Abstract
Background
Giardia lamblia, a protozoan pathogen causing diarrheal outbreaks, has characteristic cytoskeletal structures including eight flagella, a median body and a ventral disc. Gamma-giardin is a unique component protein of the cytoskeleton of this protozoan.
Results
Through comparative proteomic analysis between different stages of the cell cycle, G. lamblia γ-giardin (Glγ-giardin) was identified as an upregulated protein in the G2-phase. Increased Glγ-giardin expression in G2 was confirmed by western blot and real-time polymerase chain reaction analyses. Knockdown of this protein using a morpholino affected the formation of ventral discs, especially the microribbons of the discs, but exerted little effect on the binding ability of G. lamblia. The number of cells with four nuclei was increased in Glγ-giardin-knockdown cells. Expression of Glγ-giardin was decreased during encystation, in contrast with the G2-phase.
Conclusions
Knockdown experiments demonstrated that Glγ-giardin is a component of the trilaminar structure of the ventral disc. Expression of Glγ-giardin is induced in the G2-phase prior to active cell division, whereas its expression decreases during encystation, a dormant stage of G. lamblia.
Similar content being viewed by others
Background
Giardia lamblia is a protozoan pathogen causing gastrointestinal diseases in humans [1]. Infection is initiated by ingestion of a metabolically dormant and infective form, the cyst, which is converted into trophozoites in the small intestines of the hosts via excystation. Trophozoites are the multiplying form responsible for the pathogenesis of giardiasis. Through the encystation process, some trophozoites transform into cysts before being released outside the host.
At present, limited information is available on the mechanism of how a trophozoite divides into two progenies or how the division process is regulated. Based on the finding that the cell cycle of Giardia trophozoites can progress despite blocked DNA synthesis, double stranded DNA breaks or defective mitotic spindles, this pathogen has been reported to have defective cell cycle checkpoint systems [2]. Investigations on cell cycle control in Giardia trophozoites have been performed primarily by obtaining synchronized cell cultures using aphidicolin [3] or nocodazole/aphidicolin [4]. Attainment of synchronized Giardia cultures was improved with the use of counterflow centrifugal elutriation [5]. In this study, Giardia cells were prepared as enriched cultures at G1/S and G2 using aphidicolin in order to identify Giardia proteins showing phase-specific expression. One of the overexpressed proteins in the G2-phase was identified as γ-giardin, which is a known Giardia-specific component of the ventral disc [6].
The Giardia trophozoite has unique cytoskeletal structures essential for its survival and pathogenicity, including four pairs of flagella, a median body (MB) and a ventral disc [7]. Microtubules (MTs) composed of α-/β-tubulin are the basic constituent of the Giardia cytoskeleton [8]. In addition, a group of proteins between 29 and 38 kDa are known as giardin and have been identified as unique components of the ventral discs [9, 10]. The proteins belonging to the giardins are classified into four subgroups, α-, β-, γ-, and δ-giardins, not related to their amino acid sequences. Alpha-giardins of 33 kDa include annexin showing a phospholipid-binding ability [11], along with 21 putative α-giardin genes found in the G. lamblia database [12]. A β-giardin of ~30 kDa contains small coiled-coil segments of four heptads and comprises the ventral disc of Giardia trophozoites [13]. A γ-giardin of 38 kDa is also a component of the ventral disc, the microribbon of Giardia trophozoites [6]. Delta-giardin, localized in the ventral disc, has been reported to be involved in attachment of Giardia trophozoites to the intestinal epithelium [14].
The ventral disc is one of the characteristic structures of the Giardia trophozoite that has been the focus of ultrastructural investigation [15]. As an organelle involved in attachment, this structure is located on the ventral aspect of trophozoites, and is formed by spiral layers of MTs wound clockwise around the central bare area. Adjacent to each disc MT, microribbons are associated with the basal MT layer along the full length of MTs. These microribbons are crosslinked with horizontal bridges [8, 16, 17]. Fragmentation and shrinkage of these discs has been reported during cell division and encystation of G. lamblia [18, 19]. Analysis of Giardia structures using transmission electron microscopy with thin and semi-thin sections and cryo-techniques, and by immunofluorescence microscopy using anti-tubulin antibodies demonstrated that the ventral disc seems to play a role in the division process, participating in karyokinesis [20, 21]. In the present study, the roles of G. lamblia γ-giardin (Glγ-giardin) in the ventral disc formation and cell division of G. lamblia, was examined by decreasing its expression using a morpholino.
Methods
Cultivation of G. lamblia trophozoites
Trophozoites of G. lamblia WB strain (ATCC30957; American Type Culture Collection, Manassas, VA, USA) were grown for 72 h at 37 °C in TYI-S-33 medium (2% casein digest, 1% yeast extract, 1% glucose, 0.2% NaCl, 0.2% l-cysteine, 0.02% ascorbic acid, 0.2% K2HPO4, 0.06% KH2PO4, 10% calf serum and 0.5 mg/ml bovine bile, pH 7.1) [22].
Giardia trophozoite synchronization using aphidicolin and flow cytometry analysis
For synchronization of Giardia, aphidicolin (Sigma-Aldrich, St. Louis, MO, USA) at a concentration of 5 μg/ml was added to cells grown to about 60% confluency. Control cells were treated with 0.05% DMSO which was used to solubilize 5 µg/ml aphidicolin. After a 6-h incubation at 37 °C, the medium was replaced with drug-free fresh TYI-S-33 culture medium and incubated for an additional 3 h.
Flow cytometry analysis of various cells (DMSO-treated control, aphidicolin-treated and aphidicolin-washed trophozoites) was performed as previously described [3]. Briefly, the harvested cells were re-suspended in 50 μl of TYI-S-33 culture medium and were treated with 150 μl of cell fixative (1% Triton X-100, 40 mM citric acid, 20 mM dibasic sodium phosphate and 200 mM sucrose, pH 3.0) at room temperature for 5 min. The samples were diluted with 350 μl of diluent buffer [125 mM MgCl2 in phosphate-buffered saline (PBS: 137 mM NaCl, 2.7 mM KCl, 10.1 mM Na2HPO4 and 2 mM KH2PO4, pH 7.4)] and then stored at 4 °C until use. Fixed cells were reacted with 2.5 μg of RNase A (Sigma-Aldrich) and 10 μg/ml of propidium iodide (Sigma-Aldrich) for 30 min at 37 °C. The cells were evaluated with respect to their DNA content by flow cytometry and FlowJO software analysis (FlowJo Llc, Ashland, OR, USA).
