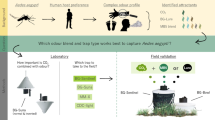
Abstract
Background
Entomological surveillance is an important means of assessing the efficacy of insect vector management programs and estimating disease transmission thresholds. Among baited traps, Biogents’ BG-Sentinel (BGS) trap baited with BG-Lure is considered to have the most similar outcome to, and be a possible replacement for, human-landing catches for the epidemiologically relevant monitoring of adult Aedes aegypti and Culex quinquefasciatus. In contrast to the BGS trap, the Black Hole ultraviolet (UV) light trap, which is widely used to catch nocturnal flying insects, is not baited with synthetic human odor-mimicking lures.
Methods
We evaluated the l-lactic acid-based Kasetsart University (KU)-lures nos. 1–6 as novel candidate chemical lures for the diurnal species Ae. aegypti and the nocturnal species Cx. quinquefasciatus using two commercial traps (the BGS trap and the Black Hole UV light trap) in a semi-field screen (SFS) house. Firstly, we optimized the dose of each KU-lure in an SFS house (140 m3). Secondly, six different candidate KU-lures were screened by comparing their percent attraction using a single discriminating dose (0.5 g). Finally, we evaluated the synergism of the KU-lures selected in this way with commercially available traps.
Results
BGS traps baited with KU-lure no. 1 exhibited the greatest percent attraction for Ae. aegypti (29.5% ± 14.3%), whereas those baited with KU-lure no. 6 most strongly attracted Cx. quinquefasciatus (33.3% ± 10.7%). Interestingly, BGS traps treated with 10 g BG-Lure did not significantly attract more Ae. aegypti or Cx. quinquefasciatus than the untreated BGS traps. CO2 at a flow rate of 250 ml/min most strongly attracted both Ae. aegypti and Cx. quinquefasciatus (42.2% ± 14.2% and 75.1% ± 16.9%, respectively). BGS and Black Hole UV light traps with KU-lure no. 6 exhibited a stronger attraction for Cx. quinquefasciatus than untreated traps, and the percent attraction did not differ between the treated traps.
Conclusions
Synergistic effects of KU-lures nos. 1 and 6 with the mosquito traps were demonstrated for both the diurnal and nocturnal species in the SFS house assays. However, further studies are urgently needed for the development of species-specific lures to increase trap efficacy in the field for local vector mosquitoes in Thailand.
Graphical Abstract

Similar content being viewed by others
Background
Dengue, the most prevalent vector-borne disease of public health importance, occurs in most countries in tropical and subtropical regions [1]. Aedes aegypti is the primary vector of dengue. This mosquito species exhibits highly anthropophilic feeding behavior, and is often found in close association with human dwellings [2, 3]. Typical larval habitats of this species include discarded tires filled with rainwater, flowerpots, empty oil drums, and large and small water storage containers [4]. Despite years of public health control activities and research progress on Ae. aegypti and dengue fever, there is still no commercially available effective and safe dengue vaccine. Prevention of dengue fever remains almost entirely reliant on the use of vector management and control practices, which are the most effective methods for reducing viral transmission in dengue-endemic areas [5]. Despite intensive efforts, the control of Ae. aegypti has proven extremely difficult because it is highly anthropophilic and thus found in close association with humans in domestic and peri-domestic environments. An accurate prediction of the abundance of Ae. aegypti females in a given area is important. One of the sampling techniques used to effectively assess and predict mosquito abundance is trapping [6].
Efforts have been made to develop better and more effective traps as integral components of surveillance systems for the monitoring and evaluation of vector control programs. Combinations of traps and lures have been successfully used for the control of several insect pests and vectors of disease [6, 7]. Devices which trap both adult and immature stages are important tools for the monitoring and surveillance of mosquito density [8, 9]. However, most traps are relatively ineffective for population sampling, and especially for adults of the day-biting Ae. aegypti [10, 11]. To overcome this critical limitation, several new traps have been developed and evaluated. The most promising of these include the BG-Sentinel (BGS) trap (Biogents, Regensburg, Germany). Several studies found that this trap efficiently captured more representative samples of adult Ae. aegypti populations than other traps [6, 7, 12].
Various chemical lures have been developed by using various types of olfactometers [13,14,15,16,17,18,19,20]. However, existing lures are not always effective in attracting Ae. aegypti from populations in different localities [21]. Therefore, lures should be developed using local field populations of species. In this study, we tested a laboratory-scale high-throughput screening system (HITSS) for the selection of candidate Kasetsart University (KU)-lures [22] for further testing in a semi-field screen (SFS) house using standard commercial mosquito traps, namely the BGS trap and the Black Hole ultraviolet (UV) light trap, for important vector mosquito species, including the diurnal Ae. aegypti, nocturnal Culex quinquefasciatus, and Anopheles minimus. In addition, traps with or without light sources were compared to assess their ability to trap Cx. quinquefasciatus. The results of the SFS house assay demonstrated the accuracy of the HITSS assay.
