Abstract
Background
Releasing considerable numbers of radiation-sterilized males is a promising strategy to suppress mosquito vectors. However, releases may also include small percentages of biting females, which translate to non-negligible numbers when releases are large. Currently, the effects of irradiation on host-seeking and host-biting behaviors have not been exhaustively investigated. Information is also lacking regarding the effects of sterilizing treatment on the endosymbiotic bacterium Wolbachia, which is known to affect the vector competence of infected mosquitos.
Methods
To ascertain the effects of irradiation on females, the pupae of two Aedes albopictus strains, differing in their natural or artificial Wolbachia infection type, and Aedes aegypti—which is not infected by Wolbachia—were treated with various doses of X-rays and monitored for key fitness parameters and biting behavior over a period of 2 weeks. The effect of radiation on Wolbachia was investigated by quantitative polymerase chain reaction (qPCR) and fluorescence in situ hybridization (FISH) analysis.
Results
Partial Aedes albopictus female sterility was achieved at 28 Gy, but the number of weekly bites more than doubled compared to that of the controls. Radiation doses of 35 and 45 Gy completely inhibited progeny production but did not significantly affect the survival or flight ability of Ae. albopictus females and caused a tripling of the number of bites per female per week (compared to untreated controls). These results were also confirmed in Ae. aegypti after treatment at 50 Gy. Wolbachia density decreased significantly in 45-Gy-irradiated females, with the greatest decreases in the early irradiation group (26 ± 2-h-old pupae). Wolbachia density also decreased as adults aged. This trend was confirmed in ovaries but not in extra-ovarian tissues. FISH analysis showed a strongly reduced Wolbachia-specific fluorescence in the ovaries of 13 ± 1-day-old females.
Conclusions
These results suggest that, under sterile insect technique (SIT) programs, the vector capacity of a target population could increase with the frequency of the irradiated females co-released with the sterile males due to an increased biting rate. In the context of successful suppression, the related safety issues are expected to be generally negligible, but they should be conservatively evaluated when large-scale programs relying on imperfect sexing and high overflooding release ratios are run for long periods in areas endemic for arboviral diseases. Also, the effects of irradiation on the vector competence deserve further investigation.
Graphical Abstract

Similar content being viewed by others
Background
Despite countless attempts, efforts to eliminate vector-borne diseases rarely produce lasting results. In recent years, some vectors have rapidly adapted to the dramatic environmental changes driven by global warming, urbanization, and deforestation, have increased in invasiveness, and have rapidly developed resistance to most pesticides [1,2,3].
Owing to their reproductive potential and ability to rapidly spread both by migration and passive transportation, mosquitoes are prominent vectors. Substantial investment and research are necessary for the development of prevention and control measures.
The negative effects of widespread insecticide use have caused many researchers to focus on developing innovative control strategies characterized by high specificity and eco-compatibility, and targeting reductions in reproductive potential or vector competence of mosquito populations. This may involve the release of modified conspecifics who mate with wild types and introduce factors that induce sterility, lethality, or virus resistance in the progeny. To establish such modifications, which may or may not be heritable, advanced biotechnological methods based on genetic modification or the exploitation of beneficial microorganisms are used [4].
Methods based on genetic modification can be targeted to specific genes using various biotechnological tools [5,6,7] but such approaches can only be employed in countries where the release of genetically modified organisms (GMOs) is legal. The sterile insect technique (SIT) involves the use of optimized doses of mutagenic radiation to sterilize laboratory-reared males (through nonspecific and nonheritable genetic modifications) and releasing large numbers of sterilized males to decrease reproduction in wild populations [8, 9].
A similar result can be obtained via a natural phenomenon of egg inviability induced by the common endosymbiotic bacterium Wolbachia [10]. Insect males infected by certain strains of this bacterium are only reproductively compatible with females harboring the same Wolbachia strain, whereas the lack of the infection or infection with a non-compatible strain causes the production of inviable offspring. This natural post-mating reproductive barrier is known as cytoplasmic incompatibility (CI) and may contribute to the spread of infected individuals in the wild, as infected females can produce viable progeny with both infected and uninfected males (unidirectional CI, Uni-CI) [11, 12]. The possibility of transferring the bacterium horizontally between species allowed researchers to exploit Wolbachia to produce incompatible males that could be released in a target area to reduce the reproductive potential of wild populations (incompatible insect technique, IIT) [13,14,15,16,17,18].
Large-scale implementation of SIT and IIT must be combined with an efficient method of sexing, since the presence of residual females inadvertently released with males can result in the occurrence of temporary spikes in vector density [13]. In the long term, the suppressive effect of control programs is expected to counterbalance the contribution of irradiated females to the mean biting rate in the target area. However, even small percentages of co-released females may translate to large numbers of individuals when high overflooding release ratios (10:1 or even 50:1) are applied for long periods [19]. Furthermore, especially when Wolbachia-induced Uni-CI occurs, released females may be invasive, possibly leading to undesired population replacement [12, 20].
The implementation of SIT using mosquito strains capable of producing incompatible males has been suggested as a method to mitigate the unpredictable effects of female co-release. Mosquito females are more sensitive to radiation than males, and this approach could utilize CI to achieve full male sterilization, with the radiation dose lowered to levels that exert no effect on male fitness but which are sufficient to induce complete sterility in females escaping the sexing procedure [21]. However, although recent trials based on this approach have been successful in terms of local Aedes albopictus suppression, the applicability of this strategy on a large scale is debated [19, 22, 23].
It should be noted that all studies aimed at implementing SIT against vector mosquitoes have been mainly focused on radiation doses aimed at the sterilization of the males [9, 24]. However, the radiation doses needed to achieve female sterilization are known to damage the ovarian tissues considerably [21], and previous studies have reported that oogenesis plays a role in regulating the tendency for biting and blood-feeding [25, 26], which are critical factors for determining the vectorial capacity of mosquitoes [27]. Therefore, an appropriate study on the effects of radiation on these physiological and behavioral traits is warranted.
A thorough investigation of the effects of irradiation on Wolbachia would also be necessary in the case of SIT or combined SIT-IIT strategy. In fact, the bacterium Wolbachia has been shown to be affected by radiation [28, 29]; thus, this aspect should also be considered before conducting large-scale control programs which involve Wolbachia infected mosquitoes, as the titer of this endosymbiont may be related to the vector competence of the host [30] and the level of induced CI [31].
Together with certain anopheline species, Aedes mosquitoes represent the main concern for human health, as they serve as vectors for several arboviruses. The impressive spread of diseases associated with these pathogens in recent decades highlights the inefficacy of the current control methods [32,33,34]. Aedes albopictus and Ae. aegypti have been the target of several SIT, IIT or SIT/IIT combined experimental trials in recent years [14, 16,17,18,19, 35]. The first species is naturally infected with two Wolbachia strains that have been demonstrated to interfere with pathogen transmission (compared to uninfected individuals) [36], while the latter is not infected by Wolbachia in nature.
Herein, we aimed to determine whether irradiation interfered with the host-biting and host-seeking behaviors of Ae. albopictus and Ae. aegypti. We also evaluated the effects of radiation on the titer of Wolbachia in Ae. albopictus in whole mosquito bodies using quantitative polymerase chain reaction (qPCR), after applying the treatment at different pupal ages and analyzing the effects at two adult ages. The ovaries and extra-ovarian tissues were similarly studied. Finally, fluorescence in situ hybridization (FISH) analysis was conducted on the irradiated ovaries to visualize the effects of the treatment on the bacterial population and to acquire further information for interpreting the results. Two Ae. albopictus lines were used in the experiments to determine whether different Wolbachia strains might affect the results. The resultant data will be useful for enhancing SIT and SIT-IIT strategies in terms of safety and sustainability, and to better evaluate their large-scale applicability.
Methods
Ae. albopictus and Ae. aegypti strains and rearing
Two Ae. albopictus strains and one Ae. aegypti strain were used in the experiments. SANG Ae. albopictus originated from wild-type individuals collected from Anguillara Sabazia (Rome) in 2006 and harbors wAlbA and wAlbB Wolbachia. ARwP Ae. albopictus was established in 2008 through the transinfection of Wolbachia-cured SANG individuals with wPip Wolbachia from Culex pipiens molestus [37] and is characterized by a bidirectional incompatibility pattern with wild-type Ae. albopictus [38]. Both lines were reared under laboratory conditions at the National Agency for New Technologies, Energy and Sustainable Economic Development (ENEA Casaccia Research Center (Rome) and were periodically outcrossed with wild-type individuals from the same area to preserve genetic variability [39]. The Ae. aegypti line (New Orleans, LA 2011) was provided by the University of Camerino (Camerino, MC, Italy), where it had been laboratory-reared since 2014 and was not infected with Wolbachia.