Two-dimensional gel electrophoresis (2-DGE) of Giardia extracts and image analysis
One milligram of protein extracts was prepared from about 3 × 108 trophozoites arrested with aphidicolin (G1/S-phase cells) and trophozoites released from the aphidicolin-mediated arrest (G2-phase cells) by resuspending them in a 2-DGE sample buffer (7 M urea, 2 M thiourea, 100 mM DTT, 4.5% CHAPS and 40 mM Tris). Immobilized pH gradient (IPG) gel strips (pH 3–10, 18 cm; GE Healthcare, Uppsala, Sweden) were soaked overnight with the extracts. The rehydrated IPG strips were treated for sequential isoelectric focusing at 80 kV. Second-dimension separation was carried out at room temperature on 9–17% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) gels (20 × 25 cm). The protein bands on the gel were visualized by Coomassie staining. The 2-DGE experiment was performed twice. Stained gels were scanned using a GS-710 imaging densitometer (Bio-Rad, Hercules, CA, USA) and analyzed with Melanie 5 image analysis software (GE Healthcare). Image labeling was processed using Adobe Photoshop v.7.0 software. Analysis of these two-dimensional (2-D) gels was performed at Yonsei Proteome Research Center (Seoul, Korea).
Liquid chromatography mass spectrometry
Protein spots on the 2D gel were excised and digested with trypsin. The trypsin-treated proteins were analyzed by quadrupole time-of-flight (Q-TOF) mass spectrometry (MS) in addition to matrix-assisted laser desorption ionization-TOF MS. Product ion spectra were collected in the information-dependent acquisition mode and were analyzed with an Agilent 6530 accurate-mass Q-TOF MS (Agilent Technologies, Santa Clara, CA, USA). For the Q-TOF liquid chromatography-tandem MS (LC–MS/MS) data sets, tandem mass spectra were submitted to our Mascot in-house database search engine (NCBI NR database downloaded on 31 July 2009). For protein identification, a Mascot ion score of > 55 was used as the criterion for a meaningful result.
Construction of G. lamblia expressing HA epitope-tagged Glγ-giardin
A 936-bp DNA fragment of the Glγ-giardin gene (GiardiaDB ORF no. GL50803_17230) was amplified from G. lamblia WB genomic DNA by PCR using two primers, γ-giardin-NcoI-F and γ-giardin-HAX3-R (Table 1). NcoI and NotI sites, located at the ends of the Glγ-giardin DNA fragment, were used for cloning into the corresponding sites of plasmid pGFP.pac [23], resulting in the plasmid, pGlγ-giardinHAX3.pac (Table 2).
The trophozoites were grown for 72 h in TYI-S-33 medium. Thirty micrograms of an episomal plasmid, pGlγ-giardinHAX3.pac, were transfected into 1 × 107 trophozoites by electroporation under the following conditions: 350 V, 1000 μF and 700 Ω (Bio-Rad). Transfection was performed with 5 independent sets of Giardia trophozoites at once. Trophozoites harboring the plasmid pGlγ-giardinHAX3.pac were initially selected by adding puromycin (AG Scientific, San Diego, CA, USA) to the TYI-S-33 medium at a final concentration of 10 µg/ml, and further enriched in the medium containing 50 µg/ml of puromycin at 4–5 days post-transfection. We obtained 4 Giardia lines showing the puromycin resistance. Among them, two Giardia cell lines demonstrated expression of HA-tagged Glγ-giardin as shown in western blot analysis using anti-HA antibodies. Giardia lamblia trophozoites carrying pΔ.pac [24] were used as a control.
Western blot analysis and antibody formation
Cell extracts were prepared from various G. lamblia cells (cells without plasmid, cells carrying pGlγ-giardinHAX3.pac, and cells carrying pΔ.pac) in PBS. The extracts were separated by SDS-PAGE and transferred onto a polyvinylidene fluoride (PVDF) membrane (Millipore, Bedford, MA, USA). The membrane was incubated with monoclonal mouse anti-HA (1:1000; Sigma-Aldrich) in a blocking solution [Tris-buffered saline with Tween 20 (TBST): 50 mM Tris-HCl, 5% skim milk and 0.05% Tween 20] at 4 °C overnight. Following incubation with horseradish peroxidase (HRP)-conjugated secondary antibody, immunoreactive proteins were visualized using an enhanced chemiluminescence (ECL) system (Thermo Fisher Scientific, Waltham, MA, USA). Membranes were incubated in a stripping buffer (Thermo Fisher Scientific) at room temperature for 20 min and then reacted with polyclonal rat antibodies specific to the protein disulfide isomerase 1 (PDI1; GiardiaDB ORF no. GL50803_29487) of G. lamblia (1:10,000) as loading control. The rat anti-GlPDI1 polyclonal antibodies were obtained by using the recombinant GlPDI1 protein [25].
To make recombinant G. lamblia cyclin B (Glcyclin B; GiardiaDB OFR no. GL50803_3977) protein, 3977-F and 3977-R primers (Table 1) were used to amplify a 1026-bp DNA fragment of the glyclin B gene. A HindIII and XhoI site were used to clone the PCR products into pET21b (Novagen, Darmstadt, Germany) resulting pET-cyclinB (Table 2). Recombinant Glcyclin B protein was overexpressed in Escherichia coli BL21 (DE3) by adding 1 mM isopropylthiogalactoside (IPTG) (Sigma-Aldrich), and then used to make Glcyclin B-specific polyclonal antibodies by immunizing Sprague-Dawley rats (three immunizations at 3-week intervals, 200 μg per immunization). Specificity of polyclonal antibodies against recombinant Glcyclin B was confirmed by reacting them with E. coli extracts overexpressing the recombinant Glcyclin B or Giardia extract (see Additional file 1: Figure S1).
Immunofluorescence assay (IFA)
To examine the localization of Glγ-giardin, G. lamblia expressing HA-tagged Glγ-giardin was attached to glass slides coated with L-lysine in a humidified chamber. The attached cells were fixed with chilled 100% methanol at − 20 °C for 10 min, and permeabilized with PBS/0.5% Triton X-100 for 10 min. After a 1 h-incubation in the blocking buffer (PBS, 5% goat serum and 3% BSA), the cells were reacted overnight with mouse anti-HA antibodies (1:100; Sigma-Aldrich) and rat anti-Glγ-giardin polyclonal antibodies (1:100). Following three 5-min washes with PBS, the cells were incubated with Alexafluor 488-conjugated anti-mouse IgG (1:100; Molecular Probes, Grand Island, NY, USA) and Alexafluor 555-conjugated anti-rat IgG (1:100; Molecular Probes) at 37 °C for 1 h. Slides were mounted with VECTASHIELD anti-fade mounting medium with 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA, USA). The slides were then examined with an Axiovert 200 fluorescent microscope (Carl Zeiss, Oberkochen, Germany).
