Abstract
Background
This study aimed to provide a systematic review on the geographical distribution of Echinococcus multilocularis in definitive and intermediate hosts in the European Union (EU) and adjacent countries (AC). The relative importance of the different host species in the life-cycle of this parasite was highlighted and gaps in our knowledge regarding these hosts were identified.
Methods
Six databases were searched for primary research studies published from 1900 to 2015. From a total of 2,805 identified scientific papers, 244 publications were used for meta-analyses.
Results
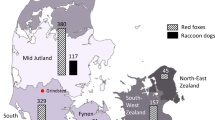
Studies in 21 countries reported the presence of E. multilocularis in red foxes, with the following pooled prevalence (PP): low (≤ 1 %; Denmark, Slovenia and Sweden); medium (> 1 % to < 10 %; Austria, Belgium, Croatia, Hungary, Italy, the Netherlands, Romania and the Ukraine); and high (> 10 %; Czech Republic, Estonia, France, Germany, Latvia, Lithuania, Poland, Slovakia, Liechtenstein and Switzerland). Studies from Finland, Ireland, the United Kingdom and Norway reported the absence of E. multilocularis in red foxes. However, E. multilocularis was detected in Arctic foxes from the Arctic Archipelago of Svalbard in Norway.
Conclusions
Raccoon dogs (PP 2.2 %), golden jackals (PP 4.7 %) and wolves (PP 1.4 %) showed a higher E. multilocularis PP than dogs (PP 0.3 %) and cats (PP 0.5 %). High E. multilocularis PP in raccoon dogs and golden jackals correlated with high PP in foxes. For intermediate hosts (IHs), muskrats (PP 4.2 %) and arvicolids (PP 6.0 %) showed similar E. multilocularis PP as sylvatic definitive hosts (DHs), excluding foxes. Nutrias (PP 1.0 %) and murids (PP 1.1 %) could play a role in the life-cycle of E. multilocularis in areas with medium to high PP in red foxes. In areas with low PP in foxes, no other DH was found infected with E. multilocularis. When fox E. multilocularis PP was >3 %, raccoon dogs and golden jackals could play a similar role as foxes. In areas with high E. multilocularis fox PP, the wolf emerged as a potentially important DH. Dogs and cats could be irrelevant in the life-cycle of the parasite in Europe, although dogs could be important for parasite introduction into non-endemic areas. Muskrats and arvicolids are important IHs. Swine, insectivores, murids and nutrias seem to play a minor or no role in the life-cycle of the parasite within the EU and ACs.
Similar content being viewed by others
Background
Human alveolar echinococcosis (AE), caused by the metacestode stage of the tapeworm Echinococcus multilocularis is considered as one of the most pathogenic zoonosis in temperate and arctic regions of Europe [1]. The life-cycle of E. multilocularis involves small rodent intermediate hosts such as arvicolids and wild or domestic canid definitive hosts such as the red fox (Vulpes vulpes), the Arctic fox (Vulpes lagopus), the raccoon dog (Nyctereutes procyonoides) or the dog (Canis lupus f. familiaris). Humans can act as aberrant intermediate hosts and are infected through the ingestion of eggs excreted in the faeces of definitive hosts. Such faecal-oral infection can be acquired by contact with definitive hosts or through contamination of soil, food or possibly water [2]. In humans, the metacestode stage resembles a malignant neoplasia as it proliferates indefinitely by exogenous budding and slowly invades the surrounding tissue to produce tumour-like lesions [3]. Human alveolar echinococcosis is characterized by an asymptomatic incubation period of around 5–15 years [4].
In Europe, the human risk of E. multilocularis infection was considered in the past to be restricted to certain geographical regions. In fact, until the 1990s, only a ‘core’ area consisting of Eastern France, southern Germany, parts of Switzerland and Austria were known to be endemic for the disease [5]. More recently, the expansion of the parasite into several new areas such as the Baltic regions, Denmark, the Netherlands, Poland, Romania, Slovakia, Slovenia and the increase of human AE incidence in ‘core’ areas such as Austria, France and Switzerland, suggested that the disease was spreading in Europe and the incidence of human AE increasing at least in some regions [6–10]. Although greater awareness and the use of advanced diagnostic tools may have contributed to an improvement in the detection of E. multilocularis infection in animals and humans, epidemiological research conducted over the past 20 years, suggested the expansion of this parasite in European countries [9]. Factors such as change in landscape composition and use, vegetation, climate change, presence of good intermediate hosts, urbanization of foxes, changing human behavioural attitudes toward foxes, wildlife reintroduction, E. multilocularis host population dynamics as well as globalization have all been proposed as potential factors influencing the increase of E. multilocularis infection risk for Europe [9, 11, 12].