Colonies were maintained by raising larvae to adulthood inside 1-l larval trays at a density of 1 larva/ml, provided with liquid food according to the methods described in a previous study [39]. Adult mosquitoes were maintained inside 40 × 40 × 40 cm cages at temperature of 28 ± 1 °C, relative humidity (RH) of 70% ± 10%, and 14:10-h light/dark cycle, and were supplied with water and 10% sucrose.
Blood meals were provided via anesthetized mice in agreement with the Bioethics Committee for Animal Experimentation in Biomedical Research and in accordance with procedures approved by the ENEA Bioethical Committee according to the EU directive 2010/63/EU. The mice belonged to a colony housed at CR ENEA Casaccia and maintained for experimentation based on the authorization no. 80/2017-PR released (on February 2, 2017) by the Italian Ministry of Health. Feeding of female mosquitoes on the blood of human hosts (i.e., the authors RM, EL, GL, and MC) during the experiments was also approved by the ENEA Bioethical Committee.
Radiation methods
Cohorts of Ae. albopictus and Ae. aegypti females belonging to the strains described above were irradiated with X-rays to enable comparison with sham-exposed individuals (pupae treated in a manner similar to the exposed pupae except for the X-ray exposure).
Aedes albopictus pupae were sexed mechanically using a specific sieving procedure described previously [16].
X-ray irradiation was performed using the Gilardoni CHF 320G X-ray generator (Gilardoni S.p.A.; Mandello del Lario, Lecco, Italy) operated at 250 kVp, and 15 mA, with filters of 2.0 mm of Al and 0.5 mm of Cu, furnished by the Physical Technologies for Security and Health Division of ENEA. Depending on the experiment and according to doses already tested for the radiation-based sterilization of the two species [9, 19, 21], Ae. albopictus pupae were subjected to 28, 35, and 45 Gy (dose rate: 0.868 ± 0.004 Gy/min, mean ± SD), while a single dose of 50 Gy was used to treat Ae. aegypti, as this dosage is known to fully inhibit egg production in the species [9]. Time of sample exposure was determined according to dose rate in order to obtain the pre-established doses. Doses were confirmed by monitoring the exposure with a PTW 7862 large-size plane-parallel transmission chamber connected to a PTW IQ4 electrometer. Groups of 100 female pupae were transferred to a Petri dish (d = 4 cm) at 36 ± 4 h of age (unless specified differently) and then transported to the irradiation facility. Immediately before commencement of irradiation, most of the residual water was removed using a glass pipette; irradiated pupae were then transferred to a larger water container to facilitate complete development and allow for adult emergence inside the experimental cages.
Survival, fecundity, and fertility in irradiated Ae. albopictus females
SANG or ARwP Ae. albopictus females irradiated at 28, 35, and 45 Gy, and untreated counterparts were allowed to emerge inside 30 × 30 × 30 cm plastic cages, which were checked for the presence of males that escaped the sexing procedure. Thirty virgin females and thirty untreated males—characterized by the same Wolbachia infection type—were placed in each cage.
These cages were used to monitor survival, fecundity, and fertility in the same treatments during the 2 weeks of observation, and for the biting rate studies described below. Five repetitions were conducted.
Mortality was recorded daily by removing dead individuals. During the two observation weeks, egg collection was initiated 3 days after the first blood meal, and the collection was stopped on the fifth day after the last meal to limit overlap with the second gonotrophic cycle. Paper-lined cups for egg collection were replaced every 3 days to avoid uncontrolled egg hatching. Eggs were maintained under wet conditions for 3 days, allowed to dry, and counted to determine the fecundity rate. Egg fertility was assessed by counting the hatched eggs after immersion in a nutrient broth [40].
Engorgement rate in irradiated Ae. albopictus and Ae. aegypti females under small enclosures
Starting from the fifth day after emergence and continuing for 5 days, irradiated SANG and ARwP females were offered a daily blood meal to monitor their feeding behavior. To allow for the study of a second gonotrophic cycle, this same procedure was also conducted from the 12th to the 16th day. The number of engorged females was tallied within 20 min of placement of the blood meal in the cage. Results obtained with the two populations of females treated at the three radiation doses were compared with those observed using control untreated females. Each of these eight treatments was repeated five times.
Although the present study was focused on Ae. albopictus, Ae. aegypti females irradiated at 50 Gy were similarly tested (in triplicate) in comparison with untreated controls to study the phenomenon in a species that is also a target of SIT and SIT/IIT programs but is not infected by Wolbachia in nature. The hatch rate of irradiated Ae. aegypti was also measured and compared to that of control females to identify a successful radiation treatment.
Host-seeking ability and biting rate of irradiated Ae. albopictus females in large enclosures
The host-seeking behavior of ARwP and SANG females irradiated at 45 Gy was studied in large enclosures and compared with that of untreated individuals based on methods described in a previous study [41]. The trials were conducted outdoor in two large cellular polycarbonate experimental units (LEU, 8.5 × 5 × 5 m L:W:H) with two large lateral openings (L = 8 m; H = 1 m) protected by a mosquito net to promote ventilation and ensure climatic conditions closer to the external environment. The latter were constantly monitored by a CR-10 data logger (Campbell Scientific, Logan, UT, USA), which registered a mean temperature of 34.0 ± 1.0 °C and RH averaging 50.0% ± 5.0%. LEUs contained benches with wet soil and potted plants, which provided refuge for the females and higher local levels of humidity (averaging 61.0% ± 5.0%).
For each experiment, an experimenter (the host) acted as a source of blood, and a second experimenter (the collector) released, recovered, and counted the Ae. albopictus females after they had fed on the host. The host wore a long-sleeved shirt and short pants, exposing only the lower legs to limit the area for the mosquitoes to land on, whereas the collector wore a white tracksuit and white shoes. Both researchers wore mosquito net hats. Four groups, differing in their infection type (SANG or ARwP) and treatment (irradiated and non-irradiated individuals), were compared. Each group consisted of 30 starved females aged 6 ± 1 days. Females were released at the side of the LEU by removing the cover of the cage. The second experimenter then immediately approached the host on the opposite side of the LEU to collect the females that had started feeding. The proportion of blood-fed females was tallied along with the time taken by each individual to reach the host, at 30-s intervals. Female mosquitoes that landed on the host, but did not bite were excluded from the count. The experiments lasted for a period of 15 min, and for each group, six repetitions were performed, alternating the experimental units and the host. At the end of each experiment, an electric mosquito swatter and a powerful aspirator were used to eliminate any mosquitoes remaining inside the LEU. The experiments were conducted in the late afternoon on sequential days during June 2020. A schematic describing the experiment is provided in the supplemental materials (Additional file 1: Figure S1).
In addition to the methods described above, 6 ± 1-day-old females from each treatment group were engorged in the laboratory and used to study the effect of the radiation treatment on their willingness to seek and bite a host 48 h after the first blood meal. After their release in the LEU, the proportion of feeding females and the time to reach the host were again noted.
Quantitative PCR analysis of Wolbachia titer in irradiated Ae. albopictus females
Considering the importance of Wolbachia in modulating the vector competence of infected mosquitoes [42], a qPCR was performed on the strains of this bacterium present in SANG and ARwP females after irradiation at 45 Gy (a dose known to induce full female sterilization in the species) [9]. Results were compared with those obtained using untreated counterparts. In operational SIT programs, mosquitoes are generally irradiated at the pupal stage (24–48 h); however, it is known that pupal age is one of the critical factors that affect the biological response to the radiation dose [43]. Therefore, the effects of the irradiation were investigated by analyzing DNA extracts of whole bodies of 6 ± 1-day-old females developed from pupae irradiated at three ages (26 ± 2, 36 ± 2, and 46 ± 2 h).
