard RK. Characterisation of P-glycoprotein-9.1 in Haemonchus contortus. Parasites Vectors. 2016;9:52.
Matoušková P, Vokřál I, Lamka J, Skálová L. The role of xenobiotic-metabolizing enzymes in anthelmintic deactivation and resistance in Helminths. Trends Parasitol. 2016;32:481–91.
Ma G, Wang T, Korhonen PK, Ang CS, Williamson NA, Young ND, et al. Molecular alterations during larval development of Haemonchus contortus in vitro are under tight post-transcriptional control. Int J Parasitol. 2018;48:763–72.
Laing R, Bartley DJ, Morrison AA, Rezansoff A, Martinelli A, Laing ST, et al. The cytochrome P450 family in the parasitic nematode Haemonchus contortus. Int J Parasitol. 2015;45:243–51.
Kotze AC, Dobson RJ, Chandler D. Synergism of rotenone by piperonyl butoxide in Haemonchus contortus and Trichostrongylus colubriformis in vitro: potential for drug-synergism through inhibition of nematode oxidative detoxification pathways. Vet Parasitol. 2006;136:275–82.
Bansal Y, Silakari O. The therapeutic journey of benzimidazoles: a review. Biorg Med Chem. 2012;20:6208–36.
Yadav G, Ganguly S. Structure activity relationship (SAR) study of benzimidazole scaffold for different biological activities: a mini-review. Eur J Med Chem. 2015;97:419–43.
Lacey E. The role of the cytoskeletal protein, tubulin, in the mode of action and mechanism of drug resistance to benzimidazoles. Int J Parasitol. 1988;18:885–936.
Lanusse CE, Prichard RK. Clinical pharmacokinetics and metabolism of benzimidazole anthelmintics in ruminants. Drug Metab Rev. 1993;25:235–79.
Lubega GW, Prichard RK. Specific interaction of benzimidazole anthelmintics with tubulin from developing stages of thiabendazole-susceptible and -resistant Haemonchus contortus. Biochem Pharmacol. 1991;41:93–101.
Driscoll M, Dean E, Reilly E, Bergholz E, Chalfie M. Genetic and molecular analysis of a Caenorhabditis elegans beta-tubulin that conveys benzimidazole sensitivity. J Cell Biol. 1989;109:2993–3003.
Friedman PA, Platzer EG. Interaction of anthelmintic benzimidazoles and benzimidazole derivatives with bovine brain tubulin. Biochim Biophys Acta Gen Subj. 1978;544:605–14.
Gupta K, Bishop J, Peck A, Brown J, Wilson L, Panda D. Antimitotic antifungal compound benomyl inhibits brain microtubule polymerization and dynamics and cancer cell proliferation at mitosis, by binding to a novel site in tubulin. Biochemistry. 2004;43:6645–55.
Acknowledgements
The authors are grateful to Dr. Malcolm Knox and Dr. Andrew Kotze from CSIRO, Australia, who provided anthelmintic-susceptible larvae isolate of H. contortus, as well as the technician Marcelo López and Dr. Pablo Alonzo, from Campo Experimental del Instituto de Higiene, Facultad de Medicina (Udelar, Uruguay), for their technical assistance with the artificial infection of sheep. This work was supported by grants from the Programa de Desarrollo de Ciencias Básicas (PEDECIBA, Uruguay), ANII (Projects FMV_1_2017_1_136597 and ANII_PR_FSA_1_2013_1_12443, Agencia Nacional de Investigación e Innovación, Uruguay). EM, MF and RT acknowledge ANII and CSIC-Udelar for the postgraduate scholarships.
Funding
This work was supported by grants from the Programa de Desarrollo de Ciencias Básicas (PEDECIBA, Uruguay), ANII (Projects FMV_1_2017_1_136597 and ANII_PR_FSA_1_2013_1_12443, Agencia Nacional de Investigación e Innovación, Uruguay). EM, MF and RT acknowledge to ANII and CSIC-Udelar for the Posgraduate scholarships.
