Abstract
Background
We have previously demonstrated that intranasal vaccination of highly susceptible BALB/c mice with whole Leishmania amazonensis antigens (LaAg) leads to protection against murine cutaneous leishmaniasis. Here, we evaluate the response of partially resistant C57BL/6 mice to vaccination as a more representative experimental model of human cutaneous leishmaniasis.
Methods
C57BL/6 mice from different animal facilities were infected with L. amazonensis (Josefa strain) to establish the profile of infection. Intranasal vaccination was performed before the infection challenge with two doses of 10 μg of LaAg alone or associated with the adjuvant ADDAVAX® by instillation in the nostrils. The lesion progression was measured with a dial caliper and the parasite load by limited dilution assay in the acute and chronic phases of infection. Cytokines were quantified by ELISA in the homogenates of infected footpads.
Results
C57BL/6 mice from different animal facilities presented the same L. amazonensis infection profile, displaying a progressive acute phase followed by a controlled chronic phase. Parasites cultured in M199 and Schneider’s media were equally infective. Intranasal vaccination with LaAg led to milder acute and chronic phases of the disease. The mechanism of protection was associated with increased production of IFN-gamma in the infected tissue as measured in the acute phase. Association with the ADDAVAX® adjuvant did not improve the efficacy of intranasal LaAg vaccination. Rather, ADDAVAX® reduced vaccination efficacy.
Conclusion
This study demonstrates that the efficacy of adjuvant-free intranasal vaccination with LaAg is extendable to the more resistant C57Bl/6 mouse model of infection with L. amazonensis, and is thus not exclusive to the susceptible BALB/c model. These results imply that mucosal immunomodulation by LaAg leads to peripheral protection irrespective of the genetic background of the host.
Similar content being viewed by others
Background
Leishmania amazonensis is a causative agent of localized and diffuse cutaneous leishmaniasis in Latin America [1, 2]. In Brazil, infections with L. amazonensis used to be concentrated in the North of the country (Amazon Forest Region) [3]. In Manaus, 8 % of cutaneous infections were caused by L. amazonensis [4]. Since 2005, the Brazilian Ministry of Health has demonstrated the presence of L. amazonensis in all regions of Brazil [3]. The concern about L. amazonensis in Brazil relates to all forms of disease, including visceral and mucosal leishmaniasis [5] and the refractoriness to treatment of serious forms of the infection [6]. Difficulty in access to the regions affected by the disease hinders treatment efforts [3], thus the best strategy is prevention through vaccination.
Leishmania amazonensis is highly virulent with capacity to infect several hosts [7]. BALB/c mice have been used for several studies; however, this model of infection is a progressive non-healing disease. This fate is not related to the most prevalent presentation of natural cutaneous infection in human populations, which is characterized by an open spontaneously healing wound, leaving an unpleasant scar containing parasites [7]. C57BL/10 mice present the same phenotype as BALB/c after experimental infection with L. amazonensis [8]. However, in C57BL/6 mice, the infection was described to have a distinctive progressive [9] and a non-progressive disease profile [10] even for the same parasite strain (MHOM/BR/77/LTB0016). Some differences in in vivo infection could be associated to differences in strains [11], time post-infection studied, challenge used, site of infection and infection route used [12]. Furthermore, the differences in microbiota is currently known to affect the immune response in mice of the same background [13, 14].
The development of a vaccine against different Leishmania parasites is the priority to control leishmaniasis [15]. Unfortunately, we do not have any vaccine approved for human use [16]. The Leishvacin® (or LaAg) vaccine, comprised of whole Leishmania amazonensis antigens, has been studied for several years. Although the safety and capacity to induce IFN-gamma production was demonstrated [17], the vaccine failed in the phase 3 of a clinical trial [18]. It is noteworthy that these trials were performed using the subcutaneous or intramuscular route of administration. Using experimental models and the same route, the immunization with LaAg in monkeys [19] or BALB/c mice [20] exacerbated the disease progression of L. amazonensis infection. However, when the same antigen was tested by intranasal route, it induced protection on BALB/c mice [21]. Mucosal vaccine elicits immune responses effective against several pathogens [22], and the intranasal route has been effective against leishmaniasis using BALB/c mice [23–28] and hamster [29, 30] models.
To improve vaccine efficacy, several adjuvants have been studied for use by the mucosal route [26, 28, 31]. Protective responses of Leish111f [26] and recombinant LACK [28] were improved when associated to cholera toxin, but this adjuvant is not approved for human use [31]. The only adjuvant approved for intranasal use is the MF59® [32]. A similar adjuvant called ADDAVAX®, a nano oil-water emulsion formulated with scalene, was developed by Invitrogen. Intranasal LaAg vaccine is effective without association of adjuvants against leishmaniasis [21] and the association with adjuvants, as ADDAVAX®, could enhance the protective immunity.
In this paper, we established the infection model of C57BL/6 from different animal facilities using L. amazonensis (strain MHOM/BR/75/Josefa). This strain was isolated from a patient with cutaneous leishmaniasis (the most common form of the disease) in 1975 by Dr. Cesar Cuba-Cuba (Universidade de Brasília, Brasília, Brazil). We evaluated the LaAg intranasal vaccine in this mouse model. The intranasal LaAg vaccine induced partial protection during the progressive and chronic phase against L. amazonensis on C57BL/6.
Methods
Animals
C57BL/6 mice were acquired from different animal breeding facilities: Universidade Federal Fluminense (C57Bl/6-UFF), Universidade Federal do Rio de Janeiro (C57Bl/6-UFRJ), Fundação Oswaldo Cruz (C57Bl/6-FIOCRUZ) and Universidade Estadual de Campinas (C57Bl/6-UNICAMP). BALB/c mice were from UFF animal facility. Animals were maintained in our own animal facility at UFRJ using sterilized bedding, filtered water and pelleted food. For experiments, females were used at 6–8 weeks of age.
Parasites
For infection experiments, L. amazonensis (strain MHOM/BR/75/Josefa) [33] and L. amazonensis (MPRO/BR/72/M1845, LV78 strain) [34] promastigotes were maintained at 26 °C in M199 medium containing 10 % heat-inactivated fetal bovine serum (HIFCS, GIBCO Laboratories, Grand Island, NY, USA) or Schneider’s medium containing 10 % HIFCS until the stationary-growth phase. The Josefa strain was originally isolated from cutaneous leishmaniasis [33], whereas the LV78 strain was isolated from skin of the rat Proechimis sp. [34]. Quantification of metacyclic promastigotes was performed routinely and was around 50 % using Ficoll density gradient.