Quantitative real-time PCR analysis
Total RNA was prepared from G1/S-phase and G2-phase cells using TRizol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Five micrograms of RNA were converted into complementary DNA (cDNA) using an Improm-II reverse transcription system (Promega, Madison, WI, USA). Real-time PCR was performed using LightCycler System and LightCycler 480 SYBR Green I Master Kit (Roche Applied Science, Mannheim, Germany). Conditions for real-time PCR were as follows: pre-incubation at 95 °C for 5 min followed by 45 amplification cycles of 94 °C for 1 min, 56 °C for 1 min and 72 °C for 1 min. The nucleotide sequences of the forward and reverse primers used for real-time PCR are listed in Table 1. The G. lamblia actin-related gene (Glactin; GiardiaDB OFR no. GL50803_15113) transcript was used for normalization of mRNA amount in the cDNA samples.
Knockdown of Glγ-giardin expression using morpholino
Glγ-giardin expression was decreased using a 25-mer morpholino for the Glγ-giardin open reading frame (ORF) from the start codon (Table 1; Gene Tools Llc, Philomath, OR, USA). The control morpholino, a non-specific oligomer, was used as a control (Table 1). Morpholinos were added to 5 × 106 cells at a final concentration of 100 μM by electroporation. The transfected cells were grown for 48 h, and then analyzed for level of Glγ-giardin by western blot and IFA as described above.
Scanning electron microscopy (SEM)
Morpholino-treated cells were prefixed in a Karnovsky fixative solution (2% glutaraldehyde, 2% paraformaldehyde, 0.5% CaCl2 in a 0.1 M cacodylate buffer, pH 7.4), followed by washing in 0.1 M cacodylate buffer (pH 7.4) and post-fixing by 1.33% osmium tetroxide in 0.1 M cacodylate buffer (pH 7.4). Thereafter, the samples were dehydrated in absolute ethanol. For observation by SEM, the dehydrated samples were rehydreated with isoamyl alcohol and treated with a 300 Å gold coating using an ion coater (Leica EM ACE600; Leica Microsystems, Vienna, Austria).
Transmission electron microscopy (TEM)
For transmission electron microscopy, morpholino-treated cells were fixed with 2% glutaraldehyde-2% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). They were post-fixed with 1% OsO4 in 0.1 M phosphate buffer (pH 7.4) for 2 h and dehydrated in an ascending graded series (50–100%) of ethanol and infiltrated with propylene oxide. Specimens were embedded using a Poly/Bed 812 kit (Polysciences Inc., Warrington, PA, USA). After embedding, the specimens were polymerized at 65 °C in an electron microscope oven (TD-700; Dosaka EM, Kyoto, Japan) for 24 h. Next, 70-nm-thin sections were double stained with 6% uranyl acetate and lead citrate (Fisher Scientific, Rockford, IL, USA) for contrast staining. Sections were cut with a Leica EM UC-7 (Leica Microsystems) using a diamond knife and transferred to copper grids. All thin sections were observed with a TEM (JEM-1011; JEOL, Tokyo, Japan) at an acceleration voltage of 80 kV.
Adherence assay
A total of 5 × 106 Giardia trophozoites treated with control morpholino or anti-Glγ-giardin morpholino were cultured in TYI-S-33 medium. After 48 h of cultivation, the culture medium was discarded to remove the non-attached cells and replaced with PBS. The tubes were incubated on ice for 20 min to detach the adherent cells. The detached cells were harvested by centrifugation at 3000× rpm for 15 min at 4 °C. The number of cells per milliliter was determined using a haemocytometer.
As a control, knockdown of expression of G. lamblia median body binding protein (GlMBP; GiardiaDB ORF no. GL50803_16343) [26] was performed by transfecting anti-GlMBP morpholino (Table 1) into G. lamblia trophozoites. Adherence of the resulting transfectants was also monitored as described above.
Determination of population of cell stage and mitotic index of G. lamblia trophozoites
Giardia cells treated with control, or anti-Glγ-giardin morpholino, were stained with Giemsa, and the percentage of cells in various stages, i.e. interphase, mitosis, cytokinesis, was then determined. The cells were attached onto glass slides, air-dried, and then fixed with 100% methanol for 10 min. They were then stained with 10% Giemsa solution for 40 min and washed with distilled water. After mounting with dibutyl phthalate xylene (Sigma-Aldrich), the slides were observed with an Axiovert 200 microscope (Carl Zeiss).
At 48 h post-treatment with control morpholino, or anti-Glγ-giardin morpholino, the ratio of cells with two nuclei to those with four nuclei was determined to monitor mitosis, as previously described [27]. The fixed cells were mounted in VECTASHIELD anti-fade mounting medium with DAPI (Vector Laboratories). The numbers of cells with four nuclei or two nuclei were counted in a total of over 300 cells per each condition.
In vitro encystation of Giardia trophozoites
To induce encystation in vitro, trophozoites were transferred into an encystation medium (TYI-S-33 medium with 10 mg/ml bovine bile, pH 7.8) [28]. At various time-points after incubation in the encystation medium, cells were harvested by centrifugation at 3000× rpm for 15 min at 4 °C. Intracellular levels of Glγ-giardin were determined using anti-Glγ-giardin antibodies. To monitor the encystation process, the intracellular level of cyst wall protein 1 (CWP1) [29] was measured in the harvested G. lamblia by western blot analysis using anti-GlCWP1 antibodies [30]. The amount of GlPDI1 was monitored as a loading control. The localization of Glγ-giardin in encysting cells was also observed by IFA as described above.
Statistical analysis
Data are presented as the mean ± standard deviation from three independent experiments. To determine the statistical significance of these results, data were analyzed using paired samples t-tests. Differences with P-values of less than 0.05 were considered significant. In the figures and tables, two asterisks indicate P-values of less than 0.01, while a single asterisk indicates P-values between 0.01 and 0.05.
Results
Identification of γ-giardin as a growth phase-controlled protein in G. lamblia
To obtain Giardia cells at specific stage of cell division, aphidicolin was used. Flow cytometry analysis of the resulting Giardia cells indicated that 6-h treatment with aphidicolin produced Giardia cells at G1/S-phase, and 3-h release from aphidicolin treatment resulted in Giardia cells at G2-phase (Fig. 1a). Control cells, i.e. Giardia trophozoites treated with 0.05% DMSO, were found to be a mixture of G1/S-, and G2-phase cells, and the cells at G2-phase were dominant (76%), as reported previously (Fig. 1a) [27].