The density of Wolbachia is known to vary with the age of Ae. albopictus females [44]. Therefore, to investigate the effects of radiation on the bacterium in the germline and somatic tissues, the titer of the bacterium was measured in ovaries and bodies lacking ovaries of 6 ± 1- and 13 ± 1-day-old females after they were treated with 45 Gy as pupae aged 36 ± 4 h. Untreated females were used as a control.
DNA was extracted from single individuals using the ZR Tissue & Insect DNA Kit MicroPrep (Zymo Research, Irvine, CA, USA), according to the manufacturer’s instructions. When necessary, females were chilled on ice and dissected in phosphate-buffered saline (PBS) to isolate the ovaries from the other tissues. Here we excluded individuals with ovaries that were not intact after the procedure.
Real-time PCR was performed using the Roche LightCycler 96 Instrument (Roche Molecular Systems, Inc., Rotkreuz, Switzerland). Each reaction was performed in triplicate with a final reaction volume of 20 μl (10 μl of the Luna Universal qPCR Master Mix (New England Biolabs, Ipswich, MA, USA), 0.03 μl of 150 nM each primer, and 2 μl of purified DNA) using the following amplification program: initial activation at 95 °C for 120 s, followed by 40 cycles at 95 °C for 15 s and 60 °C for 40 s. The presence of specific amplification products was verified using dissociation curves [44].
Strain-specific primers were used to amplify the wsp loci [45], namely, the wAlbA-wsp and wAlbB-wsp loci in the case of SANG females, and the wPip-wsp loci in the case of ARwP specimens. Wsp plasmid standards were used to generate a standard curve [44]. In order to normalize qPCR data, the Ae. albopictus actin gene was used as a reference for whole-body extracts and extracts obtained after removal of the ovaries and was amplified with the primer pair actAlbqPCR [46]. Owing to the marked decrease in actin gene copies observed preliminarily in irradiated ovaries (Additional file 2: Figure S2), the normalization of qPCR data related to these organs was performed using total DNA (2 μl of purified DNA per reaction, corresponding to 200–300 ng) as reference [47]. For this purpose, a NanoDrop 2000 spectrophotometer (Thermo Fisher, Waltham, MA, USA) was used.
FISH analysis of the ovaries in irradiated Ae. albopictus females
Based on the results of qPCR, fluorescent in situ hybridization (FISH) analysis was conducted on the ovaries of 13 ± 1-day-old irradiated and untreated females according to the protocol described by previous authors [48]. Two hundred nanograms of the Wolbachia specific 16S rRNA probe (W2: 5′-CTTCTGTGAGTACCGTCATTATC-3′) was added to the hybridization buffer [43]. Tissues were placed on a slide containing a drop of VECTASHIELD Antifade Mounting Medium with DAPI (Vector Laboratories, Burlingame, CA, USA) and visualized using the Nikon Eclipse E800 confocal microscope and NIS-Elements 4.0 software (Nikon, Tokyo, Japan). An aposymbiotic population of Ae. albopictus [37] was used as the negative control.
Data analysis
Results were expressed as mean ± SE, and the arcsine square root transformation was applied to analyze proportional data. The Levene test and the Shapiro–Wilk test were performed to assess equality of variances and normality, respectively. Statistical analysis was performed using PASW Statistics software (PASW Statistics for Windows, version 18.0. SPSS Inc., Chicago, IL, USA), with the level of significance set at P < 0.05.
The survival curves of the four different treatments for each Wolbachia infection type were compared using the Kaplan–Meier method and the log-rank (Mantel–Cox) test. The Kruskal–Wallis H-test followed by the Conover-Iman test was used to compare fecundity and egg hatch data between treatments within each infection type.
Repeated-measures analysis of variance (ANOVA) was used to analyze the bite data obtained between treatments over each week. Cages were considered the experimental units, and data are expressed as the mean percentage of engorged females per day, adjusted for cage-specific mortality. If Mauchly’s test indicated a violation of the sphericity assumption, the degrees of freedom were corrected using Huynh–Feldt estimates. Multiple comparisons between treatments were assessed using Tukey’s honestly significant difference (HSD) post hoc test. Additionally, one-way ANOVA was performed to compare the mean number of bites per female per treatment during each week of study.
Data regarding the host-seeking behavior under large enclosures were analyzed by assigning a value to each mosquito based on the median of each catching interval to compute the average time to landing. The proportion of engorged mosquitoes was measured based on those retrieved within the defined 15-min interval. Two-way ANOVA was used to analyze the differences between groups in terms of the proportions of females that had bitten the host and to compare average landing times. The Shapiro–Wilk test was conducted to ascertain that the proportions and average landing times were normally distributed.
One-way ANOVA was used to compare within each Wolbachia strain the qPCR data obtained from Ae. albopictus females treated at the three tested pupal ages or untreated. The effect of female aging on the overall titer of Wolbachia was also analyzed by performing one-way ANOVA with data of each strain of the bacterium. Data regarding ovaries and extra-ovarian tissues of the treated or untreated counterparts were similarly analyzed at the two tested female ages. In the case of rejection of the assumptions of equality of variance and/or normality, the Kruskal–Wallis rank-sum test was performed.
Results
Female survival, fecundity, and fertility in irradiated Ae. albopictus females
Regardless of the infection type, irradiation did not significantly affect the survival of female mosquitoes during the 2 weeks of observation (Fig. 1; log-rank test for SANG: χ2 = 2.43; df = 3; P = 0.49; log-rank test for ARwP: χ2 = 1.847; df = 3; P = 0.61).
A 45-Gy dose completely inhibited egg-laying in Ae. albopictus, whereas a few eggs were oviposited by females irradiated at 35 Gy. Among the latter, fertile females were very rare (Table 1). A dose of 28 Gy induced a considerable reduction in the number of oviposited eggs compared to that in untreated controls and markedly affected egg fertility, but did not fully inhibit progeny production (Table 1). These results were confirmed in both Ae. albopictus strains and both gonotrophic cycles.
Increased engorgement rate in irradiated Ae. albopictus and Ae. aegypti females
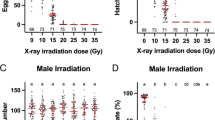
Repeated-measures ANOVA revealed significant differences between treatments with respect to the weekly biting activity (Fig. 2a; SANG-1st week: Huynh–Feldt correction, F(10.19, 54.35) = 2.99, P < 0.05; SANG-2nd week: sphericity assumed, F(12, 64) = 4.52, P < 0.05; ARwP-1st week: sphericity assumed, F(12, 64) = 5.90, P < 0.05; ARwP-2st week: Huynh–Feldt correction, F(9.12, 48.64) = 3.36, P < 0.05). Regardless of the week and the infection type, untreated mosquitoes generally showed the most biting activity during the first day of the blood meal and then they generally rested while their eggs underwent maturation. In contrast, irradiated females exhibited an anomalous blood-feeding behavior and the percentage of the daily engorged females only decreased slightly over each week (Fig. 2a).
Altered biting behavior in irradiated Ae. albopictus and Ae. aegypti. The experiment was carried out in laboratory cages by offering a blood meal each day over two sets of 5 subsequent days interrupted by 2 days of rest and starting with 5-day-old females. a Daily mean percentage of biting SANG and ARwP Ae. albopictus; b mean weekly number of bites per SANG and ARwP Ae. albopictus female over 2 weeks; c daily mean percentage of biting Ae. aegypti; d mean weekly number of bites per Ae. aegypti female over 2 weeks; a and c: within each mosquito population, different letters indicate statistically significant differences between treatments (repeated-measures ANOVA followed by Tukey's HSD test: P < 0.05); b and d: within each mosquito population, different letters indicate statistically significant differences between treatments (one-way ANOVA followed by Tukey's test: P < 0.05)
For the 35- and 45-Gy treatments, total bites per female approximately tripled weekly over those recorded for untreated females (Fig. 2b). During the first week, the mean number of bites per SANG female averaged 1.15 ± 0.07 (untreated), 2.14 ± 0.06 (28 Gy), 2.69 ± 0.07 (35 Gy), and 2.77 ± 0.07 (45 Gy). Tukey’s test showed significant differences among untreated and treated females, and among the females treated with 28 Gy and the other three groups (F(3, 16) = 118.01; P < 0.05). During the second week, the above-mentioned values were 1.07 ± 0.05 (untreated), 1.89 ± 0.05 (28 Gy), 2.63 ± 0.03 (35 Gy), and 2.84 ± 0.06 (45 Gy), and all treatments differed significantly from one another (F(3, 16) = 282.23; P < 0.05). With respect to the ARwP line, the mean number of bites per female was 1.10 ± 0.03 (untreated), 2.15 ± 0.02 (28 Gy), 2.68 ± 0.03 (35 Gy), and 2.84 ± 0.02 (45 Gy) during the first week (F(3, 16) = 371.96; P < 0.05), with the Tukey’s test indicating significant differences between all treatments. Values of 1.05 ± 0.03 (untreated), 2.12 ± 0.02 (28 Gy), 2.70 ± 0.03 (35 Gy), and 2.76 ± 0.04 (45 Gy) were obtained during the second week (F(3, 16) = 642.89; P < 0.05), with significant differences observed between all treatments except those at 35 and 45 Gy.