LaAg preparation
Leishmania amazonensis (MHOM/BR/75/Josefa strain) promastigotes were maintained at 26 °C in M199 medium containing 10 % HIFCS. Leishmania amazonensis promastigote antigens (LaAg) were prepared as previously described [35]. Briefly, stationary-growth phase promastigotes were washed three times in phosphate buffered saline (PBS) and subjected to three cycles of freezing and thawing. LaAg was lyophilized, stored at -20 °C and reconstituted with PBS immediately prior to use.
Immunization, infection challenge and evaluation of disease progression
Mouse immunization was by instillation of 10 μg of LaAg in 20 μl of PBS, 10 μl in each nostril, using a micropipette adapted with a polystyrene microtip. A booster dose was given 7 days later [21]. Controls received PBS alone. For association with adjuvant, 10 μg of LaAg (in 10 μl) was mixed by pipetting with 10 μl of ADDAVAX®, and 10 μl were administered in each nostril. Seven days post-boost, animals were infected in the right hind footpad with 5 × 105 or 2 × 106 stationary-phase L. amazonensis promastigotes. Lesion sizes were measured once a week with a dial caliper and expressed as the difference between the thicknesses of infected and contralateral non-infected footpads. The parasite load was determined at the end of the experiments, when the infected foot was skinned and individually homogenized in 1 ml of PBS using a tissue grinder. Tissue debris was removed by gravity sedimentation for 5 min. Homogenates were submitted to limited dilution assay (LDA).
Cytokine quantification
For in situ production [24], infected footpads were isolated, skinned, weighed, teased and individually homogenized in 1 ml of PBS using a glass tissue homogenizer. The footpad homogenates were centrifuged (10 min, 20,000 × g at 4 °C) and the supernatants collected. For cytokine quantification, supernatants prepared as above were assayed for TGF-β, IFN-γ, IL-10 and IL-4 by ELISA following the manufacturer’s instructions (R&D Systems, Minneapolis, USA). For TGF-β, the supernatants were pre-heated to 80 °C for 5 min prior to the assay.
Flow cytometry
Lymph node cells isolated from mice were cultured for 4 h to at 37 °C in the presence of PMA (20 ng/ml), Ionomycin (1μg/ml) and brefeldin A (Sigma-Aldrich, St. Lois, USA). Cells were surface stained with Anti-CD3-Percp and anti-CD8-FITC and anti-CD4-PE CY7 (Biolegend, San Diego, USA) and fixed and permeabilized for 1 h using Foxp3/Transcription Factor Fixation/Permeabilization Kit (e-Bioscience, Santa Clara, USA). Intracellular cytokine staining was performed with anti- IFN-γ -APC (Biolegend). At least 10,000 gated CD4+ lymphocyte events were acquired. Analytical flow cytometry was conducted with a BD FACSCanto™ II (BD Biosciences New Jersey, USA) and the data were processed with FlowJo X software.
Statistical analysis
The experiments were performed two or three times, and the result of one representative experiment is shown. For experiments illustrated in Figs. 1 and 2, differences of the peak of infection to the progressive phase and the chronic phase were tested statistically by Student's t-test. For the results provided in the remaining figures, differences between vaccinated and non-vaccinated groups were tested by Student’s t-test. We used the GraphPad Prism v. 5 software, and were considered significant when P ≤ 0.05.
Course of infection by L. amazonensis challenge (Josefa strain) in C57BL/6 mice from different sources. Leishmania amazonensis were cultured on M199 Medium. C57Bl/6-UNICAMP (a, b), C57Bl/6-FIOCRUZ (c, d), C57Bl/6-UFRJ (e, f) and C57Bl/6-UFF (g, h) were infected in the footpads with 5 × 105 stationary-phase promastigotes of L. amazonensis by subcutaneous route. Lesion sizes were measured at the indicated days and are expressed as the difference in thickness between non-infected and infected footpads (a, c, e, g). Parasite load was measured at the end of the experiment and expressed as the mean number of parasites in each footpad (b, d, f, h). The data (means ± standard deviations; n = 4–5) are representative of two (a, b) and three (c, d, e, f, g, h) independent experiments producing the same result profile. *P ≤ 0.05 in comparison to peak of infection (a, 42 days; c, 56 days; e, 56 days, g, 53 days; see Table 1 for details)
Comparison of infection of C57BL/6 mice by L. amazonensis Josefa strain versus LV78 strain. Leishmania amazonensis (Josefa or LV78 strains) were cultured on Schneider’s medium. C57Bl/6-UFF were infected with stationary-phase promastigotes of L. amazonensis Josefa strain (a, b) or LV78 strain (c, d). Lesion sizes were measured at the indicated days and expressed as the difference of thickness between non-infected and infected footpads (a, c). Parasite load was measured at the end of the experiment and expressed as the mean number of parasites per footpad (b, d). The data (means ± standard deviations; n = 4–5) are representative of two independent experiments producing the same result profile. *P ≤ 0.05 in comparison to peak of infection (a, 49 days; c, 55 days; see Table 2 for details)
Results
Characterization of the partially resistant model of L. amazonensis infection in C57BL/6 mice
To characterize the chronic mouse model of infection using L. amazonensis Josefa strain in C57BL/6 mice, we evaluated mice from different animal facilities: UNICAMP (Fig. 1a), FIOCRUZ (Fig. 1c), UFRJ (Fig. 1e) and UFF (Fig. 1g). All mice presented a similar profile after L. amazonensis infection, with lesion progression until days 42–60 post-infection followed by a partial resolution of the lesion, with chronic parasite persistence (Fig. 1, Table 1). Independently of the animal facility of origin, the parasite load was very similar in the chronic infection (Fig. 1b, d, f and h). The results demonstrated a partially resistant mouse model with chronic infection by L. amazonensis. All these experiments were performed with parasites cultured in M199 medium. To evaluate the interference of the culture medium on the infection, the assay was repeated using Schneider’s medium. Results were very similar to M199 medium, with compared lesion progression followed by partial resolution and chronic infection (Fig. 2a) and parasite load (Fig. 2b). We also evaluated this resistance model using a different strain of L. amazonensis, to test if this profile is general to the parasite species. Using L. amazonensis LV78 strain (MPRO/BR/72/M1845), we could observe a similar profile of infection (Fig. 2c, Table 2) and parasite load (Fig. 2d) in comparison to L. amazonensis Josefa strain. For data presented in Figs. 1 and 2, based on statistics, a lesion growth in the progressive phase, a partial lesion resolution and lesion stabilization in the chronic phase compared with the peak of infection, was observed in all experiments performed.