Author information
Authors and Affiliations
Contributions
BM: Conceptualization, Methodology, Research, Formal analysis, Resources, Writing—Review and editing; JS: Methodology, Research, Formal analysis, Proofreading and editing; MN: Research, Proofreading and editing; MEM: Research, Writing-review and Editing; MF: Research, Proofreading and Editing; RT: Research, Proofreading and Editing; WP: Research (supplies the new benzimidazole derivatives tested), Proofreading and Editing; EM: Resources, Proofreading and Editing; LD: Conceptualization, Validation, Resources, Writing-review and Editing, Supervision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Haemonchus contortus infection was maintained at the Campo Experimental del Instituto de Higiene de Facultad de Medicina, UdelaR. The animal protocol complied with Uruguayan Law No. 18.611. Experimental protocol no. 241 was reviewed and approved for this study by IACUC of Facultad de Medicina-UdelaR, Uruguay (file no. 070153–000586-16).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1
. The present table shows the IC90 values obtained from the set-up and verification of in vitro assays in H. contortus: exsheathed third-stage (xL3) automated motility assay and xL3 development to fourth-stage (L4). Table S2. xL3 motility in the medium and DMSO control groups at 24 h of incubation. Statistical analysis and study of the existence of significant differences between the medium control group and DMSO control group for each experiment. Table S3. xL3 motility in the medium and DMSO control groups at 48 h of incubation. Statistical analysis and study of the existence of significant differences between the medium control group and the DMSO control group for each experiment. Table S4. xL3 motility in the medium and DMSO control groups at 72 h of incubation. Statistical analysis and study of the existence of significant differences between the medium control group and the DMSO control group for each experiment. Table S5. xL3 % of development in the medium and DMSO control groups at 7 days of incubation. Statistical analysis and study of the existence of significant differences between the medium control group and the DMSO control group for each experiment. Table S6. Commercial anthelmintic drugs and their activity at different concentrations for each H. contortus assay. Bold numbers indicate that the drug was active at the concentration tested. Table S7. Activity of a novel series of benzimidazole derivatives (at 25 µM) for each H. contortus assay (incubation time of 72 h for xL3 or adult stage motility test and 7 days for L4 development test). Bold numbers indicate that the compound was considered active. Figure S1. The present figure shows the morphological differences between xL3 and L4 stages of H. contortus, where the most notable distinction is the well-developed pharynx and the presence of a complete buccal capsule in L4 stage. Figure S2. Albendazole sulfoxide dose-response curve in xL3 automated motility assay at 24 h (dotted line), 48 h (dashed line) and 72 h (solid line). Each data point represents mean ± SEM of three different experiments with six replicates for each concentration. Figure S3. Monepantel dose-response curve in xL3 automated motility assay at 24 h (dotted line), 48 h (dashed line) and 72 h (solid line). Each data point represents mean ± SEM of three different experiments, with six replicates for each concentration. Figure S4. Ivermectin dose-response curve in xL3 automated motility assay at 24 h (dotted line), 48 h (dashed line) and 72 h (solid line). Each data point represents mean ± SEM of three different experiments with six replicates for each concentration. Figure S5. Levamisole dose-response curve in xL3 automated motility assay at 24 h (dotted line), 48 h (dashed line) and 72 h (solid line). Each data point represents mean ± SEM of three different experiments with six replicates for each concentration. Figure S6. Albendazole sulfoxide dose-response curve in xL3 to L4 development assay. Each data point represents mean ± SEM, of three different experiments, with three replicates for each concentration. Figure S7. Monepantel dose-response curve in xL3 to L4 development assay. Each data point represents mean ± SEM of three different experiments with three replicates for each concentration. Figure S8. Ivermectin dose-response curve in xL3 to L4 development assay. Each data point represents mean ± SEM of three different experiments with three replicates for each concentration. Figure S9. Albendazole sulfoxide effect on motility of H. contortus adult stage at 24 h (dotted line), 48 h (dashed line) and 72 h (solid line). Each data point represents mean ± SEM motility score of three different experiments with six replicates for each concentration. Red dotted line indicates a motility score of 1.5, a value at which a product is considered active. Figure S10. Monepantel effect on motility of H. contortus adult stage at 24 h (dotted line), 48 h (dashed line) and 72 h (solid line). Each data point represents mean ± SEM motility score of three different experiments with six replicates for each concentration. Red dotted line indicates a motility score of 1.5, a value at which a product is considered active. Figure S11. Ivermectin effect on motility of H. contortus adult stage at 24 h (dotted line), 48 h (dashed line) and 72 h (solid line). Each data point represents mean ± SEM motility score of three different experiments with six replicates for each concentration. Red dotted line indicates a motility score of 1.5, a value at which a product is considered active. Figure S12. Levamisole's effect on motility of H. contortus adult stage at 24 h (dotted line), 48 h (dashed line) and 72 h (solid line). Each data point represents mean ± SEM motility score of three different experiments with six replicates for each concentration. Red dotted line indicates a motility score of 1.5, a value at which a product is considered active.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Munguía, B., Saldaña, J., Nieves, M. et al. Sensitivity of Haemonchus contortus to anthelmintics using different in vitro screening assays: a comparative study. Parasites Vectors 15, 129 (2022). https://doi.org/10.1186/s13071-022-05253-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-022-05253-3