Similarly, 50 Gy-irradiated Ae. aegypti females showed an enhanced propensity to bite compared to the untreated mosquitoes during both weeks of observation (Fig. 2c; first week: sphericity assumed, F(4, 16) = 8.38, P < 0.05; second week: sphericity assumed, F(4, 16) = 11.51, P < 0.05). Overall, the mean number of bites for the 50-Gy-treated females almost tripled that of untreated mosquitoes during the first (treated females: 2.81 ± 0.03; untreated females: 1.03 ± 0.02; F(1, 4) = 3315.67; P < 0.05) and second weeks (treated females: 2.86 ± 0.04; untreated females: 1.05 ± 0.03; F(1, 4) = 1522.09; P < 0.05) (Fig. 2d). The applied radiation dose was found to induce complete sterility in Ae. aegypti females (Table 1).
Host-seeking behavior of irradiated Ae. albopictus females in large enclosures
The host-biting behavior exhibited by the irradiated females in large enclosures reinforced the results obtained in small cages. The applied X-ray dose was not sufficient to reduce the ability of Ae. albopictus to reach the host in the LEU (compared to untreated females; Fig. 3a, b). The average times to landing were not significantly affected by the infection type (two-way ANOVA: F(1, 20) = 0.66, P = 0.43), nor were they affected by the treatment (two-way ANOVA: F(1, 20) = 0.93, P = 0.35) in starved females. On average, landing times (± SE) were 346.31 ± 15.98 and 354.30 ± 10.74 in irradiated and untreated SANG females and 331.11 ± 12.74 and 348.29 ± 12.30 in irradiated and control ARwP individuals, respectively.
Altered host-seeking and biting behavior in irradiated SANG and ARwP Ae. albopictus under large enclosures setting. The experiment was carried out in large experimental units (8.5 × 5 × 5 m) under open field climatic conditions and involved females irradiated at 45-Gy compared to untreated counterparts. Average landing time and biting proportions were compared between treatments within a 15-min interval. a Comparison between irradiated and untreated starved females (6 ± 1 days old). b Comparison between irradiated and untreated females 48 h after the engorgement (8 ± 1 days old). Two-way ANOVA demonstrated that the difference between treatments was statistically significant in the case of the engorged females (P < 0.05)
In contrast, the biting behavior was affected by irradiation. Starved females did not reveal a significant difference in the proportion of biting females between untreated and 45-Gy-treated females (two-way ANOVA: F(1, 20) = 0.59, P = 0.45) or between infection types (two-way ANOVA: F(1, 20) = 0.80, P = 0.38) (Fig. 3a). Instead, the proportion of fed females that repeated the blood meal after 48 h was significantly higher in irradiated mosquitoes than that in untreated mosquitoes (two-way ANOVA: F(1, 20) = 276.53, P < 0.05), while the infection type showed no significant effect (two-way ANOVA: F(1, 20) = 1.13 P = 0.30) (Fig. 3b). Consequently, a comparison between average landing times related to the engorged females was not performed due to the reduced number of biting individuals among the untreated controls (0 in certain repetitions). To verify this result in older females that might have experienced the occurrence of age-related damage due to irradiation, the test was also repeated in triplicate with treated and untreated individuals aged 13 ± 1 days, and similar results were obtained (Additional file 3: Figure S3).
Decreased titer of Wolbachia in irradiated Ae. albopictus females
The age at which Ae. albopictus pupae had been irradiated at 45 Gy was found to significantly affect the density of Wolbachia (wAlbA: F(3, 36) = 2.96, P < 0.05; wAlbB: F(3, 36) = 4.05, P < 0.05; wPip: F(3, 36) = 6.76, P < 0.05) regardless of the Wolbachia infection type. Early irradiation (26 ± 2-h-old pupae) resulted in significantly decreased the whole-body Wolbachia titer, by a third in wAlbA, and by half in wAlbB and wPip strains (Fig. 4a) compared to the levels observed in the controls. The Wolbachia titer did not significantly differ in pairwise comparisons between treated and untreated individuals when irradiation was performed on older pupae (36 and 46 ± 2 h old).
Decreased Wolbachia titer in irradiated SANG and ARwP Ae. albopictus. a A 45-Gy dose was applied at three different pupal ages (26, 36, and 46 ± 2 h); quantitative PCR analysis was then performed using primers targeting the specific Wolbachia strains characterizing the two Ae. albopictus populations and analyzing 6 ± 1-day-old females. b A 45-Gy dose was applied to pupae aged 36 ± 4 h; qPCR analysis was then carried out studying two female ages (6 and 13 ± 1 days). Data were normalized using the actin gene as host reference. Within each infection type and female age, different letters indicate statistically significant differences between treatments (one-way ANOVA followed by Tukey's test: P < 0.05)
Aging of adult females was also found to affect the titer of all the tested Wolbachia strains when a 45-Gy treatment was performed (Fig. 4b). The difference between treatments was not significant when the analysis was performed on 6 ± 1-day-old females (one-way ANOVA; wAlbA: F(1, 18) = 1.54, P = 0.23; wAlbB: F(1, 18) = 0.82, P = 0.89; wPip: F(1, 18) = 3.28, P = 0.09), but the differences increased significantly in females aged 13 ± 1 days (one-way ANOVA; wAlbA: F(1, 18) = 62.11, P < 0.05; wAlbB: F(1, 18) = 38.93, P < 0.05; wPip: F(1, 18) = 4.60, P < 0.05).
In agreement with the data obtained from the analysis of whole bodies, qPCR revealed a correlation between aging and a reduced titer of the bacteria in the ovaries of irradiated Ae. albopictus females compared to that in untreated Ae. albopictus females (Fig. 5a). Differences were significant in females at 13 ± 1 days after the irradiation (one-way ANOVA; wAlbA: F(1, 18) = 62.11, P < 0.05; wAlbB: F(1, 18) = 5.91, P < 0.05; wPip: F(1, 18) = 4.60, P < 0.05), whereas the density of wAlbA and wAlbB Wolbachia strains did not differ between treatments in 6 ± 1-day-old females (one-way ANOVA; wAlbA: F(1, 18) = 0.39, P = 0.54; wAlbB: F(1, 18) = 0.50, P = 0.49). A significant difference was found between the wPip Wolbachia titer in treated and untreated 6 ± 1-day-old ARwP females (F(1, 18) = 5.25, P < 0.05). The titer of wAlbA and wAlbB Wolbachia also increased with aging in the ovaries of control females and confirmed the results of previous studies (Fig. 5a) [40]. With respect to wPip, the controls exhibited an age-dependent decrease in titer and irradiation further intensified this trend in the density of the bacteria.
Decreased Wolbachia titer in the ovaries of irradiated SANG and ARwP Ae. albopictus. A 45-Gy dose was applied to 36 ± 4-h-old pupae. a qPCR analysis was performed on the dissected ovaries of 6 and 13 ± 1-day-old females using primers targeting the specific Wolbachia strains characterizing the two Ae. albopictus populations. Data were normalized using DNA (2 μl of purified DNA per reaction). b qPCR analysis was performed on bodies lacking ovaries of 6- and 13 ± 1-day-old females using primers targeting the specific Wolbachia strains characterizing the two Ae. albopictus populations. Data were normalized using the actin gene as host reference. Within each infection type and female age, different letters indicate statistically significant differences between treatments (one-way ANOVA followed by Tukey's test: P < 0.05)
When testing females without ovaries, the qPCR amplification results of Wolbachia (somatic fraction) did not reveal differences between treatments related to the titers of wAlbA, wAlbB, and wPip Wolbachia when irradiated females were tested at 6 ± 1 days (Fig. 5b) (Kruskal–Wallis test; wAlbA: χ2 = 1.85, df = 1, P = 0.17; wAlbB: χ2 = 0.32, df = 1; P = 0.57; wPip: χ2 = 1.37, df = 1, P = 0.24). In contrast to the results obtained in the ovaries, the titer of Wolbachia in the extra-ovarian tissues exhibited an apparent age-related increase in irradiated females compared to the controls, but differences between treatments were not significant (Kruskal–Wallis test; wAlbA: χ2 = 2.17, df = 1, P = 0.14; wAlbB: χ2 = 2.52, df = 1, P = 0.11; wPip: χ2 = 0.24, df = 1, P = 0.62).