To show that this infection profile was related to mice lineage and not to leishmanial strain, infection of L. amazonensis (Josefa strain) was performed on BALB/c mice to demonstrate a progressive (non-healing) disease in this mouse model (Additional file 1: Figure S1). The establishment and use of a partially resistant chronic infection mouse model is interesting because this model is more similar to the natural course of cutaneous infection in humans.
Efficacy of intranasal LaAg vaccine against L. amazonensis infection in C57BL/6 mice
Intranasal LaAg vaccine has been demonstrated to be effective on susceptible BALB/c mice against L. amazonensis infection [21]. We evaluated intranasal LaAg vaccine on C57BL/6 mice against L. amazonensis infection. As expected, non-vaccinated mice presented the lesion profile described above, with a progressive lesion until day 63 post-infection, when a partial lesion resolution ensued associated with a chronic resistant lesion (Fig. 3a). Immunized mice controlled the lesion progression from day 42 post-infection (Fig. 3a). After partial lesion resolution, both, PBS and LaAg, showed the same lesion size after day 84 post-infection (Fig. 3a). However, the parasite load at day 98 day post-infection demonstrated that intranasal LaAg vaccine reduced the number of parasites in the chronic infection (Fig. 3b).
Evaluation of intranasal LaAg vaccine efficacy in the chronic stage of infection. C57Bl/6-UFF mice received 10 μg of LaAg by the intranasal route on days -14 and -7 of infection. Non-vaccinated controls received PBS alone. On day 0, animals were infected with 5 × 105 promastigotes of L. amazonensis (Josefa strain). a Lesion sizes were measured at the indicated days and expressed as the difference of thickness between non-infected and infected footpads. b Parasite load was measured on day 98 of infection and expressed as the mean number of parasites per footpad. The data (means ± standard deviations; n = 4–5) are representative of three independent experiments producing the same result profile. *P ≤ 0.05 in comparison to PBS controls as follows: a Day 42 (t (6) = 2.853, P = 0.0291); Day 49 (t (6) = 6.113, P = 0.0009); Day 56 (t (6) = 3.970, P = 0.0074); Day 64 (t (6) = 3.416, P = 0.0142); Day 72 (t (6) = 2.481, P = 0.0478); Day 78 (t (6) = 2.921, P = 0,0266). b t(6) = 3.472, P = 0.0070
Varying the number of parasites used to infect mice, low model of infection (challenge with 5 × 105 parasites) and high model of infection (challenge with 2 × 106 parasites), we observed the same profile of lesion progression control (Additional file 2: Figure S2a) and reduction of parasite load (Additional file 2: Figure S2b) following LaAg vaccination. To determine the parasite load during lesion progression, we vaccinated mice and evaluated lesion progression and parasite load at day 44 post-infection. As expected, we could observe the control of lesion progression (Fig. 4a) and a reduction in parasite load, showing that parasite control happens in parallel to lesion progression inhibition (Fig. 3) in vaccinated mice.
Evaluation of intranasal LaAg efficacy in the progressive stage of infection. C57Bl/6-UFF mice received 10 μg of LaAg by the intranasal route on days -14 and -7 of infection. Non-vaccinated controls received PBS alone. On day 0, animals were infected with 5 × 105 promastigotes of L. amazonensis (Josefa strain). a Lesion sizes were measured at the indicated days and expressed as the difference of thickness between non-infected and infected footpads. b Parasite load was measured on day 44 of infection and expressed as the mean number of parasites. The data (means ± standard deviations; n = 5) are representative of three independent experiments producing the same result profile. P ≤ 0.01 in comparison to PBS controls as follows: a Day 39 (t (7) = 3.566, P = 0.0073); Day 44 (t (7) = 5.037, P = 0.0015). b t (7) = 4.614, P = 0.0024
Intranasal LaAg vaccine induced a Th1 response
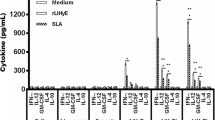
To evaluate the mechanism of vaccine protection, we quantified in situ cytokine levels in the footpad homogenates. We could observe during the lesion progression at day 44 post-infection that LaAg induced in vaccinated mice an increase in IFN-gamma release (Fig. 5a) that paralleled the lesion control (Fig. 4a) and reduction in parasite load (Fig. 4b). However, no modulation of IL-4 (Fig. 5b), TGF-beta (Fig. 5c) and IL-10 (Fig. 5d) were detected. In the chronic infection at day 98 post-infection, despite the reduction in parasite load (Fig. 3b), we could not detect any modulation of IFN-gamma (Additional file 3: Figure S3a), IL-4 (Additional file 3: Figure S3b), TGF-beta (Additional file 3: Figure S3d) and IL-10 (Additional file 3: Figure S3c). Probably, the immune modulation during the lesion progression was enough to decrease and maintain a reduced parasite load, and it is important to point out that the level of IFN-gamma is higher in the chronic phase in comparison to the progressive phase, probably associated to the self-healing (lesion resolution) process. In a preliminary experiment, we observed, in the peak of infection at 44 days post-infection, an induction of CD4+ IFN-γ+ T cells by intranasal LaAg vaccine in comparison to PBS (Additional file 4: Figure S4f) in popliteal lymph node cells. We could not detect any difference in CD8+ IFN-γ+ T cells at the peak of infection (result not shown). This result suggests CD4+ T cells as the major mechanism of Th1 response by Intranasal LaAg vaccine.