In the light of these concerns, the European Commission (EC) adopted a Commission Delegated Regulation (EU) No. 1152/2011 (14 July 2011). This was considered as a preventive health measure to control E. multilocularis infection in dogs and decrease the potential risk of AE infection in humans, in order to ensure continuous protection of Finland, Ireland, Malta and the United Kingdom (UK), countries that have remained free from E. multilocularis [13]. Regulation 1152/2011 described the obligations of these four European Union (EU) member states in implementing a pathogen surveillance programme for the detection of E. multilocularis in accordance with specific requirements regarding sampling, detection and reporting procedures [14]. It also stipulated that the EC had to review this regulation by December 2016 to assess the justification of these preventive health measures, in the light of scientific developments regarding E. multilocularis infection in animals. In response to Article 29 of Regulation (EC) No. 178/2002, in addition to an EC request, the European Food Safety Authority (EFSA) was tasked with assessing E. multilocularis infection in animals within the EU and neighbouring Adjacent Countries (ACs) (Albania, Belarus, Bosnia and Herzegovina, Iceland, Kosovo, Liechtenstein, Macedonia, Moldova, Montenegro, Norway, Russia, Serbia, Switzerland, Turkey and the Ukraine). To fulfil this requirement, EFSA funded a project to provide a comprehensive and quantitative assessment of the current knowledge on E. multilocularis using a systematic review (SR) approach (GP/EFSA/AHAW/2012/01: Echinococcus multilocularis infection in animals).
The current SR provides an overview of the distribution and prevalence of E. multilocularis in the EU and ACs derived from both scientific and grey literature. In addition, the purpose of this review was to systematically determine the geographical distribution of E. multilocularis and the known wild and domestic definitive and intermediate hosts. The retrieved information was used to compile tables on the occurrence of E. multilocularis or highlight the lack of reliable reports. When available, data on E. multilocularis prevalence and worm burden of definitive hosts was reported. The importance of the various definitive and intermediate host species in the life-cycle of E. multilocularis in different parts of the EU and ACs was assessed and gaps in our knowledge were identified.
Methods
Bibliographic searches
This SR and meta-analysis followed the Cochrane and PRISMA Group guidelines [15] and the systematic search was carried out using the Documentation Service for literature search at the Istituto Superiore di Sanità, Rome, Italy. The STN International-Fiz Karlsruhe platform [16] was used for database searching carried out on the 5th November 2013 and again on the 11th February 2015 in order to identify articles that had been published since the initial search. The results of these two searches were then combined. Searches were carried out using the Medical Literature Analysis and Retrieval System Online (MEDLINE), Excerpta Medica Database (EMBASE), Science Citation Index (SciSearch), Biological Abstracts (BIOSIS), Centre for Agricultural Bioscience International (CABI) and Google Scholar. Databases were searched using keywords associated with the Boolean operators “AND” and “OR”. The question mark (“?”) was used to expand searches by looking for words with similar prefixes using more than one letter (i.e. “echinococc?” was used to search for “echinococcus”, “echinococci”, “echinococcosis” and “echinococcoses”). The hashtag (“#”) was used to expand searches by looking for words with similar prefixes using one letter (i.e. dog# was used to search for “dog” or “dogs”).
Different combinations of words and Boolean operators were used in order to narrow results retrieved and maximise the number of relevant studies returned. The full electronic search strategy, including any limits used was: [Echinococcus multilocularis OR (Echinococcus AND Multilocularis) OR E# Multilocularis OR Alveolar Echinococcosis OR A# Echinococcosis] AND (Dog OR Dogs OR Cat OR Cats OR Canis OR Felis OR Canid? OR Felid? OR Wolf OR Wolves OR Animal OR Animals OR Fox OR Foxes OR Vulpes OR Ferret OR Ferrets OR Rodent OR Rodents OR Rodentia OR Nutria# OR Muskrat# OR Jackal# OR Arvicolid? OR Arvicolinae OR Worm Burden OR Host OR Hosts OR Hosted) AND (Occurrence# OR Geographic? Distribut? OR Geographic? Diffus? OR Incidence# OR Frequency OR Epidemic Outbreak# OR Endemic Outbreak# OR Prevalence# OR Epidemiology)]. If the title or abstract did not give a clear indication of relevance, the full text was screened. After this initial selection, full-text articles were evaluated for eligibility, in accordance with the inclusion/exclusion criteria described below. Data extraction was performed independently by two researchers and any disagreements were resolved either by consensus among researchers or through arbitration by an additional independent researcher. If database outcomes overlapped, all duplicated articles were removed. EU reports and conference proceedings were searched using the keywords “European Union report, “EU report”,”, “conference proceedings”, “Echinococcus multilocularis”, “E. multilocularis” and “alveolar echinococcosis”. Unpublished epidemiological data on E. multilocularis available within individual member states was collected from the National Reference Laboratories for Parasites in Europe [17] using a questionnaire (Additional file 1: Text S1). Searches for Bachelor, Masters and PhD theses were carried out using the keywords “Echinococcus multilocularis” and “alveolar echinococcosis”. A list of databases used for retrieving theses is available in Additional file 2: Text S2. Review Manager [18] software was used to prepare and maintain this SR.