Methods
Mosquitoes
Larvae and pupae of Ae. aegypti were collected from natural or artificial breeding habitats in Pu Tuey Village, Kanchanaburi Province, Thailand (14°77′N, 99°11′E) in January 2019. This field population showed a high phenotypic resistance to 0.25% permethrin, with only 6% mortality, in the World Health Organization (WHO) tube bioassay [23]. Aquatic stages of Cx. quinquefasciatus were collected from sewage near a local restaurant at Thawi Watthana, Bangkok, Thailand (13°77′N, 100°34′E) in March 2020. The results of the WHO bioassay confirmed that permethrin resistance in the Cx. quinquefasciatus used here is moderate, with 60% mortality at 0.75% permethrin [22].
Field-collected individuals of both populations were immediately transferred to the insectary at the Department of Entomology, Faculty of Agriculture, Kasetsart University, Bangkok, Thailand to increase the size of the test populations. Aquatic stages (larvae and pupae) of the field populations were transferred to a field insectarium in Pu Teuy, Kanchanaburi Province, Thailand for the field study. The mosquitoes were reared under the same field insectary conditions (25 ± 5 °C, 80 ± 10% relative humidity, and 12-h:12-h light:dark photoperiod). Newly emerged F2 adults were provided with 10% cotton pads soaked with sugar solution. On each day of the study, newly emerged adults were transferred to a separate screen cage [(30 × 30 × 30 cm, length (L) × width (W) × height (H)] so that the females’ ages could be determined. To increase the size of the population, sugar-fed F2 males and females were allowed to mate naturally in the screen cages for several days. Five-day-old females were blood-fed twice per week using a membrane feeding system with expired human blood obtained from the Thai Red Cross Society (Bangkok, Thailand). Two days post-blood-feeding, 10-cm-diameter oviposition dishes with (for Ae. aegypti) or without (for Cx. quinquefasciatus) moist filter paper were placed in the screen cages to collect the eggs.
Approximately 200–250 eggs were placed in individual plastic rearing trays [30 × 20 × 5 cm, L × W × H] containing clean water. A few days later, granules of Optimum Nishikigoi Carp Fish Food (Perfect Companion, Samutprakarn, Thailand) were provided daily to feed the larvae of both species. Pupae were collected and transferred to the adult rearing cage covered with damp towels for adult emergence. Fifty nulliparous, 3- to 5-day-old sugar-starved females of each species were tested. At least 12 h before each test, the female mosquitoes were marked using fluorescent powder (BioQuip, Rancho Dominquez, CA) to distinguish them from the mosquitoes used in the previous assays and from wild mosquitoes that had accidentally flown in, following the method of Achee et al. [24]. If no dead or knocked-down mosquitoes were observed post-marking, the mosquitoes were considered healthy and used for testing.
Specimens of An. minimus were originally obtained from the Malaria Division, Department of Communicable Disease Control, Ministry of Public Health (Nonthaburi, Thailand) in 1998. This laboratory strain showed 100% mortality in response to permethrin exposure using the WHO bioassay [22]. The larvae were fed powdered Tetramin tropical fish food daily. The pupae were collected and transferred to a screen cage and allowed to develop into adults. The adults were provided with 10% sugar solution as an energy source. All of the mosquito cages were covered with damp towels to retain moisture. Non-blood-fed female mosquitoes (3–5 days old) were starved (provided only with water) for 24 h prior to testing.
Experimental assays
Chemical lures
Four previously described [22] l-lactic-(+)-acid (Chemical Abstracts Service no. 79-33-4) based mixtures and two single compounds labeled as KU-lures (Table 1) were tested in an SFS house assay. A commercial product, namely the BG-Lure (Biogents), and CO2 supplied from a cylinder at a flow rate of 250 ml/min were used as the positive controls. The BG-Lure is available in a 10-g pack and consists of lactic acid, ammonia and caproic acid, which are used to mimic human sweat.
BGS trap
Each tested lure was inserted into a BGS trap (Fig. 1) which was then placed on the ground at approximately 1 m from the wall to enable air to circulate around it. All the traps were operational for the entire experimental period. The diurnal species Ae. aegypti was tested during the daytime (0600–1800 hours), and the nocturnal species Cx. quinquefasciatus was tested during the nighttime (1800–0600 hours).
Black Hole UV light trap
Two Black Hole UV light traps (manufactured from Bio-Trap Inc., Seoul, Korea, and purchased from Pan Science Co., Ltd., Bangkok, Thailand) were suspended 1.5 m above the ground from a chain attached to the top of each SFS cubicle (10 × 4 × 3.5 m, L × W × H] (Fig. 2). The total number of captured mosquitoes was determined after 12 h of collection by KU-lure no. 6-treated and untreated traps during the nighttime (1800–0600 hours).
SFS house assay
The screen house was subdivided into four 10-m-long cubicles separated by folding metal screen partitions. The volume of each cubicle was 140 m3 (4 m × 10 m × 3.5 m; Figs. 1–2), which approximated the volume of the existing SFS facility and the expected volume that Ae. aegypti would primarily use in and around a typical home in a dengue-endemic environment in Thailand. The environmental parameters temperature and relative humidity were measured for each separate cubicle using a hygrometer (Yuequing Xinyang Technology, Yuequing City, Zhejiang Province, China), and did not significantly differ between them (Table 2). This study was conducted from February to July in 2019 and in 2020.
