Abstract
Background
In Iran, both cutaneous leishmaniases (CL) and visceral leishmaniases (VL) are endemic, recording one of the 10 highest CL prevalence in the world. Parasites are transmitted by the bite of infected Phlebotomus sand fly females. Several sand fly species have been identified as vectors in the studied region of Kerman province. Residual spraying to control adult sand flies, is the only way to decrease the spreading of the diseases but, following control treatment against malaria vectors in endemic areas in Iran, resistance or tolerance to insecticides emerged in some sand fly species. The objective of this study was to survey insecticides susceptibility levels of 3 vector species in wild sand fly populations in different foci of the diseases in Kerman province. Ph. sergenti, and Ph. papatasi respectively vectors of anthroponotic and zoonotic cutaneous leishmaniases and for the first time Ph. alexandri one of the anthroponotic visceral leishmaniases vector were tested against: deltamethrin 0.05%, malathion 5%, dichloro-diphenyl-trichloroethane (DDT) 4%.
Materials and methods
In leishmaniases endemic areas species specific sand fly sites were selected in Kerman province, and specimens were collected by manual aspirators at different time intervals during the spring and summer 2019. All the susceptibility tests were performed according to the WHO tube test recommended procedure.
Results
Twenty five blood-fed female sand flies from the region's prevalent species were used in each pooled test replicates. All wild specimens died within 60 min of exposure to DDT 4%, malathion 5%, and deltamethrin 0.05%, but the mortality rate for Ph. papatasi exposed to malathion and DDT was 91.6% and 66.6%, respectively.
Conclusion
According to current study results, Ph. sergenti and Ph. alexandri are highly susceptible to all the evaluated insecticides in the study areas. However, Ph. papatasi was susceptible to deltamethrin (100% mortality), possibly resistant or tolerant to malathion (91.6% mortality), and confirmed to be resistant to DDT (66.6% mortality).
Graphical Abstract

Similar content being viewed by others
Background
Two types of leishmaniasis, cutaneous leishmaniasis (CL) and visceral leishmaniasis (VL), are endemic in Iran. Iran ranks among the 10 countries with the highest prevalence of CL worldwide, which is caused by Leishmania tropica and Leishmania major (Kinetoplastida: Trypanosomatidae) that are transmitted by the bite of infected female sand flies Phlebotomus sergenti and Phlebotomus papatasi (Diptera: Psychodidae), respectively [1]. Phlebotomus sergenti is the primary vector of anthroponotic CL (ACL), and Ph. papatasi is the main vector of zoonotic CL (ZCL) in Iran. ACL is endemic in some regions of Iran where it is considered to be anthroponotic, with the route of transmission from human to human [2, 3]. Most cases of CL in Iran are ZCL, which is endemic in many rural areas of 18 out of 31 provinces in the country, thus representing a significant public health problem [4,5,6]. VL, or kala-azar as it is referred to locally, has a very high mortality rate in the absence of timely diagnosis and treatment. VL is endemic in some foci of the country, including the provinces of North Khorasan, East Azerbaijan, Ardabil, Fars, Qom, Bushehr and Kerman. Leishmania infantum and Leishmania donovani have been reported using parasitological and molecular techniques [7, 8]. Three species of sand flies in southern Iran, namely Phlebotomus major, Phlebotomus keshishiani and Phlebotomus alexandri, and three species in the northwest and northeast regions of the country, namely Phlebotomus kandelakii, Phlebotomus transcucasicus perfiliewi and Phlebotomus tobbi, are reported to be the vectors of the disease [9]. In the southeast of Iran, massive spraying during the malaria eradication era resulted not only in a significant reduction in the number of malaria vectors in endemic areas, but also those for leishmaniasis . However, the annual incidence of leishmaniasis is increasing, and active foci of the disease are observed in both smal and large cities of Iran [10].