Study selection
Studies eligible for inclusion were defined a priori and fulfilled the following criteria: (i) studies published from 1900 to 2015; (ii) studies based on cross-sectional or cohort design; (iii) primary research studies either published or in press; (iv) reports on wild or domestic hosts of E. multilocularis; (v) studies published in English, German, French, Polish, Finnish, Dutch, Spanish or Italian.
The list of included articles is available in Additional file 3: Text S3. Studies providing data from outside Europe and ACs, case reports, reports on E. multilocularis in humans, studies on agents other than E. multilocularis (e.g. Echinococcus granulosus), reviews and letters or editorials without original data were all excluded from this SR. The list of excluded articles is available in Additional file 4: Text S4. The study selection process was carried out according to the PRISMA statement [15] and is reported using the flow chart shown in Fig. 1.
Eligibility for inclusion in the meta-analyses
Studies included in the meta-analyses were those that reported prevalence data (total number of studied animals and number of positive animals) and studies with a definition of a geographical area (whenever possible the Nomenclature of Territorial Units for Statistics designated as NUTS level 1, 2 and 3 was used) [19]. When studies originated from different geographical areas or when they were conducted within the same geographical area but at different time intervals (e.g. during distinct years or months) they were divided into sub-studies. Data were extracted from each study independently. If the same samples were tested using different diagnostic methods, only data derived from the sedimentation and counting technique (SCT) or the intestinal scraping technique (IST) were included in the analysis. Studies reporting prevalence data obtained exclusively by enzyme-linked immunosorbent assays (ELISAs), designed to detect pathogen-specific copro-antigens in DHs, were subsequently excluded from the meta-analysis because of the low specificity of this test. When it was not possible to accurately assign the proportion of data reported per country, meta-analysis was not performed.
Statistical approach and meta-analyses
Statistical analysis was conducted using the statistical software Stats Direct 2.8.0 (Stats Direct Ltd., Altrincham, UK). To perform the meta-analysis, animal species were divided into two main groups, definitive (DH) and intermediate (IH) hosts. The DHs included the red fox (Vulpes vulpes), the Arctic fox (Vulpes lagopus), the raccoon dog (Nyctereutes procyonoides), wild canids (wolf, Canis lupus; golden jackal, Canis aureus), the dog (Canis lupus f. familiaris) and the cat (Felis silvestris f. catus). The IHs included arvicolid rodents (including Arvicola spp., Myodes (syn. Clethrionomys) glareolus and Microtus spp. but excluding the muskrat Ondatra zibethicus), muskrat, nutria or coypu (Myocastor coypus), murid rodents (including Apodemus spp., Micromys minutus, Mus musculus and Rattus spp.), insectivores (including Sorex spp., Neomys fodiens and Talpa europaea) and swine (domestic Sus scrofa f. domesticus and the wild boar Sus scrofa). Each meta-analysis group included studies conducted in the same geographical area, at the European level, national level and using the three NUTS levels [19]. Meta-analyses were not stratified for the years/months in which the studies were conducted.
Since all included studies were cross-sectional, meta-analyses on proportions were performed. The Cochran’s Q test was performed to assess the degree of heterogeneity between studies, and the I2 index was used to describe the percentage of total variation across studies as a result of heterogeneity. If the p-value from the Cochran’s Q test was < 0.05 and the I2 statistic was > 50 %, heterogeneity was found and a random-effect model was applied. However, if heterogeneity was not detected, a fixed-effect model was used. A forest plot was produced to describe the pooled analysis; this showed the single prevalence of the studies and the pooled proportion with relative 95 % confidence intervals (CIs). Publication bias was quantified by inspection of funnel plots and computation of Begg and Egger’s probability values [20, 21].
Quality assessment
The quality of all included studies was assessed independently by two researchers using the Newcastle-Ottawa Scale (NOS) according to the Cochrane Handbook for Systematic Reviews [22, 23]. The NOS was modified for use on an animal model. Quality assessment could not be performed on grey literature.