FISH
The results of quantitative PCR were consistent with FISH images that revealed evident histological damage due to radiation and reduction in the mean size of the organs (Additional file 4: Figure S4), coupled with a markedly reduced Wolbachia-specific fluorescence intensity (Fig. 6; Additional files 5, 6, 7: Figures S5, S6, S7). Analysis of the Wolbachia-infected individuals revealed that fluorescent signals differed markedly between treatments (Fig. 6). In most of the ovaries dissected from SANG and ARwP females irradiated at 45 Gy, a weak fluorescent signal was observed (Fig. 6b, d; Additional files 6, 7: Figures S6, S7) compared to that in untreated controls (Fig. 6a, c; Additional files 8, 9: Figures S8, S9). In certain cases, the fluorescence was only localized in the ovarioles that possibly had not been degenerated by the irradiation (Fig. 6d, red circle). In contrast, the organs maintained their typical ovariole structure in untreated samples and the signal was abundant, specific, and confined within each oocyte (Fig. 6; Additional files 8, 9: Figures S8, S9).
Fluorescence in situ hybridization of Ae. albopictus ovaries in irradiated females. A 45-Gy dose was applied to 36 ± 4-h-old pupae of SANG and ARwP Ae. albopictus. Ovaries from sample individuals aged 13 ± 1 days were then subjected to FISH analysis and compared with untreated counterparts. The distribution of Wolbachia is evidenced in green, while blue stain is DAPI. a Ovaries of untreated SANG females; b ovaries of irradiated SANG females; c ovaries of untreated ARwP females; d ovaries of irradiated ARwP females
Discussion
Multiple blood-feeding is known to naturally occur in Ae. albopictus as not all of the ovarian follicles commence the gonotrophic cycle with a single blood meal [49]. Studies conducted on engorged Ae. aegypti females demonstrated that they were generally inhibited from host-seeking by the occurrence of distension-induced and oocyte-induced mechanisms of regulation [24, 50]; however, this inhibition does not apply to all individuals [49]. Results presented here demonstrate that the radiation doses generally used in the framework of SIT programs significantly attenuate this inhibition in both Ae. albopictus and Ae. aegypti. In fact, most females irradiated at 35 and 45 Gy fed several times a week, even if the highest number of bites was generally observed on the first day of the blood meal. Additionally, the dose of 28 Gy was found to be sufficient to induce a doubled biting rate (compared to the untreated controls). Tissue alterations in ovaries in response to irradiation might explain this phenomenon as the host-seeking behavior is interrupted through the release of hormones produced by the ovaries in the hemocoel during oogenesis [51, 52]. In virus-endemic areas, multiple blood-feeding increases the likelihood of a female mosquito being infected by a suitable arbovirus and this possibility may significantly increase the chances of virus transmission to other hosts. Furthermore, the comparison of the survival curves of irradiated and untreated females revealed that the tested irradiation doses were not adequate to induce a marked decrease in life expectancy within the first 20 days in Ae. albopictus, which is an age that only a minority of adults are expected to reach in nature [53].
The vectorial capacity of a species describes its potential to transmit a pathogen and is dependent on the ratio of mosquitoes to humans, the extrinsic incubation period of the parasite, the mosquitos’ rate of biting humans, and the survival of mosquito females [54,55,56]. SIT and SIT/IIT programs have the specific goal of reducing the vectorial capacity (together with the mosquito nuisance) via the suppression of a target population. In this context, the co-release of relatively small percentages of irradiated females with enhanced biting activity could be viewed as a negligible and transient side effect considering that the benefits are supposed to outweigh the potential risks. However, large-scale releases of sterile males do not always adapt to the ever-decreasing wild population [19, 35] and if releases rely on high overflooding release ratios and a not perfect sexing, the increasing frequency of co-released irradiated females might represent an issue to be carefully evaluated in areas endemic for arboviral diseases.
Host-seeking is another behavioral trait that determines the biting rate and that could be affected by the radiation treatment. Previously, flight ability in radiation-sterilized males was not reported to be affected when compared to that in untreated males [9]. Our experiments testing the host-seeking ability in large enclosures confirm that flying ability is not affected by radiation doses that are capable of fully suppressing fecundity in females. Indeed, starved females irradiated at 45 Gy did not exhibit a significantly different ability to reach and bite the host compared to the controls, but when engorged, their behavior was anomalous and they continued to seek hosts, unlike the untreated females, leading to a marked increase in the mean number of bites per individual.
Overall, dose–response curves related to fecundity and fertility were in agreement with the results of other studies [9] and verified the success of the radiation treatment. However, differently from previous reports [21], a dose of 28 Gy was inadequate to induce full sterilization in eggs. This result may indicate that this dose approaches the minimum threshold necessary to achieve full sterilization of Ae. albopictus females and that slight modifications of the experimental setup are sufficient to allow the viability of a small percentage of fertile eggs [19]. This issue also becomes evident when moving from laboratory conditions to large-scale operational conditions, and highlights the need for studies specific to the latter before conducting open field trials [22]. This is particularly necessary for releases involving mosquito strains with altered Wolbachia infection types that are able to spread in the wild population through a Uni-CI pattern [15]. In these cases, a few partially fertile females could initiate a local population replacement, with unpredictable consequences [20]. In this context, for safety purposes, adding a perfect sexing protocol to Uni-CI-based IIT programs [17, 18, 35] could be preferable to combining IIT with SIT [19, 21, 22].
The evaluation of the vectorial capacity of a mosquito population also builds on the measurement of the vector competence, which is mainly determined by genetic factors [57], and Wolbachia exhibited the ability to modulate these factors in infected mosquitoes. This phenomenon has been suggested to depend on the bacterial titer and the specific strain of the bacterium [30, 58]. The density of Wolbachia in naturally infected Ae. albopictus is an individual feature that may vary substantially within a population [44] and that may be affected by the environmental conditions (temperature and food availability) under which the larvae develop [59,60,61]. Although distribution in somatic tissues has been observed in various cases, Wolbachia is mainly present within the germ-line [62], and previous studies have already shown that the ovarian tissues of irradiated females are severely affected by the treatment [9, 21]. Bacteria are generally less sensitive to radiation than eukaryotes [63]; nevertheless, the density of Wolbachia is markedly affected by radiation doses employed for other insect species to achieve sterilization [28]. The results presented in this study highlight an overall decrease in the density of Wolbachia in irradiated females, and this decrease was greatest when the pupae were irradiated at younger ages, and was positively correlated with female aging. This downward trend was common in all the studied Wolbachia strains (compared to the untreated controls).
Early irradiation is generally associated with stronger efficacy of the sterilization treatment [43]; however, negative effects on fitness have been found to increase in younger treated pupae. For this reason, SIT programs are generally targeted at an intermediate pupal age to achieve full sterilization and to sufficiently preserve the fitness of adult males [9]. The damage experienced by the ovaries is consistent with complete sterilization that occurs when sufficiently high doses are employed. Our results highlighted that the destruction of these tissues—which are relatively sensitive to radiation due to intense mitotic activity [64]—seems to also affect the survival and/or the reproduction of the hosted Wolbachia. The endosymbiont may be involved—together with the host cells—in the mechanisms of apoptosis usually characterizing the oocytes subjected to irradiation [65]. However, further studies should be conducted to investigate this phenomenon and to determine why the reduction in the density of the bacterium becomes evident by qPCR only more than a week after the treatment. This occurrence may be partly explained by a gradual clearance of the bacterial DNA corresponding to dead individuals or present in extracellular form [66] in the damaged ovarian tissues of irradiated females. Selection of an effective method to normalize the qPCR data allowed us to study an otherwise difficult-to-study phenomenon, as irradiation has previously been demonstrated to compromise the suitability of various common housekeeping genes when tissues are damaged [67]. A comparison of the actin gene copy number in the ovaries of irradiated and control individuals confirms this (Additional file 2: Figure S2), and suggests the need for specific studies to identify the best candidate housekeeping genes when performing similar experiments. Investigating the levels of expression of specific Wolbachia genes, or host genes under Wolbachia control, may provide useful information to measure the activity of the bacteria, and as a consequence, to estimate their response to the irradiation treatment [68].