Several control methods are available to control sand flies, with an emphasis on insecticides. Due to the inaccessibility of the larval habitats of sand flies, it is impossible to control their larvae, so researchers have focused their efforts on the control of the adult sand flies. One of the approaches used is the application of chemical methods, such as residual spraying of indoor places and insecticide-impregnated mosquito nets [11, 12]. In some parts of the world, cases of resistance or tolerance of sand flies to insecticides have been reported [11]. In Iran, residual spraying with dichlorodiphenyltrichloroethane (DDT) against malaria vectors began in 1947 in most of the malaria-endemic areas. In 1957, insecticide resistance against DDT was reported in Anopheles stephensi [13, 14]. Rashti et al. investigated the susceptibility of Ph. papatasi to DDT in different agricultural fields of Iran between 1985 and 1988, and their results showed that sand flies in some areas of Isfahan Province had developed more tolerance to this insecticide [15]. Yaghoobi-Ershadi et al. subsequently confirmed this tolerance to DDT in Isfahan Province [4]. In 2012, Saeidi et al. evaluated and reported the level of susceptibility of Ph. papatasi species in the Badrood region of Isfahan Province to DDT and pyrethroids [14]. Aghaei Afshar et al. in 2011 declared that Ph. papatasi and Ph. sergenti were susceptible to DDT and deltamethrin in the Dehbakri area of Bam City [15]. Due to the lack of complete information on the susceptibility of sand flies in endemic areas of leishmaniasis to insecticides at the differential concentrations specified by WHO, which are recommended for periodic monitoring of insecticide resistance, we planned the present study. The objective was to survey current susceptibility levels of Ph. sergenti, the vector of urban leishmaniasis, Ph. papatasi, the vector of rural leishmaniasis and Ph. alexandri, the vector of VL, to the insecticides deltamethrin 0.05%, malathion 5% and DDT 4%, in wild sand fly populations during the season of highest activity in different foci of these diseases in Kerman province in 2019. Using the results of such studies, the WHO can develop specific guidelines for the control of sand flies, and such guidelines can be used to study and monitor resistance to insecticides, with the aim to implement measures to combat vectors in countries, including Iran.
Methods
Study areas
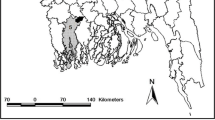
To evaluate the susceptibility of sand flies in different foci of Kerman province, we chose Bam district as the focus of ACL, Orzouieh district (Soltan Abad) as the focus of ZCL and the south of Baft district (Goushk) as the focus of VL [3, 16]. Bam district is geographically located in the southeast of Kerman Province and has a dry climate; Dehbakri, a small town surrounded by villages, was selected for sampling. Baft district is located in the southwest of Kerman Province, southeastern Iran, and has a cold climatic zone. Arzooieh district is located 125 km south of Baft district and has a hot and relatively humid climate (Fig. 1).
Sand fly collection
Sand flies were collected by manual aspirator at different time intervals in study areas (Fig. 1) during the spring and summer of 2019. The collected live sand flies were transferred to cages kept under a wet towel. Adults were fed with 10% sucrose solution soaked on cotton pads, and the cages were transported to the laboratory at Kerman Leishmaniasis Research Center where the sand flies were maintained in the insectary at 27 ± 2 °C, 60 ± 10% relative humidity and a photoperiod of 14:10 h (light: dark).
A 10% sucrose solution was provided during the recovery period.
Insecticides and susceptibility tests
Studies were conducted with the following insecticides: DDT 4.0% (batch number DD 265; expiry date: July 2022), deltamethrin 0.05% (batch number DE 381; expiry date: August 2019) and malathion 5.0% (batch number MA 234; expiry date: July 2020). The WHO provided the insecticide-impregnated test papers, and all susceptibility tests were conducted according to WHO tube-test guidelines [17].
During the tests, the wild sand flies were transferred into the holding tube, which was marked with a green dot. The exposure tubes were marked with a red dot and were lined with insecticide-impregnated test paper for different time durations (1.75, 3.5, 7, 15, 30 and 60 min). The holding tubes were transferred to the insectary for 24 h at 28 ± 2 °C, a photoperiod of 12:12 h (light:dark) and 75 ± 5% relative humidity. During the holding time, the sand flies were supplied with 20% fresh sugar solution on cotton pads. The mortality was recorded after a 24-h recovery period. Abbott’s correction formula was used to correct all mortalities compared to the control results (between 5 and 20%) [18]. The bioassay tests with a control mortality rate of > 20% were repeated. After each test, all sand flies (live and dead) were stored in 70% alcohol and subsequently mounted in a drop of Puri’s medium and identified by their morphological characteristics using a standard taxonomic key [19].
Statistical analysis
The median lethal time causing 50% mortality (LT50) and median lethal time causing 90% mortality (LT90) of sand flies, regression equation and chi-square values were determined by probit analysis (Finney’s method) [20]. Data analysis was performed using SPSS software version 20 (SPSS IBM Corp., Armonk, NY, USA). The graphs were designed with Excel 2016 (Microsoft Corp., Redmond, WA, USA).