Results and discussion
Bibliographic searches identified 2,805 scientific papers, of which 1,429 were deleted due to duplications. At the end of the search, 1,376 papers were identified of which 974 were excluded based only on title and abstract screening. A total of 402 full-text papers were assessed for eligibility, data were extracted from 255 studies and it was possible to perform meta-analyses on 244 studies (Fig. 1). The quality assessment carried out using the modified NOS, allowed the allocation of a maximum 7-star rating to any one individual study. A score of 5 or 6 was given to 108, 9, 1, 17 and 8 studies on foxes, raccoon dogs, wild canids, dogs and cats, respectively. A lower score (4 or 3) was assigned to 79 studies on foxes, 7 on raccoon dogs, 2 on wild canids, 9 on dogs and 12 on cats. A similar scoring for intermediate hosts showed that 2 studies on muskrats and 5 on arvicolids had a 5 or 6 rating. Four or three star ratings were assigned to 6 studies on muskrats, 11 on arvicolids, 4 on murids, 2 on nutria, 1 on insectivores and 1 on swine, respectively.
Geographical distribution and prevalence of Echinococcus multilocularis
Red foxes
Data regarding the geographical distribution and prevalence of E. multilocularis in red foxes were extracted from reports published for the period between 1968 and 2014 (Table 1).
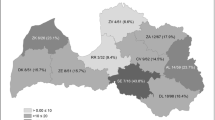
A total of 192 papers describing the distribution and prevalence of E. multilocularis in foxes were used in the meta-analyses. A preliminary ranking of E. multilocularis infection in red foxes based on pooled prevalence allowed us to identify three main groups (Table 1). A low prevalence group included countries with a pooled prevalence of ≤1 %, namely Denmark [24–27], Slovenia [28, 29] and Sweden [24, 30–36]; a medium prevalence group with a pooled prevalence of > 1 % but ≤ 10 %, which included Austria [37–44], Belgium [24, 45–55], Croatia [24, Relja Beck, personal communication], Hungary [24, 56–59], Italy [24, 60–66], the Netherlands [30, 31, 40, 49, 54, 67–72], Romania [73–75] and the Ukraine [76, 77], whereas the high prevalence territories had a pooled prevalence of > 10 % and included the Czech Republic [30, 31, 40, 78–83], Estonia [84–86, L. Laurimaa, personal communication], France [30, 31, 40, 87–104], Germany [24, 30, 31, 39–41, 105–151], Latvia [152], Lithuania [153, 154], Poland [155–171], Slovakia [24, 28, 31, 40, 164, 172–184], Liechtenstein [70] and Switzerland [24, 30, 39, 40, 185–198]. The occurrence and pooled prevalence of E. multilocularis in foxes in the EU and ACs is shown in Fig. 2. The highest prevalence estimates for E. multilocularis in red foxes seem to be concentrated in central and north-eastern Europe. A more detailed map of the geographical distribution and pooled prevalence of E. multilocularis in red foxes at a NUTS 1 level is shown in Fig. 3. Studies from four countries, namely Finland, Ireland, the UK and Norway, reported the absence of E. multilocularis in red foxes [24, 31, 32, 158, 196–203]. Echinococcus multilocularis in Arctic foxes in Norway was documented only for the Arctic Archipelago of Svalbard [207, 208].
Pooled prevalence of Echinococcus multilocularis in red and Arctic foxes within the European Union and adjacent countries at national level (data obtained from studies performed after 2000). Note: the pooled prevalence data for Norway originated only from Arctic foxes on the Svalbard islands; prevalence data from Spain originated from single studies
Pooled prevalence of Echinococcus multilocularis in red foxes within the European Union and adjacent countries at NUTS 1 level (data obtained from studies after 2000). Note: prevalence data from the Netherlands and Sweden originated from single studies; only studies reporting NUTS information were taken into account
Other definitive hosts
Five potential DHs of E. multilocularis other than red foxes were identified in the screened literature; four wild animal species, the Arctic fox [21, 24, 209], the raccoon dog [24–26, 32, 77, 152, 154, 168, 169, 175, 177, 199, 210–213], the golden jackal [24, 214] and the wolf [31, 77, 215] and two domestic animal species, dogs [24, 30, 72, 80, 95, 101, 122, 142, 168, 169, 177, 193, 195, 199, 200, 216–225] and cats [24, 37, 66, 80, 113, 115, 121, 122, 132, 133, 142, 168, 169, 177, 216, 219, 223, 226–230]. The geographical distribution and prevalence of E. multilocularis for these DHs are summarized in Table 2.
Pooled prevalence results showed that sylvatic animals, excluding red foxes, are more frequently infected than domestic species. The two species showing high E. multilocularis prevalence were the raccoon dog and the golden jackal. In general, high E. multilocularis prevalence in these two species correlated with high infection rates in foxes. Importantly, the raccoon dog is currently not established in some areas that are deemed free of E. multilocularis (e.g. Ireland, Malta and the UK), but is present in high numbers in Finland. A third species, with high prevalence rates was the Arctic fox [21, 24, 209], which is only present in a few northern countries, namely northern Russia, Iceland, and the Norwegian Arctic Archipelago of Svalbard, in addition to a small population on the Scandinavian peninsula.