In situ cytokine profile in the acute stage of infection. C57Bl/6 mice (from UFF) received 10 μg of LaAg by the intranasal route on days -14 and -7 of infection. Non-vaccinated controls received PBS alone. On day 0, animals were infected with 5 × 105 promastigotes of L. amazonensis (Josefa strain). On day 44 of infection (see Fig. 4), the levels of IFN-γ (a), IL-4 (b), TGF-β (C), IL-10 (d) were measured in the lesion homogenates. The data (means ± standard deviations; n = 4–5) are representative of two independent experiments. *P ≤ 0.05 in comparison to PBS controls (t(6) = 2.491, P = 0.0471)
Association of LaAg with Addavax® adjuvant did not enhance the protective efficacy
Scalene based adjuvant known as MF59 was the first approved adjuvant to be used by intranasal route in the Flu vaccine [32]. Addavax® is a nano emulsion based on scalane oil-water emulsion from Invitrogen. Based on the capacity to induce T cell response by intranasal route of scalene- based adjuvants [32], we hypothesized the association of LaAg with Addavax® could improve the vaccine efficacy. Surprisingly, the association of LaAg with Addavax® partially impaired the lesion control promoted by LaAg (Fig. 6a, Table 3) and reverted its parasite load control in chronic infection (Fig. 6b). The administration of Addavax® alone by intranasal route did not affect the lesion and parasite load (data not shown).
Evaluation of intranasal LaAg vaccine associated with ADDAVAX®. C57Bl/6-UFF mice received 10 μg of LaAg (10 μl) associated or not with ADDAVAX (10 μl) by the intranasal route on days -14 and -7 of infection. Non-vaccinated controls received PBS alone. On day 0, animals were infected with 5 × 105 promastigotes of L. amazonensis (Josefa strain). a Lesion sizes were measured at the indicated days and expressed as the difference of thickness between non-infected and infected footpads. b Parasite load was measured on day 70 of infection and expressed as the mean number of parasites in each footpad. The data (means ± standard deviations; n = 5–6) are representative of three independent experiments producing the same result profile. *P ≤ 0.05: LaAg in comparison to PBS controls; # P ≤ 0.05: b LaAg in comparison to LaAg + ADDAVAX; +P ≤ 0.05; LaAg + ADDVACS in comparison to PBS controls Test statistics for a are provided in Table 3. b LaAg in comparison to PBS: t (8) = 5.788, P = 0.0022; LaAg in comparison to LaAg + Addavacs: (t (8) = 6.501, P = 0.0013)
Discussion
Before clinical studies for vaccines, it is necessary to perform very robust pre-clinical studies using different infection models, such as mice, dog and non-human primates [36]. Intranasal LaAg vaccine is protective to BALB/c mice against L. amazonensis [21] and L. infantum/chagasi infection [23] and to hamsters against L. braziliensis [29]. LaAg ability to protect against different parasite species (L. amazonensis, L. chagasi and L. braziliensis) and positive results in two different species (BALB/c and Hamster) is very promising. However, it is very important to find the best model to evaluate LaAg vaccine efficacy [37]. In this study, we evaluated immunization against L. amazonensis infection in the C57BL/6 mouse model, which displays a different profile of infection.
At the beginning, we characterized the infection of L. amazonensis using Josefa strain on C57BL/6 mice. In the early infection, infected mice presented a progressive phase (42–60 days post-infection), followed by a partial resolution and chronic infection (Figs. 1 and 2). Human cutaneous leishmaniasis infection is a self-healing disease, however, parasites can be found in healed lesions [38]. Human disease is very different from the clinical outcome observed in BALB/c mice [12], being more alike to C57BL/6 mice described here. Although BALB/c mice have been used for drug trials, it is necessary to use a self-healing model that more closely reproduces the natural infection course in humans to evaluate and confirm the efficacy of these compounds [39]. The same concept has to be transposed for vaccine development. It is important that differences between experimental models and humans are accounted for in vaccine development [40]. We presented here a partially resistant mouse model using C57BL/6 mice with a chronic infection with persistent parasite load. Using this model it is possible to evaluate the efficacy of LaAg vaccine in the progressive phase (Fig. 4) and in the chronic phase (Fig. 3). In vaccinated mice, the control of lesion growth (Figs. 3a and 4a) is very important to avoid tissue destruction. The partial reduction of parasite load (Figs. 3b and 4b) could also be important to prevent disease transmission in the progressive phase and in the chronic phase [41].
For standardization of our mouse model, we evaluated mice from different animal facilities and parasites grown in different culture medium. It has previously been described that mice from different facilities could present different microbiota, and this can influence their immune response [14, 42]. We used C57BL/6 mice originally from Jackson Laboratories, however, housed and bred in UNICAMP, FIOCRUZ, UFRJ and UFF animal facilities. Our experiments demonstrated that independent of facility, the infection profiles were very similar (Fig. 1). These results minimize the possibility that results are relevant only for animals from a specific supplier.
Then, we tested different culture media for Leishmania growth and infectivity. The three more important media (199 medium, Grace’s insect tissue-culture medium and Schneider’s Drosophila Medium) have been used for a long time [43]. In this study, we evaluated L. amazonensis infectivity after growth in 199 (Fig. 1) and Schneider’s (Fig. 2) medium, and no difference was observed on the profile of infection. Besides, we evaluated different numbers of parasites used to infect mice: 2 × 105 and 2 × 106. There was no difference in the profile either (data not shown).
It is important to note that different strains of the same parasite can present different disease progression, for example, for Leishmania major, the strain V1 (MHOM/IL/80/Friedlin) has a healing model, but the strain Sd (MHOM/SN/74/SD) is a progressive non-healing model in C57BL/6 mice [11]. There are three L. amazonensis strains being used for research in Brazil: Josefa strain (used in this work), PH8 and LBT0016. LBT0016 was isolated from cutaneous leishmaniasis; Josefa strain was also isolated from cutaneous leishmaniasis [33] and not from diffuse cutaneous leishmaniasis [44]. Thus, this strain was isolated from a patient with the most prevalent presentation of the disease and reproduced the same infection profile after inoculation in mice. LV78 (results herein) and LBT0016 strains also showed the same profile of infection, and as such, are an interesting model to evaluate the impact of vaccines relevant to human leishmaniasis.
However, L amazonensis (MHOM/BR/76/Ma-5) isolated from a human patient with cutaneous diffuse leishmaniasis demonstrated a different profile, presenting a progressive lesion on C57BL/6 mice until 90 days post-infection [45]. In the chronic phase, despite the presence of a large lesion, it was not possible to detect parasites [45]. Others demonstrated that intradermal infection on ears of C57BL/6 mice using L amazonensis PH8 strain, isolated from sand flies, showed a progressive disease with a chronic lesion, in other words, in the chronic phase, the lesion was not uncontrolled; however, also did not heal [46, 47]. The different site of infection (ear) or the different route of infection (intradermal) from subcutaneous injection in the hind paw could affect the lesion progression [12]. These results demonstrate that each parasite should be empirically evaluated to determine the behaviour of infection in mice, but they seem to generally reproduce in the animal model the original behaviour in lesions of human patients. The model used herein presents a chronic phase with a high parasite load resembling the natural history of leishmaniasis and is more interesting for vaccine evaluation due to this similarity with human disease outcome (progressive phase, partial resolution and chronic phase development).