Synchronization of Giardia trophozoites using aphidicolin and proteomic analysis of the synchronized cells. a FACS analysis of various G. lamblia cells (0.05% DMSO-treated cells, 5 μg/ml of aphidicolin-treated cells, and cells released from aphidicolin arrest) on their DNA content using propidium iodide staining. The experiment was performed three times, and three replicates were analyzed for each experiment. b Comparative 2-dimensional gel electrophoresis of lysates prepared either from trophozoite arrested at G1/S-phase with aphidicolin or trophozoites at G2-phase released from aphidicolin arrest. Five protein spots (#1 to #5) present only at G1/S-phase and 14 protein spots (#6 to #19) found only at G2-phase, were analyzed by LC/MS-MS; identities of these proteins are listed in Table 3. c Parallel comparison of selected protein spots between G1/S- and G2-phase cells in extended panels
Giardia extracts enriched at G1/S and G2 cells were compared using 2-DGE (Fig. 1b). The 2-DGE experiment was performed twice. Through gel image analysis, we obtained 180 non-pairing spots (50 spots in G1/S-gel and 130 spots in G2-gel), which could be detected only in one of the two samples. Among of them, five spots were selected as upregulated proteins at G1/S-phase whereas 14 spots were chosen as upregulated proteins at G2-phase. Comparison of these spots at G1/S-phase versus G2-phase are presented as extended panels in Fig. 1c. Differential expression of these 19 proteins at G1/S-phase versus G2-phase was observed in two independent 2-DGE experiments. The identities of these protein spots were investigated with LC–MS/MS analysis (Table 3).
Three of the G1/S-phase upregulated proteins were metabolic enzymes, pyruvate-flavodoxin oxidoreductase, aldose reductase, and arginyl-tRNA synthetase. Cytosolic heat shock protein 70 (HSP70) was found to be increased at G1/S phase of G. lamblia. The other one was denoted as a hypothetical protein.
With respect to the G2-phase upregulated proteins, six encoded metabolic enzymes, i.e. enolase, phosphatase, NADP glutamate dehydrogenase, carbamate kinase, protein disulfide isomerase 4 and fructose-bis-phosphate aldolase. Four proteins showing increased expression at the G2-phase were associated with the G. lamblia cytoskeleton (α-7.1-giardin, γ-giardin, calmodulin and dynein light intermediate chain). In addition, expression of Giardia trophozoite antigen 1 was increased at the G2-phase. No functional information could be obtained for another two clones (protein 21.1 and hypothetical protein) showing increased expression in the G2-phase. Identification of the protein folding related proteins (T complex protein-1 chaperonin subunit eta and HSP70 as an upregulated protein in the G2-phase and G1/S-phase, respectively), was probably caused from the side-effect of aphidicolin [31].
Among the identified protein spots, γ-giardin, a unique cytoskeletal component of G. lamblia [6], was selected for further investigation. Giardia lamblia γ-giardin, Glγ-giardin, was previously found as a protein interacting with end-binding protein 1, one of microtubule binding proteins [32]. The intracellular location of Glγ-giardin in Giardia trophozoites was observed using transgenic Giardia cells expressing the HA-tagged Glγ-giardin. A transgenic Giardia expressing HA-tagged Glγ-giardin was constructed by transfecting the episomal plasmid, pGlγ-giardinHAX3.pac into Giardia trophozoites. Expression of HA-tagged Glγ-giardin was confirmed by western blot analysis using anti-HA antibodies (Fig. 2a). An immunoreactive protein of ~38 kDa was detected in the Giardia extracts with pGlγ-giardinHAX3.pac, whereas the protein was absent in extracts of Giardia cells carrying the vector plasmid. Localization of Glγ-giardin was examined in the transgenic Giardia using anti-HA antibodies and anti-Glγ-giardin antibodies (Fig. 2b). Glγ-giardin was found on the adhesive disc and the MB in double-stained Giardia trophozoites. To examine whether localization of Glγ-giardin in MB occurs in a phase-specific manner, percentages of cells with stained MB were measured for both G1/S- and G2-arrested cells. Percentages of Giardia cells with stained MB were significantly increased up to 13% at G2-phase from 3.2% at G1/S phase-cells (Fig. 2c).
Construction of transgenic Giardia trophozoites expressing HA-tagged Glγ-giardin and localization of HA-tagged Glγ-giardin. a Expression of HA-tagged Glγ-giardin was confirmed by western blot analysis. Extracts were prepared from G. lamblia containing pΔ.pac (Lane 1), or pGlγ-giardinHAX3.pac (Lane 2). Membrane was reacted with monoclonal mouse anti-HA (1:1000). After deprobing antibodies with stripping buffer, membrane was incubated with polyclonal rat antibodies specific to PDI1 of G. lamblia (1:10,000) as loading control. b Localization of Glγ-giardin in G. lamblia expressing HA-tagged Glγ-giardin. Giardia lamblia expressing HA-tagged Glγ-giardin attached to glass slides were reacted overnight with mouse anti-HA (1:100) and anti-Glγ-giardin (1:100) then incubated with Alexafluor 488-conjugated anti-mouse IgG (1:100) and Alexafluor 555-conjugated anti-rat IgG (1:100). DIC image was acquired to show cell morphology. Scale-bars: 2 μm. c Percentages of Giardia cells with stained MB at G1/S- and G2-phases
Increased expression of Glγ-giardin in the G2-phase was examined by western blot analysis (Fig. 3a). Giardia lamblia WB trophozoites were used to make G1/S-arrested cells, and G2-arrested cells as described above. Western blot analysis of these extracts using anti-Glγ-giardin antibodies demonstrated a 2.2-fold increase in the protein level of Glγ-giardin at G2-phase in comparison with the G1/S-phase cells. Western blot analysis of the same blot using anti-GlPDI1 antibodies served as a loading control [25]. These G1/S- and G2-phase cells were analyzed for levels of G. lamblia cyclin B (Glcyclin B; GiardiaDB ORF no. GL50803_3977), which is a G2-phase marker in G. lamblia [33] (Fig. 3a). The amount of Glcyclin B was increased 1.8-fold in the G2-phase cells, indicating that our samples were arrested at G1/S- and G2-phase by aphidicolin.
Expression of Glγ-giardin in Giardia trophozoites at G1/S- and G2-phases. a Giardia lamblia were arrested at G1/S phase with aphidicolin (Lane 1) or trophozoites at G2-phase released from aphidicolin arrest (Lane 2). Expression of Glγ-giardin was confirmed by western blot analysis. Membrane was reacted with rat anti-Glγ-giardin polyclonal antibodies (1:1000). After deprobing antibodies with stripping buffer, membrane was incubated with polyclonal rat antibodies specific to PDI1 of G. lamblia (1:10,000) as loading control. The same blot was also reacted with rat anti-Glcyclin B polyclonal antibodies (1:500). b Total RNA was prepared from G1/S-phase and G2-phase cells using TRizol. Five micrograms of RNA were converted into cDNA using an Improm-II reverse transcription system. Real-time PCR was performed using a LightCycler System and LightCycler 480 SYBR Green I Master Kit. Conditions for real-time PCR were as follows: pre-incubation at 95 °C for 5 min followed by 45 amplification cycles of 94 °C for 1 min, 56 °C for 1 min and 72 °C for 1 min. Nucleotide sequences of forward and reverse primers used for real-time PCR are listed in Table 1. Normalization of mRNA quantity in the cDNA samples was performed using glactin transcript levels
Upregulation of Glγ-giardin was also examined with an alternative method, quantitative RT-PCR (Fig. 3b). The level of Glγ-giardin transcript at G2-phase was 1.6-fold higher than that of G1/S-phase cells.