Dogs and cats do not seem to be important in terms of prevalence and are found to be infected only in some areas of high E. multilocularis pooled prevalence in red foxes such as Czech Republic [80, 212], Germany [24, 113, 115, 121, 122, 132, 133, 142, 216, 219, 227, 228], France [24, 30, 95, 101, 216–218, 226] and Switzerland [24, 193, 195, 223–225]. However, dogs can be regarded as potentially relevant hosts considering E. multilocularis introduction into areas that are free of the parasite by travelling from endemic to distant (non-endemic) areas with their owners, and also with regard to transmission in endemic areas because of their closer association with humans than sylvatic DHs.
Information on E. multilocularis worm burden in definitive hosts from EU countries and ACs was only available in a few studies for red foxes (43/190) [25–27, 29, 34, 38, 55–59, 61, 63, 68, 75–77, 79, 80, 85, 99, 102, 110, 119, 127, 140, 152–154, 161, 165–167, 173–176, 179–181, 189, 191, 192], raccoon dogs (3/17) [32, 154, 213], dogs (1/23) [224] and cats (5/19) [80, 113, 132, 169, 228]. In contrast, no data were available on E. multilocularis worm burden of wild canids and Arctic foxes for the same regions.
Intermediate hosts
Potential IHs of E. multilocularis screened in this study included the muskrat [51, 113, 121, 136, 231–241], arvicolids [24, 30, 32, 39, 51, 79, 93, 95, 101, 121, 141, 187, 189–191, 207, 209, 223, 224, 242–257], murids [51, 79, 95, 101, 168, 169, 224, 229, 242, 246, 250, 251, 253, 256], nutria [231, 233], swine [32, 73, 220, 258] and insectivores [24, 79, 101, 251] (Table 3). For the majority of countries, the distribution of the prevalence of E. multilocularis in muskrats and arvicolids matched that (although the prevalence was lower) in red foxes and was similar to the pooled prevalence of E. multilocularis in other sylvatic DHs (Table 2). Muskrats and arvicolids are thus potentially good sentinels to investigate the presence of E. multilocularis in specific settings.
Among murids, Apodemus spp. was the host with the highest E. multilocularis prevalence [24, 79, 224, 242, 246, 250, 251, 253, 256]. In France, E. multilocularis prevalence in these species was similar to that reported for Microtus spp. [256]. Only one study on E. multilocularis infection in Mus musculus in France is known to exist [95]. In general, murids have not frequently been found positive for E. multilocularis [24, 79, 101, 168, 169, 224, 229, 242, 246, 250, 251, 253]. However, the number of studies (n = 14) and the number of murids examined remains small (n = 2,610). None of the screened insectivores were positive for E. multilocularis [24, 79, 101, 251] but the number examined was small (n = 531). Although swine seem to play no role in the life-cycle of this parasite, E. multilocularis infections in swine were reported from Germany [259], Lithuania [220] and Switzerland [258] and therefore this animal species could potentially be regarded as a domestic IH sentinel (Table 3). Data regarding E. multilocularis in definitive and intermediate hosts in EU countries and ACs are summarised in Table 4.
Ranking of hosts (other than red foxes) in the life-cycle of Echinococcus multilocularis
Definitive hosts
In order to clarify the importance of other screened DHs in the life-cycle of E. multilocularis, pooled prevalence for each DH, other than red foxes were generated (Table 5). The ranking of pooled prevalence in DHs could be used to hypothesise the importance of the different DHs in the life-cycle of E. multilocularis.
Ranking based on an E. multilocularis pooled prevalence of > 3 %, resulted in the following order (high to low rank): red fox, Arctic fox, golden jackal, raccoon dog and wolf. Although data on the golden jackal and the Arctic fox are scarce [21, 24, 209, 214], they provide evidence in support of these two animal species serving as potentially important DHs of E. multilocularis. Despite some uncertainties due to the low number of studies regarding these two species, data have nevertheless been included in this report for the following reasons: (i) these are the only data available for the golden jackal and the Arctic fox; and (ii) parasite prevalence in the studied individuals was high (Arctic fox, 9 %, 95 % CI: 6–12; golden jackal, 4.7 %, 95 % CI: 0.1–15.3), which is indicative of the potentially important role that these species could play in the maintenance and transmission of E. multilocularis. Interestingly, Arctic foxes are restricted to the northern area of the EU and ACs because of their habitat needs, but the golden jackal population seems to have an increasing trend of migrating from eastern EU countries and ACs towards the west, which should be taken into account when considering the potential future spread of E. multilocularis.