Leishmania amazonensis has the capacity to induce a mixed cytokine response, Th1-IFN-gamma/Th2-IL-4 [48], IL-10 [49] and TGF-beta [20, 50]. Immunization did not modulate IL-4, IL-10 or TGF-beta, maybe indicating a secondary role of these molecules in a vaccine context. The protection observed by intranasal LaAg vaccine on C57BL/6 mice was correlated to IFN-gamma levels in the lesions (Fig. 5). IFN-gamma is a crucial cytokine to control L. major [51, 52] and L. donovani infection [53]. IFN-gamma is described to increase L. amazonensis parasite load in vitro [54], however, in vivo it is considered important for infection control [55]. Moreover, production of IFN-gamma in the site of infection in BALB/c mice is associated with protection against L. amazonensis infection [24]. The mechanism of intranasal LaAg vaccine against L. amazonensis in BALB/c [21]; L. chagasi in BALB/c [26]; L. braziliensis in hamster [29]; and now L. amazonensis in C57BL/6 mice, is associated with IFN-gamma production. These results together demonstrate the importance of IFN-gamma as the major marker for vaccine studies against leishmaniasis. In preliminary experiments, we suggested the participation of CD4+ T cells to produce Interferon-gamma (Additional file 4: Figure S4f) in LaAg vaccine, as indicated for several studies as the most important Th1 parasitic-specific response against leishmaniasis [56].
The human vaccine candidate has to be feasible to protect against different parasites and against different clinical forms [56–58]. Intranasal LaAg vaccine has demonstrated being effective in different mouse models, against different Leishmania species and with different forms of disease [21, 26, 29]. In our work, the choice of a model of infection more similar to human infection based on the self-healing in human with normal immunity using C57BL/6 mice allowed us to do consideration about the LaAg vaccine. The efficacy of the vaccine in the control of the lesion size in the progressive phase is very interesting. Besides, there is a reduction of parasite load in the chronic phase in mice, demonstrating the quality of this vaccine. When we considered the efficacy on BALB/c mice, we can transpose the vaccine against the severe form of disease to cutaneous diffuse leishmaniasis based on the uncontrolled parasite load. The perspective of LaAg intranasal vaccine as a human vaccine candidate is due its capacity to reduce the size of the lesion and control the parasite load. Intranasal LaAg vaccine has all the concepts expected for a human vaccine candidate.
The importance of adjuvants to enhance the immune response of vaccines is already known, and new adjuvants based on squalene emulsion open the possibility to development of new vaccines [32]. The association with ADDAVAX® adjuvant can enhance the protection in some vaccines [59], and hinders efficacy for others [60]. This type of adjuvant has been used to enhance both Th1 and Th2 responses [61, 62]. Here, we demonstrated that the use of LaAg associated to ADDAVAX® decreased the LaAg vaccine efficacy (Fig. 6). The protection of LaAg adjuvant free is very encouraging, but we are still looking for new adjuvants to enhance LaAg protection [26] and for characterization of LaAg components to developing more defined vaccines [24, 63–65] .
Conclusion
Adjuvant free LaAg by intranasal route is protective against L. amazonensis infection using the C57BL/6 mouse model that more closely reproduces the infection profile in humans. The efficacy against other parasites such as L. chagasi and L. braziliensis point to intranasal LaAg immunization as a promising vaccine candidate against leishmaniasis.
Abbreviations
- LaAg:
-
Leishmania amazonensis antigens
- LDA:
-
Limited dilution assay
- DPI:
-
Days post-infection
References
Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, den Boer M. WHO Leishmaniasis Control Team. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7(5), e35671. doi:10.1371/journal.pone.0035671.
Silveira FT, Lainson R, De Castro Gomes CM, Laurenti MD, Corbett CE. Immunopathogenic competences of Leishmania (V.) braziliensis and L. (L.) amazonensis in American cutaneous leishmaniasis. Parasite Immunol. 2009;31(8):423–31. doi:10.1111/j.1365-3024.2009.01116.x.
Manual de vigilência de Leishmaniose Tegumentar Americana/Ministério da Saúde, Secretaria de Vigilância em Saúde, Departamento de vigilância Epidemiológica. Série A. Normas e Manuais Técnicos. -2 ed. Brasília: Editora do Ministério da Saúde; 2007. p. 182. http://bvsms.saude.gov.br/bvs/publicacoes/manual_vigilancia_leishmaniose_2ed.pdf.
Camara Coelho LI, Paes M, Guerra JA, Barbosa M, Coelho C, Lima B, et al. Characterization of Leishmania spp. causing cutaneous leishmaniasis in Manaus, Amazonas, Brazil. Parasitol Res. 2011;108(3):671–7. doi:10.1007/s00436-010-2139-9.
Barral A, Pedral-Sampaio D, Grimaldi Júnior G, Momen H, McMahon-Pratt D, Ribeiro de Jesus A, et al. Leishmaniasis in Bahia, Brazil: evidence that Leishmania amazonensis produces a wide spectrum of clinical disease. Am J Trop Med Hyg. 1991;44(5):536–46.
de Menezes JP, Guedes CE, Petersen AL, Fraga DB, Veras PS. Advances in development of new treatment for leishmaniasis. Biomed Res Int. 2015;815023. doi: 10.1155/2015/815023
Pereira BA, Alves CR. Immunological characteristics of experimental murine infection with Leishmania (Leishmania) amazonensis. Vet Parasitol. 2008;158(4):239–55. doi:10.1016/j.vetpar.2008.09.015. Epub 2008 Sep 18.
Afonso LC, Scott P. Immune responses associated with susceptibility of C57BL/10 mice to Leishmania amazonensis. Infect Immun. 1993;61(7):2952–9.
Ji J, Sun J, Soong L. Impaired expression of inflammatory cytokines and chemokines at early stages of infection with Leishmania amazonensis. Infect Immun. 2003;71(8):4278–88.
Jones DE, Ackermann MR, Wille U, Hunter CA, Scott P. Early enhanced Th1 response after Leishmania amazonensis infection of C57BL/6 interleukin-10-deficient mice does not lead to resolution of infection. Infect Immun. 2002;70(4):2151–8.