FISH results reinforced the idea that the population of this endosymbiotic bacterium is severely affected by 45-Gy radiation treatment, at least in this organ, and that qPCR is only partially capable of highlighting this phenomenon.
Overall, our results are insufficient to determine whether radiation treatment can be associated with a loss of specific Wolbachia-controlled biological activities such as pathogen interference. Wolbachia endosymbionts mainly exert their limiting action on arbovirus dissemination in the bodies of Aedes species by acting in the midgut [69], and similar to ovarian cells, midgut cells are characterized by intense mitotic activity, and their structure and physiology have been previously shown to be affected by radiation [64, 70]. Therefore, Wolbachia and the related host–symbiont mechanisms of regulation may be affected by radiation in these tissues as well. However, this study does not provide evidence of such phenomena. Furthermore, as reported above, the detection of Wolbachia DNA does not necessarily indicate that the bacterium is alive or capable of fully exerting its effects on host physiology, because nucleic-acid-based analytical methods provide only limited information regarding the activities and physiological state of microorganisms in samples. These aspects can be detected retrospectively, but only after sufficient time has elapsed for the degradation and removal of DNA associated with inactivated cells [66, 71].
qPCR analysis specifically targeting the Wolbachia density in the gut of irradiated females may provide useful information for investigating bacterial population dynamics in these tissues. However, specific vector-competence studies will be necessary to ascertain whether a sterilizing radiation treatment leads to increased risk of virus acquisition or transmission per single bite. Coupled with the enhanced biting activity shown in the present study, increased vector competence would further boost the vectorial capacity of female mosquitos. For such a study to be feasible, oral infection trials should be conducted on mass-reared mosquito females after applying radiation treatment at the doses necessary for large-scale operational programs, and all Wolbachia-infected vector species should be included [22].
In the case of programs based on the combination of SIT and IIT, investigation of the effects of irradiation on the induced level of CI in treated males should also be performed [31], because based on the results of the ovaries, a decrease in the titer of Wolbachia in the testes following irradiation is a reasonably possible scenario.
Conclusions
The results presented in this work stress the need for more thorough scientific investigations on the radiation biology of female Aedes mosquitoes, as small percentages of females can be released together with sterile males during area-wide SIT or SIT/IIT programs. Even if this safety issue should be negligible in the context of successful population suppression, the effect of increasing frequencies of irradiated females in the target area should be conservatively evaluated with the support of opportune models analyzing the epidemiological risk [55, 72, 73], as these females could exhibit increased biting activity compared to wild types.
Measuring the vector competence of irradiated females that are infected with Wolbachia should also be opportune, because the irradiation-induced decrease in the density of this bacterium may be consistent with effects on biological phenomena such as pathogen interference.
Applying advanced and more efficient systems of sex separation capable of preventing the escape of females during the release of sterile males [17, 18, 35, 74] would be sufficient to mitigate risks. Additionally, release protocols could use constant monitoring of the wild-type population to limit the number of mosquitoes released to a minimum threshold to guarantee efficacy [75]. Certainly, in the case of release programs that involve Wolbachia infections with pathogen interference phenotypes, the irradiation of pupae at a young age should be avoided because this treatment would maximize the biting activity and the Wolbachia depletion in adult females.
The data presented here may furnish useful cues to enhance the safety level of SIT-based control programs against Aedes mosquitoes and encourage a careful comparison between the various genetic control methods in search of the most efficient, sustainable, and safe strategy for mosquito vector control.
Availability of data and materials
All relevant data are within the paper and its Supporting Information files. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- SIT:
-
Sterile insect technique
- CI:
-
Cytoplasmic incompatibility
- Uni-CI:
-
Unidirectional cytoplasmic incompatibility
- IIT:
-
Incompatible insect technique
- qPCR:
-
Quantitative polymerase chain reaction
- FISH:
-
Fluorescence in situ hybridization
- ARwP:
-
Aedes albopictus population obtained through the replacement of the native wAlbA-wAlbB Wolbachia infection with wPip Wolbachia
- SANG :
-
Wild-type Ae. albopictus from Anguillara Sabazia (Rome)
- LEU:
-
Large cellular polycarbonate experimental units
- ANOVA:
-
Analysis of variance
References
T Iwamura A Guzman-Holst KA Murray 2020 Accelerating invasion potential of disease vector Aedes aegypti under climate change Nat Commun 11 2130
CL Moyes J Vontas AJ Martins LC Ng SY Koou I Dusfour 2017 Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans PLoS Negl Trop Dis. 11 e0005625
GA Garcia AA Hoffmann R Maciel-de Freitas DA Villela 2020 Aedes aegypti insecticide resistance underlies the success (and failure) of Wolbachia population replacement Sci Rep 10 63
L Alphey M Benedict R Bellini GG Clark DA Dame MW Service 2010 Sterile-insect methods for control of mosquito-borne diseases: an analysis Vector Borne Zoonotic Dis. 10 295 311
A Hammond R Galizi K Kyrou A Simoni C Siniscalchi D Katsanos 2016 A CRISPR-Cas9 gene drive system targeting female reproduction in the malaria mosquito vector Anopheles gambiae Nat Biotechnol 34 78 83
DD Thomas CA Donnelly RJ Wood LS Alphey 2000 Insect population control using a dominant, repressible, lethal genetic system Science 287 2474 2476
B Massonnet-Bruneel N Corre-Catelin R Lacroix RS Lees KP Hoang D Nimmo 2013 Fitness of transgenic mosquito Aedes aegypti males carrying a dominant lethal genetic system PLoS One. 8 e62711
RS Lees JR Gilles J Hendrichs MJ Vreysen K Bourtzis 2015 Back to the future: the sterile insect technique against mosquito disease vectors Curr Opin Insect Sci 10 156 162
JG Bond AR Osorio N Avila Y Gomez-Simuta CF Marina I Fernandez-Salas 2019 Optimization of irradiation dose to Aedes aegypti and Ae. albopictus in a sterile insect technique program PloS One. 14 e0212520
JH Werren L Baldo ME Clark 2008 Wolbachia: master manipulators of invertebrate biology Nat Rev Microbiol 6 741 751
S Sinkins S O’Neill 2000 Wolbachia as a vehicle to modify insect populations AMJA Handler Eds Insect transgenesis CRC Press Boca Raton 271 287
SL Dobson CW Fox FM Jiggins 2002 The effect of Wolbachia-induced cytoplasmic incompatibility on host population size in natural and manipulated systems Proc Royal Soc B 269 437 445
K Bourtzis SL Dobson Z Xi JL Rasgon M Calvitti LA Moreira 2014 Harnessing mosquito-Wolbachia symbiosis for vector and disease control Acta Trop 132 S150 S163
J Mains C Brelsfoard R Rose S Dobson 2016 Female adult Aedes albopictus suppression by Wolbachia-infected male mosquitoes Sci Rep UK 6 33846
R Moretti GA Marzo E Lampazzi M Calvitti 2018 Cytoplasmic incompatibility management to support Incompatible insect technique against Aedes albopictus Parasites Vectors 11 649
B Caputo R Moretti M Manica P Serini E Lampazzi M Bonanni 2020 A bacterium against the tiger: preliminary evidence of fertility reduction after release of Aedes albopictus males with manipulated Wolbachia infection in an Italian urban area Pest Manag 76 1324 1332