Intermediate hosts
In order to clarify the importance of the screened IHs in the life-cycle of E. multilocularis, the pooled prevalence for each IH group was determined (Table 6). Pooled prevalence in the screened IH groups showed that muskrats and arvicolids (muskrats, n = 25,985; arvicolids, n = 65,956) (and more specifically Arvicola spp.) are important in the life-cycle of E. multilocularis. For nutria (n = 650) and murids (n = 2,610), the number of animals screened was too low to draw any robust conclusions, although it seems that they could play a role in the life-cycle of E. multilocularis in areas with a sustained medium to high pooled prevalence in red foxes [24, 79, 95, 101, 168, 169, 224, 229, 231, 233, 242, 246, 250, 251, 253, 256]. Swine and insectivores seem to play no role in the life-cycle of E. multilocularis within the EU and ACs.
The importance of different definitive hosts in countries classified as having low, medium and high prevalence rates of Echinococcus multilocularis
Definitive hosts
Considering that the number of studies and the number of animals screened in many cases were too low for drawing robust conclusions, the following comments should be regarded as tentative.
The importance of each screened DH, according to country, was stratified by the pooled prevalence of E. multilocularis in red foxes (or Arctic foxes in Svalbard, Norway). The resulting classification, with regard to E. multilocularis infection, enabled us to group countries into zero, low, medium or high prevalence regions (Table 7). The raccoon dog [24–26, 32, 199, 200], the wolf [31], the dog [31, 216] and cat [216] were screened in countries with low (including absence of the parasite) E. multilocularis prevalence in foxes. None of these DHs, at this level of fox prevalence, seem to sustain the life-cycle of E. multilocularis, although issues relating to the representativeness of the sample number should be taken into account since, occasionally, the number of screened animals was low (raccoon dogs, n = 3,833; dogs, n = 27,638; cats, n = 13,498).
For countries stratified in the medium E. multilocularis prevalence group, golden jackals [24, 214], if present, seem to participate in the life-cycle of the parasite, with prevalence estimates roughly similar to those reported for red foxes in the same countries [56–59, 120]. By contrast, wolves [77], dogs [24, 72, 216] and cats [216] seem to play no role in countries with medium E. multilocularis prevalence levels in foxes [24, 30, 31, 37–44, 49, 54, 60–72]. For countries with high E. multilocularis prevalence levels, raccoon dogs [24, 152, 154, 168, 169, 175, 177, 210–213] are also important in the life-cycle of the parasite, with prevalence estimates of between one-seventh and two-thirds of the pooled prevalence in foxes. An exception is evident in Slovakia, where the pooled E. multilocularis prevalence in foxes [24, 31, 40, 164, 172–184, 260] was similar to the prevalence found in raccoon dogs (~27 %) [24, 175, 177]. Importantly, in countries with a high prevalence, an additional DH (i.e. wolf) seems to join the life-cycle of E. multilocularis, although with a lower prevalence (one-sixth) than that reported for foxes and raccoon dogs [215].
With regard to domestic hosts (dogs and cats), only a very low prevalence of E. multilocularis could be found and only in highly endemic situations (Table 7), and thus these hosts seem to be of minor importance in the life-cycle of the parasite in Europe and ACs, especially when a zero, low or medium E. multilocularis prevalence is found in foxes. In addition, cats have been shown to be unsuitable hosts for E. multilocularis, because full maturity of the parasite is often not attained in the feline intestine [261].
Intermediate hosts
In countries with a low (including 0) E. multilocularis pooled prevalence in foxes, only two types of IHs have been screened, namely arvicolids (in Finland) [24] and swine (in Finland and Sweden) [32] whereas in other countries such as Ireland, Slovenia and the UK no IHs have been inspected for the prevalence of E. multilocularis. Therefore, to interpret these results, the potential importance of those IHs in medium- and high-prevalence situations should first be assessed. Muskrats and arvicolids seem to be the only IHs for E. multilocularis in medium-prevalence rated countries. In muskrats, a pooled prevalence of 16 % was recorded in Belgium [51, 232] and a prevalence of 0.06 % in the Netherlands [236] where the pooled prevalence for E. multilocularis in foxes was 8 and 4.7 %, respectively. Similarly, in Norway (Arctic fox pooled prevalence 5.8 %), Romania (fox pooled prevalence 4.5 %) and Belgium (fox pooled prevalence 8 %), the pooled prevalence for E. multilocularis in arvicolids was 27.5 % [30, 39, 207, 209], 1.4 % [262] and 0.2 % [51], respectively. In countries with a high E. multilocularis prevalence, the prevalence estimates were high for arvicolids (13.3 %) [187, 189–191, 223, 224, 252–254] and pigs (10 %) [258] in Switzerland (fox pooled prevalence 17 %), muskrats (3.8 %) [113, 121, 136, 231, 235–237, 240, 241] and pigs (5.3 %) [259] in Germany (fox pooled prevalence 29.2 %) and arvicolids (4.8 %) [93, 95, 101, 242–249, 255, 257] and nutria (5.8 %) [233] in France (fox pooled prevalence 13.9 %) (Table 7).