Effect of knockdown of Glγ-giardin on cell division of G. lambla
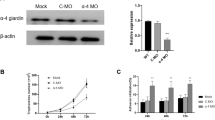
To define the role of Glγ-giardin in G. lamblia, we designed an anti-Glγ-giardin morpholino to block the translation of Glγ-giardin mRNAs (Table 1). A control morpholino (non-specific oligomers) was also made and transfected into G. lamblia WB trophozoites by electroporation (Table 1). These extracts were examined to determine their intracellular levels of Glγ-giardin at 48 h post-transfection by western blot using anti-Glγ-giardin antibodies (Fig. 4a). Cells treated with anti-Glγ-giardin morpholino demonstrated decreases in the amount of Glγ-giardin at 48 h post-transfection to 47% of that of the cells treated with the control morpholino. IFA images using anti-Glγ-giardin antibodies indicated that cells treated with anti-Glγ-giardin morpholino had less fluorescence in the adhesive disc compared with control cells. This result demonstrated the decreased expression of Glγ-giardin in the knockdown cells (Fig. 4b).
Effect of morpholino-mediated knockdown of Glγ-giardin on Giardia cell division. a Giardia trophozoites were collected 48 h after electroporation with control morpholino (control mph; Lane 1), or anti-Glγ-giardin morpholino (anti-Glγ-giardin mph; Lane 2). Extracts of these cells were reacted with anti-Glγ-giardin or anti-GlPDI1 antibodies. Abundance of each immunoreactive protein was quantified by densitometry and normalized to that of Giardia treated with control morpholino. b Expression of Glγ-giardin was monitored by IFAs. Cells were reacted overnight with rat anti-Glγ-giardin polyclonal antibodies (1:100) then treated with Alexafluor 555-conjugated anti-rat IgG (1:100). Slides were mounted with VECTASHIELD anti-fade mounting medium with DAPI then observed with an Axiovert 200 fluorescent microscope. c Effect of anti-Glγ-giardin morpholino on cytokinesis of Giardia cells. Cells were stained with 10% Giemsa solution and observed with a light microscope to count numbers of cells in interphase (gray columns), mitosis (closed columns) and cytokinesis (open columns). d Cell number was determined by counting a least 500 cells per each condition. Cells were attached on coverslips and mounted in anti-fade mounting medium with DAPI. To determine numbers of cells with four or two nuclei, more than 300 cells per condition were counted. Scale-bars: b, c, d, 2 µm
The effect of Glγ-giardin knockdown on cell division was determined by Giemsa staining of these cells to distinguish Giardia at different stages (i.e. interphase, mitosis and cytokinesis) (Fig. 4c). A small proportion of the cells were in mitosis (1–2%) or cytokinesis (3–5%), while the majority of cells were in interphase (94–95%). Percentages of these cells at different stages were maintained in a similar pattern between control cells and Glγ-giardin knockdown cells. In contrast, an additional assay to determine the mitotic index showed that the proportion of cells with four nuclei increased from 4% of the cells treated with the control morpholino to 10% in cells treated with anti-Glγ-giardin morpholino (Fig. 4d; P = 0.003).
Effect of Glγ-giardin knockdown on formation and function of the ventral disc of Giardia
Giardia cells treated with the control morpholino, or the anti-Glγ-giardin morpholino, were observed by SEM to determine the structural integrity of the adhesive disc (Fig. 5a). The overall structure of the ventral disc appeared intact. However, flattened margins, particularly the ventral groove, were more frequently found in cells treated with anti-Glγ-giardin morpholino. The ventral discs were also observed using TEM (Fig. 5b). When cells were cut in transverse sections, the adhesive discs were observed as a periodical array of filaments connected with MTs in Giardia trophozoites treated with control morpholino. On the other hand, the transverse TEM images of Giardia treated with anti-Glγ-giardin morpholino clearly demonstrated disruption of the periodical array of filaments in the ventral discs. That is, the portion of the filaments was shortened in the anti-Glγ-giardin morpholino-treated cells.
Effect of morpholino-mediated knockdown of Glγ-giardin on structure and function of adhesive disc in Giardia. Giardia trophozoites were collected 48 h after electroporation with control morpholino, or anti-Glγ-giardin morpholino. a Scanning electron microgram showing adhesive disc of G. lamblia. Shape of the adhesive discs are presented as cartoons. b Transmission electron micrographs demonstrating microribbons of adhesive discs. c After 48-h cultivation, culture media were discarded to remove non-attached cells and replaced with PBS. Tubes were incubated on ice for 20 min to detach adherent cells. Detached cells were counted using hemocytometer. As a control, adherence of G. lamblia trophozoites transfected with anti-GlMBP morpholino was evaluated as described above. The experiment was performed three times, and three replicates were analyzed for each experiment. The error bars are from three independent experiments. Scale-bars: a, b, 2 µm
As a result of the Glγ-giardin knockdown, the shape of the adhesive disc was changed; thus, the following experiment was performed to determine whether the adherence was also affected (Fig. 5c). An additional morpholino was made against G. lamblia median body protein (GlMBP; GiardiaDB ORF no. GL50803_16343), which had been previously reported to be involved in adhesion of Giardia, and used as a positive control for Giardia adherence ability [26]. As expected, treatment with anti-GlMBP morpholino resulted in a 40% reduction of Giardia adherence (P = 0.0067). In contrast, treatment of Giardia with anti-Glγ-giardin morpholino did not induce any change in adherence, indicating that Glγ-giardin may be a structural component of the adhesive disc, but is not a key factor in adherence.
Expression and localization of Glγ-giardin during encystation
Since Glγ-giardin was identified as one of the proteins upregulated during the G2-phase, we examined its expression pattern during encystation (Fig. 6a). Giardia extracts at various time points of encystation were evaluated for GlCWP1, GlPDI1, and Glγ-giardin. The intracellular level of GlPDI1 served as a loading control. As expected, expression of GlCWP1 was dramatically increased in the encysting cells. Conversely, the intracellular quantity of Glγ-giardin in encysting cells was decreased to 36% of that found in trophozoites.