Anderson CF, Mendez S, Sacks DL. Nonhealing infection despite Th1 polarization produced by a strain of Leishmania major in C57BL/6 mice. J Immunol. 2005;174(5):2934–41.
Mears ER, Modabber F, Don R, Johnson GE. A review: The current in vivo models for the discovery and utility of new anti-leishmanial drugs targeting cutaneous leishmaniasis. PLoS Negl Trop Dis. 2015;9(9), e0003889. doi:10.1371/journal.pntd.0003889.
de Moura Lopes ME, Carneiro MB, Santos LM, Vieira LQ. Indigenous microbiota and leishmaniasis. Parasite Immunol. 2015. doi:10.1111/pim.12279.
Ivanov II, Frutos Rde L, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4(4):337–49. doi:10.1016/j.chom.2008.09.009.
Domínguez-Bernal G, Horcajo P, Orden JA, Ruiz-Santa-Quiteria JA, De La Fuente R, Ordóñez-Gutiérrez L, Martínez-Rodrigo A, Mas A, Carrión J. HisAK70: progress towards a vaccine against different forms of leishmaniosis. Parasit Vectors. 2015;8:629.
Alvar J, Croft SL, Kaye P, Khamesipour A, Sundar S, Reed SG. Case study for a vaccine against leishmaniasis. Vaccine. 2013;18(31 Suppl 2):B244–9.
De Luca PM, Mayrink W, Coutinho SG, Oliveira MP, Bertho AL, Toledo VP, et al. Evaluation of the stability and immunogenicity of autoclaved and nonautoclaved preparations of a vaccine agaisnt American tegumentar leishmaniasis. Vaccine. 1999;17(9-10):1179–85.
Velez ID, Gilchrist K, Arbelaez MP, Rojas CA, Puerta JA, Antunes CM, et al. Failure of a killed Leishmania amazonensis vaccine against American cutaneous leishmaniasis in Colombia. Trans R Soc Trop Med Hyg. 2005;99:593–8.
Kenney RT, Sacks DL, Sypek JP, Vilela L, Gam AA, Evans-Davis K. Protective immunity using recombinant human IL-12 and alum as adjuvants in a primate model of cutaneous leishmaniasis. J Immunol. 1999;163(8):4481–8.
Pinheiro RO, Pinto EF, Lopes JR, Guedes HL, Fentanes RF, Rossi-Bergmann B. TGF-beta-associated enhanced susceptibility to leishmaniasis following intramuscular vaccination of mice with Leishmania amazonensis antigens. Microbes Infect. 2005;7(13):1317–23.
Pinto EF, Pinheiro RO, Rayol A, Larraga V, Rossi-Bergmann B. Intranasal vaccination against cutaneous leishmaniasis with a particulated leishmanial antigen or DNA encoding LACK. Infect Immun. 2004;72(8):4521–7.
Woodrow KA, Bennett KM, Lo DD. Mucosal vaccine design and delivery. Annu Rev Biomed Eng. 2012;14:17–46. doi:10.1146/annurev-bioeng-071811-150054.
Leal JM, Mosquini M, Covre LP, Stagmiller NP, Rodrigues RR, Christensen D, et al. Intranasal vaccination with killed Leishmania amazonensis promastigotes antigen (LaAg) associated with CAF01 adjuvant induces partial protection in BALB/c mice challenged with Leishmania (infantum) chagasi. Parasitology. 2015;142(13):1640–6.
de Matos Guedes HL, da Silva Costa BL, Chaves SP, de Oliveira Gomes DC, Nosanchuk JD, De Simone SG, Rossi-Bergmann B. Intranasal vaccination with extracellular serine proteases of Leishmania amazonensis confers protective immunity to BALB/c mice against infection. Parasit Vectors. 2014;7:448. doi:10.1186/1756-3305-7-448.
De Oliveira Gomes DC, Schwedersky RP, Barbosa De-Melo LD, Da Silva Costa Souza BL, De Matos Guedes HL, Lopes UG, Rossi-Bergmann B. Peripheral expression of LACK-mRNA induced by intranasal vaccination with PCI-NEO-LACK defines the protection duration against murine visceral leishmaniasis. Parasitology. 2012;139(12):1562–9. doi:10.1017/S0031182012000868.
Sakai S, Takashima Y, Matsumoto Y, Reed SG, Hayashi Y, Matsumoto Y. Intranasal immunization with Leish-111f induces IFN-gamma production and protects mice from Leishmania major infection. Vaccine. 2010;28(10):2207–13. doi:10.1016/j.vaccine.2009.12.055.
Pinheiro RO, Pinto EF, de Matos Guedes HL, Filho OA, de Mattos KA, Saraiva EM, et al. Protection against cutaneous leishmaniasis by intranasal vaccination with lipophosphoglycan. Vaccine. 2007;25(14):2716–22.
McSorley SJ, Rask C, Pichot R, Julia V, Czerkinsky C, Glaichenhaus N. Selective tolerization of Th1-like cells after nasal administration of a cholera toxoid-LACK conjugate. Eur J Immunol. 1998;28(2):424–32.
da Silva-Couto L, Ribeiro-Romão RP, Saavedra AF, da Silva Costa Souza BL, Moreira OC, Gomes-Silva A, et al. Intranasal vaccination with leishmanial antigens protects golden hamsters (Mesocricetus auratus) against Leishmania (Viannia) braziliensis infection. PLoS Negl Trop Dis. 2015;9(1), e3439. doi:10.1371/journal.pntd.0003439.
DE Oliveira Gomes DC, DA Silva Costa Souza BL, DE Matos Guedes HL, Lopes UG, Rossi-Bergmann B. Intranasal immunization with LACK-DNA promotes protective immunity in hamsters challenged with Leishmania chagasi. Parasitology. 2011;138(14):1892–7. doi:10.1017/S0031182011001417.
Thompson AL, Staats HJ. Cytokines: the future of intranasal vaccine adjuvants. Clin Dev Immunol. 2011;2011:289597.
O’Hagan DT, Ott GS, Nest GV, Rappuoli R, Giudice GD. The history of MF59(®) adjuvant: a phoenix that arose from the ashes. Expert Rev Vaccines. 2013;12(1):13–30. doi:10.1586/erv.12.140. Review.