Gaps and conclusions
Generally gaps were found in the literature regarding the following aspects (i) NUTS level specifications beyond the national level were absent in many reports, making it difficult to localise infection foci within specific areas for each country; (ii) many EU countries and ACs (n = 18) had no data on E. multilocularis prevalence in definitive or intermediate hosts, even in cases where E. multilocularis infection was probable because the parasite had been found in surrounding countries; (iii) data on the prevalence of the parasite in DHs, other than red foxes, and in some IHs were scarce and often reported in only one single study; (iv) the number of screened animals was considered insufficient in some reports in which the estimated prevalences were low; and (v) publication bias (for example there may be unpublished studies regarding the absence of E. multilocularis within the EU and/or ACs).
Furthermore, inadequacies were identified with regard to the assessment of E. multilocularis prevalence in red foxes. Specific gaps were that (i) the vast majority of studies were concentrated in six countries (Germany, France, Slovakia, Switzerland, Poland and Hungary, whereas the estimates of the pooled prevalence for other countries was based on few studies or further to this, in two cases (Liechtenstein and Spain) on single studies; (ii) sampling in some countries had been done in only specific areas in which it was assumed that the prevalence might be high and thus extrapolation of the data at national level could be biased; and (iii) bias may arise as a result of the sampling strategy used. The sampling strategy data for red foxes are summarised in Additional file 5: Table S1. In the current SR, 50/190 studies relating to fox sampling and E. multilocularis control programmes, excluding those based on coproELISA (n = 10), were included in the analysis. In addition, data were obtained from 20/190 papers describing rabies control programmes, in which foxes were probably mainly obtained by shooting. A further 38/190 papers included in this study did not report the type of sampling methods utilized. Additionally, in 133 studies examined, fox carcasses were made available to authors/authorities through other sources (road kill; hunting season). This type of sampling strategy can cause bias with regard to restrictions in sampling locations, since hunting for example is generally conducted in areas distant from human habitation. Therefore, in more than half of the prevalence studies, synanthropic fox populations living in villages, towns or cities were not included in the sampled animals. This may be the case for all fox sampling within the EU and ACs.
Specific gaps and weaknesses were also found for data relating to DHs other than red foxes. These were that (i) the number of studies was very low (n = 44) for the five DHs; (ii) some of the DHs are geographically restricted, for example, Arctic foxes are limited to northern latitudes [21, 24, 209] and golden jackals are found in only a few countries [24, 93, 209, 214]; (iii) some of the DHs such as raccoon dogs were not found on island countries (Ireland, Malta and the UK) and (iv) some of the DHs are protected species (e.g. the wolf). Specific gaps and deficiencies in data for IHs were that (i) the number of studies were very low (n = 27) for all screened IHs, excluding arvicolids and muskrats; and (ii) some of the IHs were geographically restricted.
In addition, in terms of the importance of definitive and intermediate hosts of E. multilocularis, this systematic review identified gaps regarding the following aspects: (i) the number of studies for the different hosts and the number of screened animals was very low, excluding red foxes, muskrats and arvicolids; and (ii) data on worm burden and worm maturity for the different DHs or fertility of protoscoleces in different IHs were lacking, precluding the assessment of the real role of each host in the maintenance of the life-cycle of E. multilocularis. However, the ranking of animals according to their importance as hosts may be useful in providing recommendations for the screening of DHs to better ascertain the presence of E. multilocularis in a given area. Host screening strategy should be as follows: in the absence of the most important DH, the second most important DH should be screened and so forth. Nevertheless, both the presence of hosts and the protected status of some species (e.g. wolves) are a matter to be taken into account when a recommendation for screening is given.
When conducting epidemiological studies, particularly if the absence of the parasite or a low to medium prevalence is expected and if red foxes cannot be screened, sylvatic animals should, preferably, be screened if the aim is to demonstrate the absence or presence of E. multilocularis. When the presence or maintenance of the life-cycle is to be assessed, the suitability of each DH to allow the full maturation of the parasite (worms producing infective eggs), and the evaluation of worm burden, should be taken into account. In a similar manner, when the presence or maintenance of the life-cycle needs to be assessed, the suitability of each IH to allow full maturation of the parasite (protoscolex production) should be considered.