Expression pattern of Glγ-giardin in G. lamblia during encystation. a Intracellular level of Glγ-giardin during G. lamblia encystation was performed by western blot analysis. Giardia extracts were prepared at various time-points in trophozoites (0) and encysting cells (6, 12, 24 and 48 h post-induction to encystation). Immunoreactive bands were observed at 35 or 21 kDa, expected sizes of Glγ-giardin or GlCWP1, respectively. After deprobing antibodies with stripping buffer, membrane was incubated with polyclonal rat antibodies specific to PDI1 of G. lamblia (1:10,000) as loading control. An immunoreactive band was detected at 26 kDa. Relative expression of Glγ-giardin during encystation is presented as a bar graph. b Localization of Glγ-giardin in HA-tagged Glγ-giardin expressing G. lamblia during encystation. Giardia lamblia attached to glass slides were reacted overnight with mouse anti-HA (1:100) and rat anti-GlCWP1 polyclonal antibodies (1:400) then incubated with Alexafluor 488-conjugated anti-mouse IgG (1:100) and Alexafluor 555-conjugated anti-rat IgG (1:200). Cells were mounted with DAPI containing anti-fade mounting medium and observed. DIC image was acquired to show cell morphology. Scale-bars: 2 μm
The intracellular location of Glγ-giardin was examined by IFA using anti-HA antibodies and anti-GlCWP1 antibodies (Fig. 6b). As expected, expression of GlCWP1 started to be detected from the cells at 6 h post-encystation, and increased dramatically to 48 h. Localization of GlCWP in the encystation specific vesicles was clearly demonstrated in encysting cells at 12 h. In the cells at 48 h post-induction, GlCWP was found on the surface of the cells, i.e. cyst wall. Glγ-giardin was mainly observed at the ventral disc in trophozoites. As the encystation process occurred, Glγ-giardin was mainly found in the traces of the adhesive disc of G. lamblia.
Discussion
Giardia lamblia is a protozoan, which has a cell cycle composed of two forms, dividing trophozoite and infective cyst. Giardia trophozoites have two identical nuclei, both of which are transcriptionally active [34]. Most trophozoites are present at the G2-phase, which is a restriction point determining the pathway for binary division or for differentiation to cyst [35, 36]. Only limited information is available regarding the cell cycle control of G. lamblia. Several studies have been conducted to identify cell cycle regulators in Giardia differentiation conditions to control the process of Giardia division. Reiner et al. [3] demonstrated synchronization of Giardia trophozoites using aphidicolin, and monitored the expression of well-known stage-specific genes. Expression of the cyclin B gene was found to be maximal at the G2-phase, whereas expression of the histone genes was upregulated at the S-phase [3]. An additional study using a counterflow centrifugal elutriation method indicated that several well-known cell stage genes (thymidine kinase, minichromosome maintenance 5, polo-like kinase, and proliferating cell nuclear antigen) were upregulated in G2-phase cells [5]. Several investigations on encystation using both transcriptomics [37,38,39] and proteomics [40,41,42] resulted in identification of the following as upregulated proteins: metabolic, secretory, trafficking, cytoskeletal-related, transcription factor, variant-specific and hypothetical. To identify a new cell stage-specific protein in Giardia, we used aphidicolin to synchronize trophozoites to prepare G1/S-phase and G2-phase cells. Comparative proteomic analysis of G1/S-phase versus G2-phase extended the list of these cell stage-specific proteins in G. lamblia (Fig. 1 and Table 3). Disappointingly, the majority of the identified genes were categorized as metabolic and hypothetical proteins, for which it is hard to conjecture a relationship with the cell cycle control of Giardia trophozoites.
Among the identified proteins showing upregulation at G2-phase, Glγ-giardin, a known component of the ventral disc, was selected for further investigation to monitor the structural change of the ventral disc during the cell cycle. Upregulation of Glγ-giardin at G2-phase was confirmed by two different methods, western blot and quantitative RT-PCR (Fig. 3). IFA indicated that Glγ-giardin was localized at the ventral disc (Fig. 2b), as reported in previous studies [6, 43]. Interestingly, Glγ-giardin was also found in the MB in 12% of interphase trophozoites (data not shown) and localization of Glγ-giardin in MB varied in a phase-dependent manner. That is, more G2-phase cells showed MB labelled with anti-Glγ-giardin antibodies than G1/S-phase cells (Fig. 2c). The MB is a unique cytoskeletal structure hypothesized as a dynamic reservoir of MTs [26, 44]. Recent studies have suggested that the volume of MBs varies depending on the cell stage; this volume gradually increased from G1-phase to G2-phase [5], and the MB has been shown to contribute to the biogenesis of the ventral disc and mitotic spindle, as well as the flagella [45]. In addition, GlMBP isolated from ventral disc was localized in both the ventral disc and MB and played a role in disc biogenesis [26].
Knockdown of expression of Glγ-giardin was performed in trophozoites, and the effect of Glγ-giardin knockdown on cell division was monitored (Fig. 4c, d). Two different assays demonstrated distinct results for cells arrested at cytokinesis, i.e. Giemsa-stained cells and DAPI-stained cells with a less and more dramatic difference, respectively. Despite this difference, knockdown of Glγ-giardin expression resulted in defects in the cell cycle progression of G. lamblia.
In addition, G. lamblia with lower level of Glγ-giardin was examined with respect to organization and function of the ventral disc (Fig. 5). The effect of Glγ-giardin knockdown on the surface of the ventral disc was not obvious in SEM images (Fig. 5a). Deformation was only found in the ventral groove, a posterior region of the ventral disc with convex curvature, in Glγ-giardin knockdown cells. It was obvious in transverse TEM images of Giardia expressing less Glγ-giardin that the formation of microribbons was affected. Shortening of the microribbon occurring uniformly only in daughter cells during division of Giardia trophozoites had been previously reported [21]. The Giardia ventral disc is composed of MTs, trilaminar microribbons, microribbon-connecting crossbridges, and MT-associated sidearm structures [8, 16, 17, 43]. Recent analysis using negative staining electron tomography and cryo-electron tomography suggested the existence of different functions in different regions of the ventral disc [46]. Glγ-giardin is a unique protein that has no homology with other eukaryotic proteins [6] and has not yet been shown to function. The shortening of microribbon structures and flatting of the ventral groove suggest that Glγ-giardin will play a role in this region of the ventral disc. In our previous research, we found Glγ-giardin that binds to the G. lamblia EB1 (GlEB1) protein, a well-known MT-binding protein, by yeast two-hybrid assay using GlEB1 bait [47]. This finding is presumed to support the assumption that various proteins are present in the ventral groove and that these will play a role in curvature of the ventral disc [43, 48]. Further investigation is required to examine how the Glγ-giardin and GlEB1 proteins interactions are involved in the biogenesis of the ventral disc.
Despite the flattening of the ventral groove in Giardia with lower levels of Glγ-giardin, there was no defect in the adherence ability of Giardia trophozoites (Fig. 5c). The adherence assay appeared to be properly performed, as we observed the knockdown effect of GlMBP [26]. It has been reported that the lateral crest and the bare area play more important roles in attachment than the ventral groove [12, 26].