Cuba-Cuba CA, Marsden PD, Barreto AC, Rocha R, Sampaio RR, Patzlaff L. Parasitologic and immunologic diagnosis of American (mucocutaneous) leishmaniasis. Bull Pan Am Healph Organ. 1981;15(3):249–59.
Killick-Kendrick R, Molyneux DH, Ashford RW. Leishmania in phlebotomid sandflies. I. Modifications of the flagellum associated with attachment to the mid-gut and oesophageal valve of the sandfly. Proc R Soc Lond B Biol Sci. 1974;187(1089):409–19.
de Matos Guedes HL, Pinheiro RO, Chaves SP, De-Simone SG, Rossi-Bergmann B. Serine proteases of Leishmania amazonensis as immunomodulatory and disease-aggravating components of the crude LaAg vaccine. Vaccine. 2010;28(33):5491–6. doi:10.1016/j.vaccine.2010.04.109.
Mbow ML, De Gregorio E, Valiante NM, Rappuoli R. New adjuvants for human vaccines. Curr Opin Immunol. 2010;22(3):411–6. doi:10.1016/j.coi.2010.04.004.
Gerdts V, Wilson HL, Meurens F, van Drunen Littel-van den Hurk S, Wilson D, Walker S, et al. Large animal models for vaccine development and testing. ILAR J. 2015;56(1):53–62. doi:10.1093/ilar/ilv009.
Mendonça MG, de Brito ME, Rodrigues EH, Bandeira V, Jardim ML, Abath FG. Persistence of Leishmania parasites in scars after clinical cure of American cutaneous leishmaniasis: Is there a sterile cure? J Infect Dis. 2004;189(6):1018–23.
Yarley V, Croft SL. Chapert 93.Animal models of cutaneous leishmaniasis. In: Zak SO, Merle A, editors. Handbook of Animals models of infection. London: Academic Press, p. 775-781
Modabber F. Experiences with vaccines against cutaneous leishmaniasis: of men and mice. Parasitology. 1989;98(Suppl):S49–60.
Podaliri Vulpiani M, Iannetti L, Paganico D, Iannino F, Ferri N. Methods of control of the Leishmania infantum dog reservoir: State of the art. Vet Med Int. 2011;2011:215964. doi:10.4061/2011/215964.
Russell SL, Gold MJ, Hartmann M, Willing BP, Thorson L, Wlodarska M, et al. Early life antibiotic-driven changes in microbiota enhance susceptibility to allergic asthma. EMBO Rep. 2012;13(5):440–7. doi:10.1038/embor.2012.32.
Hendricks LD, Wood DE, Hajduk ME. Haemoflagellates: commercially available liquid media for rapid cultivation. Parasitology. 1978;76(3):309–16.
Martiny A, Vannier-Santos MA, Borges VM, Meyer-Fernandes JR, Assreuy J, Silva NL C e, de Souza W. Leishmania-induced tyrosine phosphorylation in the host macrophage and its implication to infection. Eur J Cell Biol. 1996;71(2):206–15.
Cupolilo SM, Souza CS, Abreu-Silva AL, Calabrese KS, da Costa SC G. Biological behavior of Leishmania (L.) amazonensis isolated from a human diffuse cutaneous leishmaniasis in inbred strains of mice. Histol Histopathol. 2003;18(4):1059–65.
Lima-Junior DS, Costa DL, Carregaro V, Cunha LD, Silva AL, Mineo TW, et al. Inflammasome-derived IL-1β production induces nitric oxide-mediated resistance to Leishmania. Nat Med. 2013;19(7):909–15. doi:10.1038/nm.3221.
Côrtes DF, Carneiro MB, Santos LM, Souza TC, Maioli TU, Duz AL, et al. Low and high-dose intradermal infection with Leishmania major and Leishmania amazonensis in C57BL/6 mice. Mem Inst Osw Cruz. 2010;105(6):736–45.
Ji J, Sun J, Qi H, Soong L. Analysis of T helper cell responses during infection with Leishmania amazonensis. Am J Trop Med Hyg. 2002;66(4):338–45.
Qi H, Popov V, Soong L. Leishmania amazonensis-dendritic cell interactions in vitro and the priming of parasite-specific CD4(+) T cells in vivo. J Immunol. 2001;167(8):4534–42.
Barral A, Teixeira M, Reis P, Vinhas V, Costa J, Lessa H, et al. Transforming growth factor-beta in human cutaneous leishmaniasis. Am J Pathol. 1995;147(4):947–54.
Glennie ND, Yeramilli VA, Beiting DP, Volk SW, Weaver CT, Scott P. Skin-resident memory CD4+ T cells enhance protection against Leishmania major infection. J Exp Med. 2015;212(9):1405–14. doi:10.1084/jem.20142101.
Scott P. Th cell development and regulation in experimental cutaneous leishmaniasis. Chem Immunol. 1996;63:98–114.
Murray HW, Mitchell-Flack M, Taylor GA, Ma X. IFN-γ-induced macrophage antileishmanial mechanisms in mice: A role for immunity-related GTPases, Irgm1 and Irgm3, in Leishmania donovani infection in the liver. Exp Parasitol. 2015;157:103–9. doi:10.1016/j.exppara.2015.07.005.
Qi H, Ji J, Wanasen N, Soong L. Enhanced replication of Leishmania amazonensis amastigotes in gamma interferon-stimulated murine macrophages: implications for the pathogenesis of cutaneous leishmaniasis. Infect Immun. 2004;72(2):988–95.
Pinheiro RO, Rossi-Bergmann B. Interferon-gamma is required for the late but not early control of Leishmania amazonensis infection in C57Bl/6 mice. Mem Inst Osw Cruz. 2007;102(1):79–82.
Kumar R, Engwerda C. Vaccines to prevent leishmaniasis. Clin Transl Immunology. 2014;3(3), e13.
Mendonça SC. Differences in immune responses against Leishmania induced by infection and by immunization with killed parasite antigen: implications for vaccine discovery. Parasit Vectors. 2016;9:492.
Srivastava S, Shankar P, Mishra J, Singh S. Possibilities and challenges for developing a successful vaccine for leishmaniasis. Parasit Vectors. 2016;9(1):277.
Goff PH, Eggink D, Seibert CW, Hai R, Martínez-Gil L, Krammer F, Palese P. Adjuvants and immunization strategies to induce influenza virus hemagglutinin stalk antibodies. PLoS One. 2013;8(11), e79194. doi:10.1371/journal.pone.0079194.