Results
The susceptibility bioassay tests on wild specimens of 25 blood-fed females of Ph. papatasi, Ph. sergenti and Ph. alexandri sand flies collected at the study sites in Kerman province were performed with different insecticide-impregnated papers, as shown in Tables 1, 2 and 3. The LT50 and LT90 values with their respective 95% CI for each species exposed to insecticides are given. The regression lines for mortality of these three sand fly species exposed to the three insecticides used are plotted against exposure times. Figures 2, 3 and 4, show the regression lines for each species separately when exposed to the three insecticides. All wild specimens died within 60 min of exposure to DDT 4%, malathion 5% and deltamethrin 0.05%; however, the mortality rate for Ph. papatasi following exposure to malathion and DDT was 91.6% and 66.6%, respectively.
Discussion
In order to plan for spraying endemic areas of leishmaniasis for control of sand flies and to consider the use of effective pesticides to protect the environment, the extent of insecticide susceptibility and resistance in sand fly vectors should be periodically reviewed. Because there are no available test procedures for monitoring insecticide resistance in sand flies, in the present study we used WHO procedures for malaria vectors. In accordance with the WHO guideline, we classified the bioassay results into three resistance classes: (i) 98–100% mortality, indicating susceptibility; (ii) 90–97% mortality, indicating resistance candidate and that more investigation is needed to confirm resistance; and (iii) mortality < 90%, indicating resistance [17].
Based on the results of the present study, Ph. sergenti and Ph. alexandri in the study areas are highly susceptible to the three insecticides tested. However, Ph. papatasi was found to be susceptible to deltamethrin (100% mortality), possibly resistant or tolerant to malathion (91.6% mortality) and resistant to DDT (66.6% mortality), with a higher susceptibility to malathion than to deltamethrin and DDT. Phlebotomus sergenti, although susceptible to all three insecticides tested, was more sensitive to DDT than to malathion and deltamethrin. In comparison, Ph. alexandri needed more time than Ph. sergenti to be killed at the same concentration of DDT in the study area at the LT50 level. Nevertheless, Ph. alexandri was found to be more susceptible to malathion and deltamethrin than Ph. sergenti and Ph. papatasi; Ph. sergenti was found to be more susceptible to malathion than Ph. papatasi to malathion; and Ph. papatasi was more susceptible to deltamethrin than Ph. sergenti.
Since the 1950s, malaria has been endemic in the south of Kerman province, and DDT and malathion have been employed in indoor residual spraying programs to combat the disease [13]. Sand flies have developed resistance to DDT and other pesticides due to spraying against malaria vectors [21].
In Iran, tolerance (existence resistance) of Ph. papatasi to DDT has been reported in the sand fly populations from Isfahan province [3]; however, this result showed that Ph. papatasi from Isfahan was more tolerant to DDT than Ph. papatasi from our study. Surveying the susceptibility status of Ph. papatasi showed that this species is also resistant to DDT in CL foci in western regions of Iran [22]. Moreover, resistance to DDT was reported in Ph. sergenti in the endemic focus of CL in northern Iran [23]. Also, it seems that Ph. kandelakii and Ph. perfiliewi are possibly resistant to DDT in the VL endemic foci in northwestern Iran [24]. There have been reports of insecticide resistance in phlebotomine sand flies in a number of parts of the world; for example resistance to DDT was reported for Ph. papatasi in India [25] and for Ph. argentipes in India and Nepal [26]. The emergence of resistance to DDT was also shown in Sergentomyia shorttii in India [27]. In the present study, 91.6% mortality indicated possible resistance to malathion in Ph. papatasi, similar to the possible resistance to malathion reported in Ph. kandelakii and Ph. perfiliewi in northwestern parts of Iran [24].
Leishmaniasis has long been present in the area, and insecticides have been employed to combat the disease’s vectors [28]. After the devastating earthquake in this area, insecticides have significantly been the main reason for the emergence of resistant species. Moreover, surveys of Ph. papatasi in the western parts of Iran showed that this species is possibly resistant to deltamethrin, permethrin and bendiocarb [22]. Given that there is no guideline on susceptibility tests in phlebotomine sand flies, the results of our study, based on guidelines for malaria vectors, indicate that more investigation is needed to confirm resistance in those species with < 90% mortality against a pesticide in our study.
Conclusion
The findings of the present study indicate that resistance to DDT is emerging in Ph. papatasi, which could be an important factor for any future vector control program in the study area, as evidenced by several reports on different aspects of leishmaniasis in the country [28,29,30,31,32]. Our results provide a guideline for the Ministry of Health to control disease in different parts of the country.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE. 2012;7: e35671.
Afshar AA, Hakimi Parizi M, Sharifi I, Amin Gorouhi M, Sharifi F, Baafi B, et al. Evaluation of the ecological characteristics in the vector of anthroponotic cutaneous leishmaniasis in a new focus of Mohammad Abad, Kerman, southeast of Iran. Asian Pac J Trop Dis. 2017;7:84–7.