We optimized the dose of the previously selected [22] KU-lure no. 1 (for Ae. aegypti) and KU-lure no. 6 (for Cx. quinquefasciatus) by testing them at five different concentrations in the SFS house cubicles (Table 3). Then, to test the accuracy of the HITSS assay, six different KU-lures at the optimized dose were screened in a SFS house, which was 50,000 times larger in volume than the space used in the laboratory-scale assay (2.75 L). In addition, the percent attraction of lure-treated and untreated traps was compared to assess synergism.
The screen house and traps were regularly cleaned to remove predators that otherwise may have consumed the trapped mosquitoes. Fifty marked healthy females were released in the middle of a single SFS room in which two BGS traps had been placed for Ae. aegypti and for Cx. quinquefasciatus (Fig. 1) or two Black Hole UV light traps had been placed for Cx. quinquefasciatus and An. minimus (Fig. 2) [25]. Eight (n = 400) and nine (n = 450) replicates were used for the BGS traps and Black Hole UV light traps, respectively.
Statistical analysis
Environmental parameters (temperature and relative humidity) data are presented as the mean ± SD for four SFS cubicles as determined using the Kruskal–Wallis H-test for multiple comparisons for nighttime (P < 0.05). Data for daytime were subjected to one-way ANOVA, and means were compared using Tukey’s honest significant difference test at 95% confidence level. The numbers of mosquitoes in lure-treated and untreated traps were compared using Student’s t–test to determine statistically significant differences. The percent attraction was calculated from the obtained values using following formula:
where Nt is the number of mosquitoes captured in baited traps and Nu is the number of mosquitoes in untreated traps. If the tested lure is attractive, the percent attraction is positive. Conversely, a negative value indicates that the lure is not attractive. The species-specific percent attraction of the tested lures was compared using ANOVA. All the statistical analyses were performed with SPSS version 28 (IBM, Armonk, NY). Statistical significance is indicated by P < 0.05.
Results
Optimization assay
Discriminating doses for the SFS house assay were selected for Ae. aegypti and Cx. quinquefasciatus. Five different amounts of KU-lures nos. 1 and 6 (0.1, 0.5, 1.0, 1.5, 2.0 g) and no lure (0 g) were evaluated for each species of mosquito (Table 3). The BGS traps baited with 0.1 g or 0.5 g of KU-lure no. 1 captured significantly more Ae. aegypti than untreated BGS traps [0.1 g, t(14) = −4.891, P = 0.000; 0.5 g, t(14) = −6.654, P = 0.000]. Specifically, BGS traps baited with 0.5 g of KU-lure no. 1 had the highest percent attraction (29.5% ± 14.3%). When no KU-lure was used (control, 0 g), the number of captured Ae. aegypti mosquitoes did not significantly differ between the BGS traps [22.3 ± 2.1 vs 22.9 ± 4.1; t(14) = −0.387, P = 0.705]. BGS traps treated with 1.0 g, 1.5 g or 2.0 g of KU-lure no. 1 captured significantly fewer Ae. aegypti than untreated traps [1.0 g, t(14) = 5.933, P = 0.000; 1.5 g, t(14) = 9.605, P = 0.000; 2.0 g, t(14) = 16.995, P = 0.000]. The results were similar for Cx. quinquefasciatus with KU-lure no. 6, as baiting the traps with 0.1 g or 0.5 g of this lure significantly increased the number of mosquitoes captured [0.1 g, t(14) = −2.327, P = 0.035; 0.5 g, t(14) = −4.390, P = 0.001]. From the results, we selected 0.5 g as the discriminating dose for the 140-m3 SFS house for all candidate KU-lures and tested species. The optimization assay indicated that there was no significant difference among the cubicles for either mean temperature or relative humidity (Table 2).
Screening assay
BGS traps baited with standard BG-Lure, CO2 or KU-lures were evaluated to compare their percent attraction for Ae. aegypti and Cx. quinquefasciatus in the SFS system. The standard BG-Lure at 10 g did not attract either Ae. aegypti or Cx. quinquefasciatus. CO2 at a flow rate of 250 ml/min exhibited the greatest percent attraction for both species. BGS traps baited with CO2 had a significantly higher percent attraction [t(14) = 4.218, P = 0.001] for Cx. quinquefasciatus (75.1% ± 16.9%) than for Ae. aegypti (42.2% ± 14.2%). There was no significant difference [t(14) = 0.591, P = 0.564] in the high attractancy of BGS traps baited with 0.5 g of KU-lure no. 6 for Cx. quinquefasciatus (33.3% ± 10.7%) and those baited with 0.5 g of KU-lure no. 1 for Ae. aegypti (29.5% ± 14.3%). The other candidate KU-lures did not attract mosquitoes, with the exception of KU-lure no. 4, which had a positive percent attraction for Cx. quinquefasciatus, albeit this was not statistically significant relative to the control [t(14) = 1.003, P = 0.333] (Table 4).