JW Mains PH Kelly KL Dobson WD Petrie SL Dobson 2019 Localized control of Aedes aegypti (Diptera: Culicidae) in Miami, FL, via inundative releases of Wolbachia-infected male mosquitoes J Med Entomol 56 1296 1303
NW Beebe D Pagendam BJ Trewin A Boomer M Bradford 2021 Releasing incompatible males drives strong suppression across populations of wild and Wolbachia-carrying Aedes aegypti in Australia PNAS. 118 e2106828118
X Zheng D Zhang Y Li C Yang Y Wu X Liang 2019 Incompatible and sterile insect techniques combined eliminate mosquitoes Nature 572 56 61
PS Yen AB Failloux 2020 A review: Wolbachia-based population replacement for mosquito control shares common points with genetically modified control approaches Pathogens 9 404
D Zhang RS Lees Z Xi JR Gilles K Bourtzis 2015 Combining the sterile insect technique with Wolbachia-based approaches: II-a safer approach to Aedes albopictus population suppression programmes, designed to minimize the consequences of inadvertent female release PloS One. 10 e0135194
R Moretti M Calvitti 2021 Issues with combining incompatible and sterile insect techniques Nature 590 E1 E2
Y Li LA Baton D Zhang J Bouyer AG Parker AA Hoffmann 2021 Reply to: issues with combining incompatible and sterile insect techniques Nature. 590 E3 E5
ME Helinski AG Parker BG Knols 2009 Radiation biology of mosquitoes Malar J 8 S6
MJ Klowden AO Lea 1979 Humoral inhibition of host-seeking in Aedes aegypti during oocyte maturation J Insect Physiol 25 231 235
R Beach 1979 Mosquitoes: biting behavior is inhibited by ecdysone Science 205 829 831
LD Kramer AT Ciota 2015 Dissecting vectorial capacity for mosquito-borne viruses Curr Opin Virol 15 112 118
G Demirbas-Uzel L Vooght De AG Parker MJ Vreysen RL Mach J Abbeele Van Den 2018 Combining paratransgenesis with SIT: impact of ionizing radiation on the DNA copy number of Sodalis glossinidius in tsetse flies BMC Microbiol 18 160
F Balestrino A Puggioli R Bellini D Petric J Gilles 2014 Mass production cage for Aedes albopictus (Diptera: Culicidae) J Med Entomol 51 155 163
P Lu G Bian X Pan Z Xi 2012 Wolbachia induces density-dependent inhibition to dengue virus in mosquito cells PLoS Negl Trop Dis. 6 e1754
PA Ross SA Ritchie JK Axford AA Hoffmann 2019 Loss of cytoplasmic incompatibility in Wolbachia-infected Aedes aegypti under field conditions PLoS Negl Trop Dis. 13 e0007357
SV Mayer RB Tesh N Vasilakis 2017 The emergence of arthropod-borne viral diseases: a global prospective on dengue, chikungunya and Zika fevers Acta Trop 166 155 163
A Wilder-Smith DJ Gubler SC Weaver TP Monath DL Heymann TW Scott 2017 Epidemic arboviral diseases: priorities for research and public health Lancet Infect Dis 17 101 106
F Baldacchino B Caputo F Chandre A Drago A della Torre F Montarsi 2015 Control methods against invasive Aedes mosquitoes in Europe: a review Pest Manag Sci. 71 1471 1485
JE Crawford DW Clarke V Criswell M Desnoyer D Cornel B Deegan 2020 Efficient production of male Wolbachia-infected Aedes aegypti mosquitoes enables large-scale suppression of wild populations Nat Biotechnol 38 482 492
L Mousson K Zouache C Arias-Goeta V Raquin P Mavingui A-B Failloux 2012 The native Wolbachia symbionts limit transmission of dengue virus in Aedes albopictus PLoS Negl Trop Dis. 6 e1989
M Calvitti R Moretti E Lampazzi R Bellini SL Dobson 2014 Characterization of a new Aedes albopictus (Diptera: Culicidae)-Wolbachia pipientis (Rickettsiales: Rickettsiaceae) symbiotic association generated by artificial transfer of the wPip strain from Culex pipiens (Diptera: Culicidae) J Med Entomol 47 179 187
M Calvitti R Moretti AR Skidmore SL Dobson 2012 Wolbachia strain wPip yields a pattern of cytoplasmic incompatibility enhancing a Wolbachia-based suppression strategy against the disease vector Aedes albopictus Parasites Vectors 5 254
R Moretti M Calvitti 2013 Male mating performance and cytoplasmic incompatibility in a wPip Wolbachia trans-infected line of Aedes albopictus (Stegomyia albopicta) Med Vet Entomol 27 377 386
R Bellini M Calvitti A Medici M Carrieri G Celli S Maini 2007 Use of the sterile insect technique against Aedes albopictus in Italy: first results of a pilot trial MJB Vreysen J Hendrichs AS Robinson Eds Area-wide control of insect pests Springer Dordrecht, The Netherlands 505 515
M-J Lau NM Endersby-Harshman JK Axford SA Ritchie AA Hoffmann PA Ross 2020 Measuring the host-seeking ability of Aedes aegypti destined for field release Am J Trop Med and Hyg 102 223 231
SM Rainey P Shah A Kohl I Dietrich 2014 Understanding the Wolbachia-mediated inhibition of arboviruses in mosquitoes: progress and challenges J Gen Virol 95 517 530
H Yamada H Maiga J Juarez DDO Carvalho W Mamai A Ali 2019 Identification of critical factors that significantly affect the dose-response in mosquitoes irradiated as pupae Parasites Vectors 12 435
M Calvitti F Marini A Desiderio A Puggioli R Moretti 2015 Wolbachia density and cytoplasmic incompatibility in Aedes albopictus: concerns with using artificial Wolbachia infection as a vector suppression tool PloS One. 10 e0121813
W Zhou F Rousset S O’Neill 1998 Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences Proc Royal Soc B 265 509 515
R Moretti P-S Yen V Houé E Lampazzi A Desiderio A-B Failloux M Calvitti 2018 Combining Wolbachia-induced sterility and virus protection to fight Aedes albopictus-borne viruses PLoS Negl Trop Dis. 12 e0006626
C Braquart-Varnier M Altinli R Pigeault FD Chevalier P Greve D Bouchon 2015 The mutualistic side of Wolbachia-isopod interactions: Wolbachia mediated protection against pathogenic intracellular bacteria Front Microbiol 6 1388
MV Mancini CS Herd TH Ant SM Murdochy SP Sinkins 2020 Wolbachia strain wAu efficiently blocks arbovirus transmission in Aedes albopictus PLoS Negl Trop Dis. 14 e0007926
T Farjana N Tuno 2013 Multiple blood feeding and host-seeking behavior in Aedes aegypti and Aedes albopictus (Diptera: Culicidae) J Med Entomol 50 838 846
MJ Klowden AO Lea 1979 Abdominal distention terminates subsequent host-seeking behaviour of Aedes aegypti following a blood meal J Insect Physiol 25 583 585
MJ Klowden 1997 Endocrine aspects of mosquito reproduction Arch Insect Biochem Phys Publ Collab Entomol Soc Am 35 491 512
MJ Klowden 1981 Initiation and termination of host-seeking inhibition in Aedes aegypti during oocyte maturation J Insect Physiol 27 799 803
OJ Brady MA Johansson CA Guerra S Bhatt N Golding DM Pigott 2013 Modelling adult Aedes aegypti and Aedes albopictus survival at different temperatures in laboratory and field settings Parasites Vectors 6 351
OJ Brady HC Godfray AJ Tatem PW Gething JM Cohen FE McKenzie 2016 Vectorial capacity and vector control: reconsidering sensitivity to parameters for malaria elimination Trans R Soc Trop Med Hyg 110 107 117
A Catano-Lopez D Rojas-Diaz H Laniado S Arboleda-Sánchez MA Puerta-Yepes DP Lizarralde-Bejarano 2019 An alternative model to explain the vectorial capacity using as example Aedes aegypti case in dengue transmission Heliyon. 5 e02577
SL Wu CHM Sánchez JM Henry DT Citron Q Zhang K Compton 2020 Vector bionomics and vectorial capacity as emergent properties of mosquito behaviors and ecology PLoS Comput Biol. 16 e1007446
BT Beerntsen AA James BM Christensen 2000 Genetics of mosquito vector competence Microbiol Mol Biol Rev 64 115 137
KN Johnson 2015 The impact of Wolbachia on virus infection in mosquitoes Viruses 7 5705 5717
PA Ross I Wiwatanaratanabutr JK Axford VL White NM Endersby-Harshman AA Hoffmann 2017 Wolbachia infections in Aedes aegypti differ markedly in their response to cyclical heat stress PLoS Pathog. 13 e1006006
I Wiwatanaratanabutr P Kittayapong 2009 Effects of crowding and temperature on Wolbachia infection density among life cycle stages of Aedes albopictus J Invertebr Pathol 102 220 224
IJH Foo AA Hoffmann PA Ross 2019 Cross-generational effects of heat stress on fitness and Wolbachia density in Aedes aegypti mosquitoes Trop Med Infect Dis 4 13