Sharifi I, Aflatoonian MR, Fekri ARA, Hakimi Parizi M, Aghaei Afshar A, Khosravi A, et al. A comprehensive review of cutaneous leishmaniasis in Kerman Province, southeastern Iran—narrative review article. Iran J Public Health. 2015;44:299.
Yaghoobi-Ershadi MR, Javadian E. Susceptibility status of Phlebotomus papatasi to DDT in the most important focus of zoonotic cutaneous leishmaniasis, Isfahan Province, Iran. Iran J Public Health. 1995;24:11–20.
Yaghoobi-Ershadi M, Hanafi-Bojd A, Akhavan A, Zahrai-Ramazani A, Mohebali M. Epidemiological study in a new focus of cutaneous leishmaniosis due to Leishmania major in Ardestan town, central Iran. Acta Trop. 2001;79:115–21.
Akhavan A, Yaghoobi-Ershadi M, Hasibi F, Jafari R, Abdoli H, Arandian M, et al. Emergence of cutaneous leishmaniasis due to Leishmania major in a new focus of southern Iran. J Arthropod Borne Dis. 2007;1:1–8.
Arzamani K. Visceral leishmaniasis in North Khorasan province, north east of Iran. Int J Infect Dis. 2012;16:e340–1.
Moradi-Asl E, Rassi E, Hanafi-Bojd AA, Saghafipour A. Spatial distribution and infection rate of leishmaniasis vectors (Diptera: Psychodidae) in Ardabil Province, northwest of Iran. Asian Pac J Trop Biomed. 2019;9:181–7.
Moradi-Asl E, Rassi Y, Adham D, Hanafi-Bojd AA, Saghafipour A, Rafizadeh S. Spatial distribution of sand flies (Diptera: Psychodidae; Larroussius group), the vectors of visceral leishmaniasis in Northwest of Iran. Asian Pac J Trop Biomed. 2018;8:425–30.
Sabzevari S, Teshnizi SH, Shokri A, Bahrami F, Kouhestani F. Cutaneous leishmaniasis in Iran: a systematic review and meta-analysis. Microb Pathog. 2021;152:104721.
Balaska S, Fotakis EA, Chaskopoulou A, Vontas J, Guizani I. Chemical control and insecticide resistance status of sand fly vectors worldwide. PLoS Negl Trop Dis. 2021;15:e0009586.
World Health Organization (WHO). WHO releases new guidance on insecticide-treated mosquito nets. 2007. https://www.who.int/news/item/15-08-2007-who-releases-new-guidance-on-insecticide-treated-mosquito-nets. Accessed 22 Dec 2021.
Rashti MA, Panah HY, Mohamadi HS, Jedari M. Susceptibility of Phlebotomus papatasi (Diptera: Psychodidae) to DDT in some foci of cutaneous leishmaniasis in Iran. J Am Mosq Contr Assoc. 1992;8:99–100.
Saeidi Z, Vatandoost H, Akhavan AA, Yaghoobi-Ershadi MR, Rassi Y, Sheikh Z, et al. Baseline susceptibility of a wild strain of Phlebotomus papatasi (Diptera: Psychodidae) to DDT and pyrethroids in an endemic focus of zoonotic cutaneous leishmaniasis in Iran. Pest Manag Sci. 2012;68:669–75.
Afshar AA, Rassi Y, Sharifi I, Abai M, Oshaghi M, Yaghoobi-Ershadi M, et al. Susceptibility status of Phlebotomus papatasi and P. sergenti (Diptera: Psychodidae) to DDT and deltamethrin in a focus of cutaneous leishmaniasis after earthquake strike in Bam, Iran. Iran J Arthropod Borne Dis. 2011;5:41.
Sharifi I, Aflatoonian MR, Daei Parizi MH, Hosseininasab A, Mostafavi M, Bamorovat M, et al. Visceral leishmaniasis in Southeastern Iran: a narrative review. Iran J Parasitol. 2017;12:1–11.
World Health Organization (WHO). Test procedures for insecticide resistance monitoring in malaria vectors, bio-efficacy and persistence of insecticides on treated surfaces: report of the WHO informal consultation, Geneva, 28–30 September 1998. https://apps.who.int/iris/handle/10665/64879. Accessed 24 Aug 2021.
Abbott WS. A method of computing the effectiveness of an insecticide. J Econ Entomol. 1925;18:265–7.
Theodor O, Mesghali A. On the Phlebotominae of Iran. J Med Entomol. 1964;1:285–300.
Finney DJ. Probit analysis. III. Cambridge: Cambridge University Press; 1971.