Using Glγ-giardin as a marker, we monitored the fate of the ventral disc during encystation (Fig. 6). Because ventral disc formation may occur in newly generated trophozoites, we expected lower expression of Glγ-giardin in the cyst stage. Western blot analysis clearly indicated decreased expression of Glγ-giardin during encystation. This result is consistent with the finding of previous studies that transcription of Glγ-giardin was decreased during encystation [19]. Decreased expression of α2-, β-, and δ-giardin was reported in the encysting G. lamblia [49] whereas no information is available for the expression of Glγ-giardin during encystation. Therefore, our study extends the list of giardins of which expression was diminished during encystation. IFA indicated that Glγ-giardin was mainly localized to the trail structure of the adhesive disc of the encysting cells (Fig. 6b). During encystation, the ventral disc components have generally been found to be broken down and shrunken [49, 50].
Conclusions
In the present study, we searched for new proteins related to Giardia differentiation through preparation of cell stage-specific cells using aphidicolin, and analyzed the lysate using LC–MS/MS. Glγ-giardin was identified as an upregulated protein in the G2-phase of the Giardia cell cycle and a downregulated protein during encystation. Knockdown experiments demonstrated that Glγ-giardin is a component of the trilaminar structure of the ventral disc, which is important in cell division of G. lamblia.
Availability of data and materials
The data supporting the conclusions of this article are included within the article and its additional file.
Abbreviations
- Glγ-giardin:
-
Giardia lamblia γ-giardin
- MT:
-
microtubule
- Q-TOF:
-
quadrupole time-of-flight
- LC–MS/MS:
-
liquid chromatography–tandem mass spectrometry/mass spectrometry
- IFA:
-
immunofluorescence assay
- DAPI:
-
4′,6-diamidino-2-phenylindole
- SEM:
-
scanning electron microscopy
- TEM:
-
transmission electron microscopy
- CWP1:
-
cyst wall protein 1
- 2-DGE:
-
2-dimensional gel electrophoresis
- MBP:
-
median body binding protein
- MB:
-
median body
- GlEB1:
-
Giardia lamblia EB1
References
Lane S, Lloyd D. Current trends in research in the waterborne parasite Giardia. Crit Rev Microbiol. 2002;28:123–47.
Markova K, Uzlikova M, Tumova P, Jirakova K, Hagen G, Kulda J, et al. Absence of a conventional spindle mitotic checkpoint in the binucleated single-celled parasite Giardia intestinalis. Eur J Cell Biol. 2016;95:355–67.
Reiner DS, Ankarklev J, Troell K, Palm D, Bernander R, Gillin FD, et al. Synchronisation of Giardia lamblia: identification of cell cycle stage-specific genes and a differentiation restriction point. Int J Parasitol. 2008;38:935–44.
Poxleitner MK, Dawson SC, Cande WZ. Cell cycle synchrony in Giardia intestinalis cultures achieved by using nocodazole and aphidicolin. Eukayot Cell. 2008;7:569–74.
Horlock-Roberts K, Reaume C, Dayer G, Ouellet C, Cook N, Yee J. Drug-free approach to study the unusual cell cycle of Giardia intestinalis. mSphere. 2017;2:e00384–416.
Nohria A, Alonso RA, Peattie DA. Identification and characterization of gamma-giardin and the gamma-giardin gene from Giardia lamblia. Mol Biochem Parasitol. 1992;56:27–37.
Elmendorf HG, Dawson SC, McCaffery JM. The cytoskeleton of Giardia lamblia. Int J Parasitol. 2003;33:3–28.
Holberton DV, Ward AP. Isolation of the cytoskeleton from Giardia. Tubulin and a low-molecular-weight protein associated with microribbon structures. J Cell Sci. 1981;47:139–66.
Crossley R, Marshall J, Clark JT, Holberon DV. Immunocytochemical differentiation of microtubules in the cytoskeleton of Giardia lamblia using monoclonal antibodies to alpha-tubulin and polyclonal antibodies to associated low molecular weight proteins. J Cell Sci. 1986;80:233–52.
Peattie DA, Alonso RA, Hein A, Caulfield JP. Ultrastructural localization of Giardins to the edges of disk microribbons of Giardia lamblia and the nucleotide and deduced protein sequence of alpha giardin. J Cell Biol. 1989;109:2323–35.
Szkodowska A, Muller MC, Linke C, Scholze H. Annexin XXI (ANX21) of Giardia lamblia has sequence motifs uniquely shared by giardial annexins and is specifically localized in the flagella. J Biol Chem. 2002;277:25703–6.
Morrison HG, McArthur AG, Gillin FD, Aley SB, Adam RD, Olsen GJ, et al. Genomic minimalism in the early diverging intestinal parasite Giardia lamblia. Science. 2007;317:1921–6.
Holberton D, Baker DA, Marshall J. Segmented alpha-helical coiled-coil structure of the protein giardin from the Giardia cytoskeleton. J Mol Biol. 1988;204:789–95.
Jenkins MC, O’Brien CN, Murphy C, Schwarz R, Miska K, Rosenthal B, et al. Antibodies to the ventral disc protein delta-giardin prevent in vitro binding of Giardia lamblia trophozoites. J Parasitol. 2009;95:895–9.
Adam RD. Biology of Giardia lamblia. Clin Microbiol Rev. 2001;14:447–75.
Holberton DV. Fine structure of the ventral disk apparatus and the mechanism of attachment in the flagellate Giardia muris. J Cell Sci. 1973;13:11–41.
Crossley R, Hoberton DV. Characterization of proteins from the cytoskeleton of Giardia lamblia. J Cell Sci. 1983;59:81–103.
Sheffield HG, Bjorvat B. Ultrastructure of the cyst of Giardia lamblia. Am J Trop Med Hyg. 1977;26:23–30.
Ghosh S, Frisardi M, Rogers R, Samuelson J. How Giardia swim and divide. Infect Immun. 2001;69:7866–72.
Benchimol M. Participation of the adhesive disc during karyokinesis in Giardia lamblia. Biol Cell. 2004;96:291–301.
Tumova P, Kulda J, Nohynkova E. Cell division of Giardia intestinalis: assembly and disassembly of the adhesive disc, and the cytokinesis. Cell Motil Cytoskeleton. 2007;64:288–98.
Keister DB. Axenic culture of Giardia lamblia in TYI-S-33 medium supplemented with bile. Trans R Soc Trop Med Hyg. 1983;77:487–8.
Singer SM, Yee J, Nash TE. Episomal and integrated maintenance of foreign DNA in Giardia lamblia. Mol Biochem Parasitol. 1998;92:59–69.