Cayatte C, Schneider-Ohrum K, Wang Z, Irrinki A, Nguyen N, Lu J, et al. Cytomegalovirus vaccine strain towne-derived dense bodies induce broad cellular immune responses and neutralizing antibodies that prevent infection of fibroblasts and epithelial cells. J Virol. 2013;87(20):11107–20. doi:10.1128/JVI.01554-13.
Calabro S, Tritto E, Pezzotti A, Taccone M, Muzzi A, Bertholet S, et al. The adjuvant effect of MF59 is due to the oil-in-water emulsion formulation, none of the individual components induce a comparable adjuvant effect. Vaccine. 2013;31(33):3363–9. doi:10.1016/j.vaccine.2013.05.007.
Ott G, Barchfeld GL, Chernoff D, Radhakrishnan R, van Hoogevest P, Van Nest G. MF59. Design and evaluation of a safe and potent adjuvant for human vaccines. Pharm Biotechnol. 1995;6:277–96.
de Matos Guedes HL, Duarte Carneiro MP, de Oliveira Gomes DC, Rossi-Bergmann B, Giovanni D-SS. Oligopeptidase B from Leishmania amazonensis: molecular cloning, gene expression analysis and molecular model. Parasitol Res. 2007;101(4):865–75.
de Matos Guedes HL, de Carvalho RSN, de Oliveira Gomes DC, Rossi-Bergman B, De Simone SG. Oligopeptidase B-2 from Leishmania amazonensis with an unusual C-terminal extension. Acta Parasitolog. 2008;53:197.
Chaves SP, de Oliveira Gomes DC, De Simone SG, Rossi-Bergman B, de Matos Guedes HL. Serine proteases and vaccines against Leishmaniasis: a dual role vaccines vaccin. 2015;6:1. http://dx.doi.org/10.4172/2157-7560.1000264, http://www.omicsonline.org/open-access/serine-proteases-and-vaccines-against-leishmaniasis-a-dual-role-2157-7560.1000264.pdf.
Acknowledgments
We thank Dr. Beatriz Lilian Costa Souza for help in the initial experiments. We thank prof. Lynn Soong (UTMB) for the donation of the LV78 strain. We thank Professor Celio Freire de Lima (UFRJ) for support for cytokine quantification.
Funding
This work was financially supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro, Brazil (FAPERJ). This study was funded by projects FAPERJ JCE 212425, FAPERJ APQ1 207296 and CNPQ Universal 486580/2013.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article and its additional files.
Authors’ contributions
Designed experiments and idea: HLMG. Performed the animal experiments: JESP, TDR, AMFM, DOM, JPC, GO, MFM. Performed vaccine experiments: JESP, DOM. Cytokines in situ: JESP, JPCAMFM and BLD. Discussion of the results: HLMG, SPC, BLD, DCOG, BRB. Manuscript preparation: HLMG, SPC, BRB, BLD. All authors read and approved the final version of the manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Experimental protocols were previously approved by the Animal Use Committee of the Institute of Biophysics/Federal University of Rio de Janeiro (Brazil) under number IBCCF157.
Author information
Authors and Affiliations
Corresponding author
Additional files
Additional file 1: Figure S1.
Course of infection by L. amazonensis in BALB/c mice. BALB/c mice were infected with 5 × 105 stationary-phase promastigotes of L. amazonensis (Josefa strain) in the footpads. a Lesion sizes were measured at the indicated days and are expressed as the difference of thickness between non-infected and infected footpads. b Parasite load was measured in the chronic infection phase and expressed as mean number of parasites. The data (means ± standard deviations; n = 5) are representative of three independent experiments producing the same result profile. (TIF 210 kb)
Additional file 2: Figure S2.
Efficacy of intranasal LaAg against high challenge of L. amazonensis infection on C57BL/6. C57Bl/6-UFF received 10 μg of LaAg by the intranasal route on days -14 and -7 of infection. Non-vaccinated controls received PBS alone. On day 0, animals were infected with 2 × 106 stationary-phase promastigotes of L. amazonensis (7 days of culture). a Lesion sizes were measured at the indicated days and are expressed as the difference of thickness between non-infected and infected footpads. b The parasite load was measured on day 98 of infection and is expressed as number of parasites. The data (means ± standard deviations; n = 4–5) are representative of three independent experiments producing the same result profile. *P ≤ 0.05 in comparison to PBS controls. a Day 38 (t(6) = 3.303, P = 0.0164), Day 45 (t(6) = 2.813, P = 0.0306), Day (t(6) = 3.743, P = 0.0096), Day 61 (t(6) = 6.917, P = 0.0010); Day 70 (t(6) = 3.757, P = 0.0198). b t(6) = 6.778, P = 0.0025. (TIF 232 kb)
Additional file 3: Figure S3.
In situ cytokine profile in the chronic phase. Mice (from UFF) were intranasally vaccinated with LaAg and infected with L. amazonensis as described for data in Fig. 3. On day 110 of infection (see Fig. 3), the levels of IFN-γ (a), IL-4 (b), TGF-β (c), IL-10 (d) were measured in the lesion homogenates, and expressed as ng per g of footpad. The data (means ± standard deviations; n = 4–5) are representative of three independent experiments. *P ≤ 0.05 in comparison to PBS controls. (TIF 285 kb)
Additional file 4: Figure S4.
LaAg vaccine induced CD4+ IFN-γ+ T cells in progressive stage of infection. Mice were vaccinated and infected as in the experiment in Fig. 4. Lymph nodes were removed at day 44 post-infection. Frequency CD3+CD4+ cells from PBS mice (a) and from LaAg-vaccinated mice (b). Dot plot (frequency) of IFN-γ staining of CD4+ from PBS mice (c) or LaAg-vaccinated mice (D). e Number of CD3+CD4+ cells for each mouse from PBS and LaAg-vaccinated groups. f The production of IFN-γ+ by CD3+CD4+ cells was calculated for each mouse. The data (means ± standard deviations; n = 4–6) are from one experiment. *P ≤ 0.01 in comparison to PBS controls (cf. Fig. 4f, t (10) = 3.410, P = 0.0067). (TIF 4245 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Pratti, J.E.S., Ramos, T.D., Pereira, J.C. et al. Efficacy of intranasal LaAg vaccine against Leishmania amazonensis infection in partially resistant C57Bl/6 mice. Parasites Vectors 9, 534 (2016). https://doi.org/10.1186/s13071-016-1822-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-016-1822-9