Nezamzadeh-Ezhiyeh H, Mirhendi H, Jafari R, Veysi A, Rassi Y, Oshaghi MA, et al. An eco-epidemiological study on zoonotic cutaneous leishmaniasis in central Iran. Iran J Public Health. 2021;50:350.
Rassi Y, Asadollahi H, Abai MR, Kayedi MH, Vatandoost H. Efficiency of two capture methods providing live sand flies and assessment the susceptibility status of Phlebotomus papatasi (Diptera: Psychodidae) in the foci of cutaneous leishmaniasis, Lorestan Province, western Iran. J Arthropod Borne Dis. 2020;14:408.
Arzamani K, Vatandoost H, Rassi Y, Abai MR, Akhavan AA, Alavinia M, et al. Susceptibility status of wild population of Phlebotomus sergenti (Diptera: Psychodidae) to different imagicides in a endemic focus of cutaneous leishmaniasis in northeast of Iran. J Vector Borne Dis. 2017;54:282.
Rassi Y, Moradi-Asl E, Vatandoost H, Abazari M, Saghafipour A. Insecticide susceptibility status of wild population of Phlebotomus kandelakii and Phlebotomus perfiliewi transcaucasicus collected from visceral leishmaniasis endemic foci in Northwestern Iran. J Arthropod Borne Dis. 2020;14:277.
Joshi G, Kaul S, Wattal B. Susceptibility of sandflies to organochlorine insecticides in Bihar (India)-further reports. J Commun Dis. 1979;11:209–13.
Dinesh DS, Das ML, Picado A, Roy L, Rijal S, Singh SP, et al. Insecticide susceptibility of Phlebotomus argentipes in visceral leishmaniasis endemic districts in India and Nepal. PLoS Negl Trop Dis. 2010;4: e859.
Kaul SM, Sharma RS, Borgohain BK. D.D.T. resistance in Sergentomyia shorttii (Diptera: Psychodidae) in Kamrup, Assam–first report in Sergentomyia genus. J Commun Dis. 1994;26:100–2.
Saeidi Z, Vatandoost H, Akhavan A, Yaghoobi-Ershadi M, Rassi Y, Arandian M, et al. Baseline insecticide susceptibility data of Phlebotomus papatasi in Iran. J Vector Borne Dis. 2013;50:57–61.
Rassi Y, Jalali M, Vatandoost H. Susceptibility status of Phlebotomus papatasi to DDT in Arsanjan country in Fars province. Iran Iran J Public Health. 2000;29:21–3.
Shirani-Bidabadi L, Zahraei-Ramazani A, Yaghoobi-Ershadi MR, Rassi Y, Akhavan AA, Oshaghi MA, et al. Assessing the insecticide susceptibility status of field population of Phlebotomus papatasi (Diptera: Psychodidae) in a hyperendemic area of zoonotic cutaneous leishmaniasis in Esfahan Province. Central Iran Acta Trop. 2017;176:316–22.
Rassi Y, Ebrahimi S, Abai MR, Vatandoost H, Akhavan AA, Afshar AA. Comparative testing of susceptibility levels of Phlebotomus sergenti, the main vector of anthroponotic cutaneous leishmaniasis, to conventional insecticides using two capture methods in Kerman City, Southeastern Iran. J Arthropod Borne Dis. 2021;15:82–96.
Razavi MR, Shirzadi MR, Mohebali M, Yaghoobi-Ershadi MR, Vatandoost H, Shirzadi M, et al. Human cutaneous leishmaniosis in Iran, up to date—2019. J Arthropod-Borne Dis. 2021;15:143–51.
Acknowledgements
This study results from a research project approved by the Vice-Chancellor for Research and Technology of Kerman University of Medical Sciences.
Funding
Kerman University of Medical Sciences supported this investigation under Grant Number 95/114.
Author information
Authors and Affiliations
Contributions
YSA analyzed and interpreted the data and drafted the manuscript. ASD analyzed the data. MHP and AAA conceived and designed the study and reviewed the manuscript. IS reviewed and edited the manuscript. MAG supervised the field and laboratory experiment. LSB and IA collected samples. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Vice-Chancellor for Research and Technology of Kerman University of Medical Sciences (ethics code IR.KMU.REC.1394.102).
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Salim Abadi, Y., Sanei-Dehkordi, A., Hakimi Parizi, M. et al. Baseline susceptibility of a wild strain of main vectors of leishmaniasis to WHO-recommended insecticides in southeastern Iran. Parasites Vectors 15, 42 (2022). https://doi.org/10.1186/s13071-022-05154-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-022-05154-5