Kim J, Park SJ. Roles of end-binding 1 protein and gamma-tubulin small complex in cytokinesis and flagella formation of Giardia lamblia. MicrobiologyOpen. 2018. https://doi.org/10.1002/mbo3.748.
Kim J, Lee HY, Lee KH, Park SJ. Phosphorylation of serine 148 in Giardia lamblia end-binding 1 protein is important for cell division. J Eukaryot Microbiol. 2017;64:464–80.
Woessner DJ, Dawson SC. The Giardia median body protein is a ventral disc protein that is critical for maintaining a domed disc conformation during attachment. Eukaryot Cell. 2012;11:292–301.
Kim J, Nagami S, Lee KH, Park SJ. Characterization of microtubule-binding and dimerization activity of Giardia lamblia end-binding 1 protein. PLoS One. 2014;9:e97850.
Kane AV, Ward HD, Keusch GT, Pereira ME. In vitro encystation of Giardia lamblia: large-scale production of in vitro cysts and strain and clone differences in encystation efficiency. J Parasitol. 1991;77:974–81.
Mowatt MR, Luján HD, Cotten DB, Bowers B, Yee J, Nash TE, et al. Developmentally regulated expression of a Giardia lamblia cyst wall protein gene. Mol Microbiol. 1995;15:955–63.
Kim J, Sim S, Kim J, Song K, Yong TS, Park SJ. Giardia lamblia EB1 is a functional homolog of yeast Bim1p that binds to microtubules. Parasitol Int. 2008;57:465–71.
Reaume C, Moore B, Hernández P, Ruzzini A, Chlebus M, Wasserman M, Yee J. Evaluation of drugs and stationary growth on the cell cycle of Giardia intestinalis. Mol Biochem Parasitol. 2013;187:72–6.
Kang K, Kim J, Yong TS, Park SJ. Identification of end-binding 1 (EB1) interacting proteins in Giardia lamblia. Parasitol Res. 2010;106:723–8.
Gourguechon S, Holt LJ, Cande WZ. The Giardia cell cycle progresses independently of the anaphase-promoting complex. J Cell Sci. 2013;126:2246–55.
Yu LZ, Birky CW Jr, Adam RD. The two nuclei of Giardia each have complete copies of the genome and are partitioned equationally at cytokinesis. Eukaryot Cell. 2002;1:191–9.
Bernander R, Palm JE, Svärd SG. Genome ploidy in different stages of the Giardia lamblia life cycle. Cell Microbiol. 2001;3:55–62.
Hofstetrova K, Uzlikova M, Tůmová P, Troell K, Svärd SG, Nohýnková E. Giardia intestinalis: aphidicolin influence on the trophozoite cell cycle. Exp Parasitol. 2010;124:159–66.
Birkeland SR, Preheim SP, Davids BJ, Cipriano MJ, Palm D, Reiner DS, et al. Transcriptome analyses of the Giardia lamblia life cycle. Mol Biochem Parasitol. 2010;174:62–5.
Morf L, Spycher C, Rehrauer H, Fournier CA, Morrison HG, Hehl AB. The transcriptional response to encystation stimuli in Giardia lamblia is restricted to a small set of genes. Eukaryot Cell. 2010;9:1566–76.
Einarsson E, Troell K, Hoeppner MP, Grabherr M, Ribacke U, Svärd SG. Coordinated changes in gene expression throughout encystation of Giardia intestinalis. PLoS Negl Trop Dis. 2016;10:e0004571.
Kim J, Bae SS, Sung MH, Lee KH, Park SJ. Comparative proteomic analysis of trophozoites versus cysts of Giardia lamblia. Parasitol Res. 2009;104:475–9.
Faso C, Bischof S, Hehl AB. The proteome landscape of Giardia lamblia encystation. PLoS One. 2013;8:e83207.
Emery SJ, Pascovi D, Lacey E, Haynes PA. The generation gap: Proteome changes and strain variation during encystation in Giardia duodenalis. Mol Biochem Parasitol. 2015;201:47–56.
Hardin WR, Li R, Xu J, Shelton AM, Alas GCM, Minin VN, et al. Myosin-independent cytokinesis in Giardia utilizes flagella to coordinate force generation and direct membrane trafficking. Proc Natl Acad Sci USA. 2017;114:E5854–63.
Campanati L, De Souza W. The cytoskeleton of Giardia lamblia. Trend Cell Mol Biol. 2009;4:49–61.
Hagen KD, Hirakawa MP, House SA, Schwartz CL, Pham JK, Cipriano MJ, et al. Novel structural components of the ventral disc and lateral crest in Giardia intestinalis. PLoS Negl Trop Dis. 2011;5:e1442.
Brown JR, Schwartz CL, Heumann JM, Dawson SC, Hoenger A. A detailed look at the cytoskeletal architecture of the Giardia lamblia ventral disc. J Struct Biol. 2016;194:38–48.
Nosala C, Hagen KD, Dawson SC. ‛Disc-o-Feverʼ: Getting down with Giardia’s groovy microtubule organelle. Trends Cell Biol. 2018;28:99–112.
Palm D, Weiland M, McArthur AG, Winiecka-Krusnell J, Cipriano MF, Birkeland SR, et al. Developmental changes in the adhesive disk during Giardia differentiation. Mol Biochem Parasitol. 2005;141:199–207.
Jenkins MC, O’Brien CN, Macarisin D, Miska K, Fetterer R, Fayer R. Analysis of giardin expression during encystation of Giardia lamblia. J Parasitol. 2012;98:1266–70.
Midlej V, Benchimol M. Giardia lamblia behavior during encystment: how morphological changes in shape occur. Parasitol Int. 2009;58:72–80.
Acknowledgements
Not applicable.
Funding
This research was supported by the Basic Science Research Program (2015R1D1A1A09057595 to SJP) and the Research Fellow Program (2016R1A6A3A11933823 to JK) through the National Research Foundation of Korea (NRF) funded by the Ministry of Education of Korea.
Author information
Authors and Affiliations
Contributions
JK and SJP designed this study. JK performed the experiments. JK and SJP analyzed the data and wrote the manuscript. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1: Figure S1.
Determination of the specificity of the polyclonal antibodies against recombinant Giardia lamblia cyclin B protein. a Western blot analysis of Escherichia coli lysates expressing recombinant Glcyclin B using rat anti-Glcyclin B polyclonal antibodies. b Western blot analysis of Giardia extracts using rat anti-Glcyclin B polyclonal antibodies. The immunoreactive recombinant Glcyclin B or native Glcyclin B are indicated with arrows.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Kim, J., Park, SJ. Role of gamma-giardin in ventral disc formation of Giardia lamblia. Parasites Vectors 12, 227 (2019). https://doi.org/10.1186/s13071-019-3478-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-019-3478-8