Synergism assay
The Black Hole UV light trap treated with the optimized KU-lure no. 6 was evaluated to compare the percent attraction for Cx. quinquefasciatus and An. minimus to that of untreated traps in an SFS house assay. The two species were captured similarly by the UV light trap without lures [Cx. quinquefasciatus, t(16) = 0.585, P = 0.567; An. minimus, t(16) = 0.333, P = 0.743]; the respective capture rates were 74.0% ± 13.2% and 76.4% ± 13.0%. Significantly more Cx. quinquefasciatus mosquitoes were captured (24.9 ± 6.1) when the trap was baited with 0.5 g of KU-lure no. 6 than when the trap was not baited (15.6 ± 5.4) [t(16) = −3.424, P = 0.003]. Although more An. minimus were captured in traps baited with KU-lure no. 6 (23.8 ± 8.7) than in the control (18.9 ± 9.6), the difference was not statistically significant [t(16) = −1.134, P = 0.274]. The capture rates increased for Cx. quinquefasciatus and for An. minimus with KU-lure no. 6, to 80.9% ± 10.2% and 85.3% ± 6.6%, respectively, but the differences were not statistically significant. Overall, although the percent attraction for both species was positive (22.8 ± 25.1% for Cx. quinquefasciatus and 12.4 ± 43.5% for An. minimus), significant result was only found from Cx. quinquefasciatus with the optimized and screened KU-lure no. 6 treated Black Hole UV light trap compared to untreated trap (Table 5).
Discussion
This aim of this study was to optimize lures used to attract mosquitoes for their possible use as integral components of surveillance systems for the monitoring and evaluation of vector control programs. Trap and lure combinations were tested in an SFS house trial with the aim of improving future mosquito surveillance. Trap and lure combinations have been successfully applied to the control of several insect taxa, including mosquitoes [26] and tsetse flies [27]. Although traps are important tools for the surveillance of mosquito abundance [9], most of them are relatively ineffective, and especially so for day-biting mosquitoes such as Ae. aegypti [10, 28].
The BGS trap has been suggested as a potential replacement for human-landing catches in the case of Ae. aegypti [7]. However, the BGS trap baited with the BG-Lure is expensive and, as it is licensed in the USA, cannot be manufactured in lower-income countries, some of which have dengue rates of up to 75%, in particular in Southeast Asia. There are various types of lower priced traps on the market, most of which use UV light-emitting diode (LED) light sources. However, there is little documented scientific evidence of the efficacy of these types of traps. In addition, populations of a mosquito species may respond differently according to their geographical location. To overcome these problems, the local development of lures using field-collected mosquitoes is necessary. In particular, economical and simple methodologies are critical for the development of mosquito attractants in less well-equipped laboratories. Recently, Kim et al. [22, 23] evaluated a potential tool for lure development, the HITSS assay. However, more data are needed to demonstrate its accuracy in larger-scale SFS house set ups.
In the present study, the results of the BGS trap baited with an appropriate amount of the KU-lures (0.5 g) were extremely promising, as they were similar to those of other lure-baited traps. Although CO2 alone was the strongest attractant for Ae. aegypti, the results were not significantly different from those when traps were baited with 0.5 g of KU-lure no. 1. CO2 at the appropriate concentration is considered the strongest cue for trapping mosquitoes [29]. However, inappropriate concentrations of CO2 repel female mosquitoes [30, 31]. Although Ae. aegypti was attracted to CO2 at the appropriate concentration, the percent attraction for this species was significantly lower than that for Cx. quinquefasciatus. This difference was also seen in a previous study using the HITSS assay, where Cx. quinquefasciatus was more strongly attracted by 0.1 g of dry ice than Ae. aegypti [23]. We also found that the amounts of the lure ingredients were among the most crucial factors for mosquito attraction and repellence. For example, KU-lures no. 1 and no. 6, and BG-Lure, contain the common component, lactic acid, at different proportions (10%, 2%, and 20%–40%, respectively). However, the KU-lures contain lactic acid as a primary component at higher proportions than the BG-Lure. In addition to lactic acid, the BG-Lure contains other important mosquito attractants such as ammonium hydrogen carbonate and hexanoic acid along with inert ingredients in different proportions. Therefore, the response of mosquitoes to these lures varies according to their chemical components and the proportions of these.
Octenol, the other key component of KU-lures, is present in human breath and sweat, and is known to play a significant role in mosquito responses to human hosts [32]. However, pure (100%) octenol alone does not attract mosquitoes, and Salazar et al. [33] found that octenol works synergistically with CO2 to attract An. gambiae and Ae. aegypti (anthropophilic species) but not Cx. quinquefasciatus, which prefers non-human hosts (it is ornithophilic) that do not emit octenol [34,35,36]. A small amount of octenol (0.25%) exerted a synergetic effect when mixed with 2% lactic acid (KU-lure no. 6) on Cx. quinquefasciatus attraction, whereas Ae. aegypti was attracted to mixtures containing higher amounts of lactic acid (10%) and octenol (2%). These results strongly indicate that the most effective species-specific attractants are mixtures (as opposed to single compounds) that contain compounds at different proportions and ratios. Therefore, to develop the best chemical lure for different populations of species, multiple candidate lures should be evaluated using a simple olfactometer in the laboratory, and the results confirmed by large-scale SFS house assays.