SL Dobson K Bourtzis HR Braig BF Jones W Zhou F Rousset 1999 Wolbachia infections are distributed throughout insect somatic and germ line tissues Insect Biochem Mol Biol 29 153 160
F Confalonieri S Sommer 2011 Bacterial and archaeal resistance to ionizing radiation J Phys Conf Ser 261 012005
A Bakri K Mehta DR Lance 2005 Sterilizing insects with ionizing radiation VA Dyck J Hendrichs AS Robinson Eds Sterile insect technique. Principles and practice in area-wide integrated pest management Springer Dordrecht, The Netherlands 233 268
HJ Shim EM Lee LD Nguyen J Shim YH Song 2014 High-dose irradiation induces cell cycle arrest, apoptosis, and developmental defects during Drosophila oogenesis PLoS One. 9 e89009
G Rogers P Marsh A Stressmann C Allen T Daniels M Carroll 2010 The exclusion of dead bacterial cells is essential for accurate molecular analysis of clinical samples Clin Microbiol Infect 16 1656 1658
G Iyer AR Wang SR Brennan S Bourgeois E Armstrong P Shah 2017 Identification of stable housekeeping genes in response to ionizing radiation in cancer research Sci Rep 7 43763
ARI Lindsey T Bhattacharya RW Hardy ILG Newton 2021 Wolbachia and virus alter the host transcriptome at the interface of nucleotide metabolism pathways mBio. 12 e03472 20
T Chouin-Carneiro TH Ant C Herd F Louis AB Failloux SP Sinkins 2020 Wolbachia strain wAlbA blocks Zika virus transmission in Aedes aegypti Med Vet Entomol 34 116 119
DA Kheirallah LM El-Samad 2020 Midgut cells alteration in gamma-irradiated beetles (Blaps polycresta, Coleoptera: Tenebrionidae) Braz J Biol 80 465 473
GA Cangelosi JS Meschke 2014 Dead or alive: molecular assessment of microbial viability Appl Environ Microbiol 80 5884 5891
EH Mayton AR Tramonte HJ Wearing RC Christofferson 2020 Age-structured vectorial capacity reveals timing, not magnitude of within-mosquito dynamics is critical for arbovirus fitness assessment Parasites Vectors 13 310
J Velázquez-Castro A Anzo-Hernández B Bonilla-Capilla M Soto-Bajo A Fraguela-Collar 2018 Vector-borne disease risk indexes in spatially structured populations PLoS Negl Trop Dis. 12 e0006234
P Koskinioti AA Augustinos DO Carvalho M Misbah-Ul-Haq G Pillwax LD de la Fuente G Salvador-Herranz RA Herrero K Bourtzis 1818 Genetic sexing strains for the population suppression of the mosquito vector Aedes aegypti Philos Trans R Soc B Biol Sci. 2021 20190808
DE Pagendam BJ Trewin N Snoad SA Ritchie AA Hoffmann KM Staunton C Paton N Beebe 2020 Modelling the Wolbachia incompatible insect technique: strategies for effective mosquito population elimination BMC Biol 18 161
Acknowledgements
We thank Marta Piscitelli (Division for Health Protection Technologies, ENEA-Casaccia Research Center, Rome, Italy), who was responsible for the facility for the housing and care of the mice used for blood-feeding. We also thank Alessia Cappelli (School of Biosciences and Medical Veterinary, University of Camerino, MC, Italy) for her assistance with the FISH analysis and Alessia Fiore (Biotechnology and Agroindustry Division, ENEA Casaccia Research Center, Rome, Italy) for her contribution to the normalization of the qPCR data through the measurement of the DNA content in the extracts from the ovarian tissues.
Funding
The authors received no specific funding for this work.
Author information
Authors and Affiliations
Contributions
RM and MC conceptualized the research. The methodology was planned by RM, EL, MC, AD, CD, and CP. RM, EL, GL, CD, CP, AD, and MC conducted the investigation. RM, EL, GF, and MC organized and managed the datasets. RM, GF, and MC conducted the statistical analysis of the data. MC and AS took care of funding acquisition. MC and CD supervised the experimentation. RM wrote the original draft of the manuscript. RM, MC, CD, EL, AS, GF, and GL contributed to writing, reviewing, and editing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Blood meals were provided via anesthetized mice in agreement with the Bioethics Committee for Animal Experimentation in Biomedical Research and in accordance with procedures approved by the ENEA Bioethical Committee according to the EU directive 2010/63/EU. The mice belonged to a colony housed at CR ENEA Casaccia and maintained for experimentation based on the authorization N. 80/2017-PR released (on February 2, 2017) by the Italian Ministry of Health. Feeding female mosquitoes on the blood of human hosts (i.e., the authors RM, EL, GL, and MC) during the experiments was also approved by the ENEA Bioethical Committee.
Consent for publication
Two of the authors (EL and MC) are present in Additional file 1: Figure S1 and consent to the publication of the image.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Schematic of host-seeking behavior trials conducted under large enclosures
Additional file 2: Figure S2.
Actin gene copies in the ovaries of Ae. albopictus females irradiated at 45 Gy in comparison with untreated counterparts
Additional file 3: Figure S3.
Host-seeking and biting behavior of irradiated SANG and ARwP Ae. albopictus under large enclosures compared to untreated controls. Biting proportions and average times to landing were compared between treatments within a 15-min interval. a Comparison between untreated and irradiated starved females aged 13 ± 1 days; b comparison between irradiated and untreated engorged females 48 h after the engorgement (i.e., 15 ± 1 days old). Two-way ANOVA demonstrated that the difference between treatments was statistically significant in the case of the engorged females (P < 0.05).
Additional file 4: Figure S4.
Structural damage induced by irradiation at 45 Gy in the ovaries of 13 ± 1-day-old Ae. albopictus females in bright field. a Ovaries of untreated SANG females; b ovaries of irradiated SANG females; c ovaries of untreated ARwP females; d ovaries of irradiated ARwP females.
Additional file 5: Figure S5.
FISH analysis of the ovaries of 13 ± 1-day-old Ae. albopictus belonging to a line (AR) cured of Wolbachia infection. a and d DAPI-stained; b and e FITC-stained; c and f bright field. No specific green-fluorescent signal was detected in the aposymbiotic line.
Additional file 6: Figure S6.
Additional images related to the FISH analysis of the ovaries of 13 ± 1-day-old SANG Ae. albopictus irradiated at 45 Gy. The distribution of Wolbachia is evidenced in green, while the blue stain is DAPI. a and d DAPI-stained; b and e FITC-stained; c and f bright field. The green-fluorescent signal related to Wolbachia is weak and not homogeneously distributed.
Additional file 7: Figure S7.
Additional images related to the FISH analysis of the ovaries of 13 ± 1-day-old ARwP Ae. albopictus irradiated at 45 Gy. The distribution of Wolbachia is evidenced in green, while the blue stain is DAPI. a and d DAPI-stained; b and e FITC-stained; c and f bright field. The green-fluorescent signal related to Wolbachia is weak and not homogeneously distributed.
Additional file 8: Figure S8.
Additional images related to the FISH analysis of the ovaries of 13 ± 1-day-old untreated ARwP Ae. albopictus. Blue stain is DAPI. a and d DAPI-stained; b and e FITC-stained; c and f bright field. The green-fluorescent signal related to Wolbachia is strong and regularly distributed.
Additional file 9: Figure S9.
Additional images related to the FISH analysis of the ovaries of 13 ± 1-day-old untreated SANG Ae. albopictus. Blue stain is DAPI. a and d DAPI-stained; b and e FITC-stained; c and f bright field. The green-fluorescent signal related to Wolbachia is strong and regularly distributed.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Moretti, R., Lampazzi, E., Damiani, C. et al. Increased biting rate and decreased Wolbachia density in irradiated Aedes mosquitoes. Parasites Vectors 15, 67 (2022). https://doi.org/10.1186/s13071-022-05188-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-022-05188-9