Mosquitoes can detect specific odors that stimulate various behaviors (e.g., nectar-seeking, mating, host-seeking, oviposition) from among thousands of different chemicals. Olfactory receptors, located in hair-like sensory organs (sensilla) on antennae and palps, participate in host-seeking behaviors [37]. In general, there is a single olfactory receptor neuron for each odor molecule [38,39,40,41]. However, some olfactory receptor neurons respond to several odor compounds, and thus may elicit a stronger attraction to a mixture than to a single compound [42]. The lure development process should include a large-scale SFS house assay to determine the actual efficacy of potential candidate lures developed in the laboratory. SFS house assay results could guide developers with regard to adjustments to the composition of potential candidate lures to increase their attractiveness. In other words, the process of lure development requires both SFS and laboratory assays to assess and increase the attractancy of lures for specific species. Additionally, the use of local mosquito populations for this is critical, as different populations and also strains of a mosquito species may display diverse response patterns. It was also confirmed in this study that the sensitivity of the diurnal and nocturnal species to the lures significantly differed. Although the diurnal species was attracted to visual cues (e.g., high contrast), the nocturnal species may have been more sensitive to chemical cues in the dark. We confirmed that both in the presence (Black Hole) and absence (BGS) of a light source, lure treatment increased the attractancy of both traps. However, the light source also attracted flying insects other than mosquitoes, such as beetles, moths, and fireflies, which can influence trap efficacy and the accuracy of entomological surveillance.
Effective mosquito surveillance tools combined with a chemical lure can be used as key components of a push–pull system along with a spatial repellent near residential areas. Salaza et al. [25] confirmed that there was no significant difference in the capture rate of BGS traps surrounding experimental huts between mosquitoes that had been previously exposed to the spatial repellent and those that had not. Furthermore, no meaningful relation between pesticide resistance and host-seeking behavior could be confirmed in laboratory experiments using the HITSS assay [22, 23]. However, further study of the persistence of the chemical lures in the material to which they are applied should be considered. Also, the performance of the lures should be compared when human hosts are present in residential buildings to assess the epidemiological effectiveness of these vector control measures.
Trap location and density are important factors for trapping efficacy [20]. Salazar et al. [25] examined trap density using different numbers of mosquitoes in a SFS. They confirmed that two traps were a suitable number for each SFS cubicle regardless of mosquito density in a range of 10–250 females. In addition, they found that the traps had the highest impact (impact period) in the first few hours. In this study, we observed a similar phenomenon for Ae. aegypti active between 0600 and 0900 hours (data not shown).
During our SFS experiments, which were conducted from February to July, the lower temperature range was 24.2–25.9 °C and the higher one 33.9–37.3 °C. However, on average, in the morning (0600–0900 hours) during the whole study period, the temperature and relative humidity were consistently lower than 30 °C and nearly 90%, respectively. As the mosquitoes tested here are most active at around 25 °C, and prefer high humidity [10, 16, 43], the number of mosquitoes captured during periods of high temperature (over 33 °C) and low relative humidity (less than 55%) dramatically reduced in the SFS facility at 1000–1500 hours. These patterns are in agreement with those of previous studies on the peak period of host-seeking in mosquitoes when a human landing collection method is used [44,45,46]. In short, mosquitoes search for hosts by detecting multiple cues, which can be visual, mechanical, or chemical [47, 48]. Environmental factors such as air temperature, relative humidity and air movement also affect a female mosquito’s host-seeking behavior [49]. For instance, it was found that when air had a higher moisture content and was warm, significantly more mosquitoes were attracted by CO2 [43].
Semi-field-scale screen houses allow researchers to determine a selected lure’s effectiveness for target species under free-flying conditions [19]. Recent WHO guidelines for the development of trapping methods for mosquitoes [20] state that the benefits of using screen-enclosed facilities are that results can be determined for exact numbers of mosquitoes of known ages and species-specific behavior recorded under local natural conditions of temperature, light, humidity and air movement, all of which are measurable.
In this study, synergistic effects between selected novel KU-lures and commercial traps were successfully demonstrated in the SFS house. However, the numbers of wild nocturnal mosquitoes captured by the lure-treated and untreated Black Hole UV light traps were not significantly different when we conducted field trials in experimental huts located in Pu-Teuy Village, Kanchanaburi Province in July 2021 (data not shown). These results are similar to those for the lure-treated and untreated BGS traps during the daytime. Furthermore, in terms of the number of mosquitoes trapped during nighttime, the BGS traps with KU-lures could not beat either the lure-treated or untreated Black Hole UV light trap. It is thus clear that a UV light source is a powerful attractant for the nocturnal species Cx. quinquefasciatus (Kim et al., unpublished data).
Recent studies that evaluated the efficacy of multiple low-cost light traps revealed that light of different colors, wavelengths and types of UV light sources (e.g., fluorescent or LED) directly affect trap performance [50, 51]. Both wavelength and light intensity are important factors for mosquito traps [52]. Fluorescent lights and LEDs differ in the range of wavelengths that they emit, with the former emitting a broad range and the latter a narrow one, although these can be adjusted by changing the diode settings from lower to higher wavelengths [53]. A recent study, conducted in an urban area of Bangkok, Thailand which used Black Hole traps [51] with multiple colored LED and fluorescent lamps, showed that the UV fluorescent lamp attracted the highest number of nocturnal mosquitoes; in addition, the selected LED UV-A wavelengths (315–400 nm) had an outstanding effect elsewhere [54, 55]. LEDs are cost-effective because they have a longer lifespan and are also less fragile than fluorescent glass lamps, which need to be replaced more frequently [56]. UV LED lamps are considered a sustainable alternative to high-energy fluorescent lamps, which are actually no longer available for sale in some countries to help cut emissions and increase energy saving. In England, for example, it is predicted that LEDs will account for 85% of total sales of light sources by 2030; this should lead to a reduction of 1.26 million tons of emitted carbon, which is equal to half the CO2 emissions of a million cars. As various types of low-cost UV LED light traps are commercially available [50], further studies of the synergistic effects of various wavelength ranges with combinations of chemical lures are urgently needed to increase the efficacy of these affordable and sustainable tools so that they can be effectively used in the entomological surveillance of national vector management programs.
Conclusions
The SFS house assay used here effectively demonstrated the synergism of traps with KU-lures for the attraction of both diurnal and nocturnal mosquito species. However, further research is urgently needed for the development of species-specific lures to increase trap efficacy.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article. Raw data are available from the corresponding author on reasonable request.
Abbreviations
- BGS:
-
BG-Sentinel
- KU:
-
Kasetsart University
- SFS:
-
Semi-field screen
- WHO:
-
World Health Organization
References
Gubler DJ. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev. 1998;11:480–96.
Watts DM, Burke DS, Harrison BA, Whitmire RE, Nisalak A. Effect of temperature on the vector efficiency of Aedes aegypti for dengue virus. Am J Trop Med Hyg. 1987;36:143–52.
Chan YC, Chan KL, Ho BC. Aedes aegypti (L.) and Aedes albopictus (Skuse) in Singapore City. 1. Distribution and density. Bull World Health Organ. 1971;44:617–27.
Gubler DJ. Epidemic dengue and dengue hemorrhagic fever: a global public health problem in the 21st century. Dengue Bull. 1997;21:1–19.
Reiter P. Surveillance and control of urban dengue vectors. In: Gubler DJ, Ooi EE, Vasudevan S, Farrar J, editors. Dengue and dengue hemorrhagic fever. Wallingford: CAB International; 2014. p. 481.
Maciel-de-Freitas R, Eiras AE, Lourenco-de-Oliveira R. Field evaluation of effectiveness of the BG-Sentinel, a new trap for capturing adult Aedes aegypti (Diptera: Culicidae). Mem Inst Oswaldo Cruz. 2006;101:321–5.
Krockel U, Rose A, Eiras AE, Geier M. New tools for surveillance of adult yellow fever mosquitoes: comparison of trap catches with human landing rates in an urban environment. J Am Mosq Control Assoc. 2006;22:229–38.
Rupp HR, Jobbins DM. Equipment for mosquito surveys: two recent developments. Proceedings of the 56th Annual Meeting of the New Jersey Mosquito Extermination Association. 1969:183–8.
Kline DL. Traps and trapping techniques for adult mosquito control. J Am Mosq Control Assoc. 2006;22:490–6.
Silver JB. Mosquito ecology: field sampling methods. 2008; New York: Springer, 3.
Jones JW, Sithiprasasna R, Schleich S, Coleman RE. Evaluation of selected traps as tools for conducting surveillance for adult Aedes aegypti in Thailand. J Am Mosq Control Assoc. 2003;19:148–50.
Williams CR, Long SA, Russell RC, Ritchie SA. Field efficacy of the BG-sentinel compared with CDC backpack aspirators and CO2-baited EVS traps for collection of adult Aedes aegypti in Cairns, Queensland, Australia. J Am Mosq Control Assoc. 2006;22:296–300.
Dogan EB, Rossignol PA. An olfactometer for discriminating between attraction, inhibition, and repellency in mosquitoes (Diptera: Culicidae). J Med Entomol. 1999;36:788–93.
Mayer M, James J. Attraction of Aedes aegypti (L.): responses to human arms, carbon dioxide, and air currents in a new type of olfactometer. Bull Entomol Res. 1969;58:629–42.
Geier M, Sass H, Boeckh. A search for components in human body odour that attract females of Aedes aegypti. In: Olfaction in mosquitoes-host interactions. Ciba Foundation Symposium. Wiley: England; 1996. p. 132.
Clements AN. Chapter 38. Host finding. In: The biology of mosquitoes, vol 2. Sensory reception and behaviour. CAB International, University Press, Cambridge; 1990. p. 460–78.
Geier M, Bosch OJ, Boeckh J. Ammonia as an attractive component of host odour for the yellow fever mosquito, Aedes aegypti. Chem Senses. 1999;24:647–53.
Kline D, Takken W, Wood J, Carlson D. Field studies on the potential of butanone, carbon dioxide, honey extract, 1-octen-3-ol,l-lactic acid and phenols as attractants for mosquitoes. Med Vet Entomol. 1990;4:383–91.
World Health Organization. Guidelines for efficacy testing of spatial repellents. Geneva: WHO; 2013.
World Health Organization. Efficacy-testing of traps for control of Aedes spp. mosquito vectors. Geneva: WHO; 2018.
Yu JJ, Bong LJ, Panthawong A, Chareonviriyaphap T, Neoh KB. Repellency and contact irritancy responses of Aedes aegypti (Diptera: Culicidae) against deltamethrin and permethrin: a cross-regional comparison. J Med Entomol. 2021;58:379–89.
Kim DY, Leepasert T, Bangs MJ, Chareonviriyaphap T. Evaluation of mosquito attractant candidates using a high-throughput screening system for Aedes aegypti (L.), Culex quinquefasciatus Say and Anopheles minimus Theobald (Diptera: Culicidae). Insects. 2021;12:528.
Kim DY, Leepasert T, Bangs MJ, Chareonviriyaphap T. Dose-response assay for synthetic mosquito (Diptera: Culicidae) attractant using a high-throughput screening system. Insects. 2021;12:355.
Achee NL, Grieco JP, Andre RG, Rejmankova E, Roberts DR. A mark-release-recapture study using a novel portable hut design to define the flight behavior of Anopheles darlingi in Belize, Central America. J Am Mosq Control Assoc. 2015;21:366–79.
Salazar FV, Achee NL, Grieco JP, Prabaripai A, Eisen L, Shah P, et al. Evaluation of a peridomestic mosquito trap for integration into an Aedes aegypti (Diptera: Culicidae) push-pull control strategy. J Vector Ecol. 2012;37:8–19.
Salazar FV, Angeles J, Sy AK, Inobaya MT, Aguila A, Toner T, et al. Efficacy of the In2Care® auto-dissemination device for reducing dengue transmission: study protocol for a parallel, two-armed cluster randomized trial in the Philippines. Trials. 2019;20:1–12.
Torr S. The tsetse (Diptera: Glossinidae) story: implications for mosquitoes. J Am Mosq Control Assoc. 1994;10:258–65.
Facchinelli L, Koenraadt CJ, Fanello C, Kijchalao U, Valerio L, Jones JW, et al. Evaluation of a sticky trap for collecting Aedes (Stegomyia) adults in a dengue-endemic area in Thailand. Am J Trop Med Hyg. 2008;78:904–9.
Webster B, Lacey ES, Carde RT. Waiting with bated breath: opportunistic orientation to human odor in the malaria mosquito, Anopheles gambiae, is modulated by minute changes in carbon dioxide concentration. J Chem Ecol. 2015;41:59–66.
Reeves W. Quantitative field studies on a carbon dioxide chemotropism of mosquitoes. Am J Trop Med Hyg. 1953;2:325–31.
Mullens BA, Gerry AC. Comparison of bait cattle and carbon dioxide-baited suction traps for collecting Culicoides variipennis sonorensis (Diptera: Ceratopogonidae) and Culex quinquefasciatus (Diptera: Culicidae). J Med Entomol. 1998;35:245–50.
Takken W, Kline D. Carbon dioxide and 1-octen-3-ol as mosquito attractants. J Am Mosq Control Assoc. 1989;5:311–6.
Salazar FV, Chareonviriyaphap T, Grieco JP, Prabaripai A, Polsomboon S, Gimutao KA, et al. BG-SentinelTM trap efficacy as a component of proof-of-concept for push-pull control strategy for dengue vector mosquitoes. J Am Mosq Control Assoc. 2017;33:293–300.
Cook J, Majeed S, Ignell R, Pickett J, Birkett M, Logan J. Enantiomeric selectivity in behavioural and electrophysiological responses of Aedes aegypti and Culex quinquefasciatus mosquitoes. Bull Entomol Res. 2011;101:541–50.
Xu P, Zhu F, Buss GK, Leal WS. 1-Octen-3-ol-the attractant that repels. F1000. 2015; 4:156.
Majeed S, Hill SR, Birgersson G, Ignell R. Detection and perception of generic host volatiles by mosquitoes modulate host preference: context dependence of (R)-1-octen-3-ol. R Soc Open Sci. 2016;3:160467.
Takken W, Knols BG. Odor-mediated behavior of Afrotropical malaria mosquitoes. Annu Rev Entomol. 1999;44:131–57.
Ghaninia M, Ignell R, Hansson BS. Functional classification and central nervous projections of olfactory receptor neurons housed in antennal trichoid sensilla of female yellow fever mosquitoes, Aedes aegypti. Eur J Neuro Sci. 2007;26:1611–23.
Hill SR, Hansson BS, Ignell R. Characterization of antennal trichoid sensilla from female southern house mosquito, Culex quinquefasciatus Say. Chem Senses. 2009;34:231–52.
Syed Z, Leal WS. Acute olfactory response of Culex mosquitoes to a human-and bird-derived attractant. PNAS. 2009;106:18803–8.
Ye Z, Liu F, Liu N. Olfactory responses of southern house mosquito, Culex quinquefasciatus, to human odorants. Chem Senses. 2016;41:441–7.
Karner T, Kellner I, Schultze A, Breer H, Krieger J. Co-expression of six tightly clustered odorant receptor genes in the antenna of the malaria mosquito Anopheles gambiae. Front Ecol Evol. 2015;3:26.
Wright R. Why mosquito repellents repel. Sci Am. 1975;233:104–11.
Thavara U, Tawatsin A, Chansang C, Kong-ngamsuk W, Paosriwong S, Boon-Loong J, et al. Larval occurrence, oviposition behavior and biting activity of potential mosquito vectors of dengue on Samui Island, Thailand. J Vector Ecol. 2001;26:172–80.
Suwannachote N, Grieco JP, Achee NL, Suwonkerd W, Wongtong S, Chareonviriyaphap T. Effect of environmental conditions on the movement patterns of Aedes aegypti (Diptera: Culicidae) into and out of experimental huts in Thailand. J Vector Ecol. 2009;34:267275.
Chareonviriyaphap T, Grieco JP, Suwonkerd W, Prabaripai A, Polsomboon S, Thainchum K, et al. An improved experimental hut design for the study of Aedes aegypti (Diptera: Culicidae) movement patterns in Thailand. J Vector Ecol. 2010;35:428–31.
Brown A, Sarkaria D, Thompson R. Studies on the responses of the female Aedes aegypti mosquito. Part 1. The search for attractant vapours. Bull Entomol. 1951;42:105–14.
Mitchell CJ. Differentiation of host-seeking behavior from blood-feeding behavior in overwintering Culex pipiens (Diptera: Culicidae) and observations on gonotrophic dissociation. J Med Entomol. 1983;20:157–63.
Takken W. The role of olfaction in host-seeking of mosquitoes: a review. Int J Trop Insect Sci. 1991;12:287–95.
Huang R, Song H, Fang Q, Qian J, Zhang Y, Jiang H. Laboratory and field performance of five cheap commercial light traps for capturing mosquitoes in China. Res Sq. 2021. (preprint).
Saeung M, Jhaiaun P, Bangs MJ, Ngoen-Klan R, Chareonviriyaphap T. Transmitted light as attractant with mechanical traps for collecting nocturnal mosquitoes in urban Bangkok, Thailand. J Am Mosq Control Assoc. 2021;37:132–42.
Smith KC, The science of photobiology. New York. NY: Plenum Press; 1977.
Schubert EF, Gessmann T, Kim JK. Light emitting diodes. In: Kirk-Othmer encyclopedia of chemical technology. 2005. New York; Wiley.
Kim HC, Kim MS, Choi KS, Hwang DU, Johnson JL, Klein TA. Comparison of adult mosquito blacklight and light-emitting diode traps at three cowsheds located in malaria-endemic areas of the Republic of Korea. J Med Entomol. 2017;54:221–8.
Peach DA, Ko E, Blake AJ, Gries G. Ultraviolet inflorescence cues enhance attractiveness of inflorescence odour to Culex pipiens mosquitoes. PLoS ONE. 2019;14:e0217484.
Hoel DF, Butler JF, Fawaz E, Watany N, El-Hossary S, Villinski J. Response of phlebotomine sand flies to light-emitting diode-modified light traps in southern Egypt. J Vector Ecol. 2007;32:302–8.
Acknowledgements
The authors would like to thank the anonymous reviewers for their valuable comments and suggestions. We would like to thank Bruce Wilcox and Kriangkrai Klerdthusanee for scientific feedback on this manuscript. We appreciate Ratchadawan Ngoen-klan for statistical advice related to this study.
Funding
This research was supported by Kasetsart University through a Postdoctoral Fellowship at Kasetsart University Research and Development Institute (KURDI) and Fundamental Fund program [KU (FF) 14.64].
Author information
Authors and Affiliations
Contributions
Conceptualization: MJB and TC. Data curation: MJB. Analysis: D-YK and TL. Funding acquisition: TC. Investigation: D-YK and TL. Methodology: D-YK and TL. Project administration: TC. Software: D-YK, TL and TC. Supervision: TC. Validation: D-YK and TL. Visualization: D-YK and TL. Writing–original draft: D-YK. Writing, reviewing and editing: MJB and TC. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflicting interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kim, DY., Leepasert, T., Bangs, M.J. et al. Semi-field evaluation of novel chemical lures for Aedes aegypti, Culex quinquefasciatus, and Anopheles minimus (Diptera: Culicidae) in Thailand. Parasites Vectors 14, 606 (2021). https://doi.org/10.1186/s13071-021-05108-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-021-05108-3