The prevalence in muskrats and arvicolids seems to parallel those found in red foxes and if foxes cannot be screened, a larger number of muskrats and arvicolids than foxes would need to be screened to confirm the absence of E. multilocularis. This is necessary because the prevalence in foxes as compared to Arvicola spp. appear to correlate at a ratio of around 3:1. Similarly, in areas where both M. glareolus and Microtus spp, were found, E. multilocularis prevalence correlated with that in foxes at a ratio of 1:4–6 (Table 7). An exceptional case is Svalbard in Norway, where Microtus spp. had a 27 % E. multilocularis prevalence and the DH (Arctic fox) showed around 9 % prevalence [207, 209]. This could be attributed to ecological variables specific for this DH-IH interaction, since the IH (Microtus levis) has a very limited spatial distribution, while Arctic foxes are able to stroll on ice and can cover long distances and are therefore not limited to either the Spitsbergen Island nor to the Svalbard Archipelago. The only additional potential DH in this area is the dog, but this DH has to date not been screened in this region.
This SR has also highlighted gaps in our knowledge regarding mustelids and the role they may potentially play in the life-cycle of this parasite. Studies on E. multilocularis infection in mustelids (including Mustela spp., Neovison vison, Lutra lutra, Meles meles and Martes spp.) from Europe initially formed part of this meta-analysis. None of the studied mustelids from the Czech Republic (n = 6) [80], Denmark (n = 29) [24], Germany (n = 1142) [24, 122, 133, 142, 263], Poland (n = 22) [168, 169, 251], Slovakia (n = 18) [175, 177] and the Ukraine (n = 26) [77] were found infected with E. multilocularis. Interestingly, mustelids (Martes spp.) from Ryazan district, Russia were recently found to harbor adults of E. multilocularis [264]. As far as we are aware this is the only known report on the occurrence of E. multilocularis in mustelids and is the only known study that identified mustelids as ‘definitive hosts’ based on the presence of E. multilocularis adults in the intestine of 4/31 Martes species. While this infection can evidently occur, no information on E. multilocularis worm maturity, worm burden or prepatency was provided. Additionally, no mustelid-derived faecal samples have been unequivocally confirmed by molecular methods to be positive for E. multilocularis. Furthermore, we speculate that this infection may occur as a result of the predator-prey relationship of carnivorous mustelids and small rodents. In the absence of studies in which a larger number of mustelids are examined and/or experimental data we were reluctant to include data on mustelids in this analysis. Although the absence of E. multilocularis in mustelids in Europe suggests that they may not be important hosts of this parasite, further studies are required in order to clarify their role.
Importantly, the presence of E. multilocularis in red foxes cannot be excluded from countries where data may have been published using languages other than those represented by this SR consortium, but where this host is known to be present. For example there are many publications (albeit in Russian) on E. multilocularis in animals in the former Soviet Union ([265], Paul Torgerson personal communication]). High E. mulitlocularis infection rates in foxes (33.1 %) and raccoon dogs (15.4 %) were reported from Ryazan district [264] and in foxes from Bryansk Oblast (41 %) [266] and Kamchatka (14.7 %) in the east, respectively [267]. Similarly, high E. multilocularis infection rates of 40 % and 98 % were found in Arctic foxes from Krasnoyarsk region [268] and Sakha, Yakutia [269], respectively. In addition, reports on rodents have documented E. multilocularis infection in Apodemus uralensis and Microtus arvalis [270] in Kabardin-Balkar and Clethrionomys spp. in Sakha [271]. In a similar manner, the absence of E. multilocularis in foxes in countries for which only a few studies were available, may not be representative of the infection status of foxes in those particular areas.
Conclusion
In conclusion, this SR confirmed the status of the red fox as the most important definitive host of E. multilocularis in the EU and ACs. If the prevalence in foxes was zero or low in a given area, there was no indication that the life-cycle of E. multilocularis was maintained by other DHs. In contrast, when the prevalence level in red foxes was greater than 3 %, both raccoon dogs and golden jackals, if present, seemed to play a similar role as the fox in the life-cycle of the parasite. In terms of IHs, muskrats and M. glareolus, if present, are important hosts in the life-cycle of E. multilocularis. Under specific conditions, Arvicola spp. and Microtus spp. could be important in the life-cycle of the parasite. Swine and insectivores seem to play no role in the life-cycle of E. multilocularis within the EU and ACs.
Abbreviations
- AC:
-
Adjacent countries
- AE:
-
Alveolar echinococcosis
- AHAW:
-
Animal health and welfare
- BIOSIS:
-
Biological Abstracts
- CABI:
-
Centre for Agricultural Bioscience International
- CI:
-
Confidence intervals
- DH:
-
Definitive host
- EC:
-
European Commission
- EFSA:
-
European Food Safety Authority
- ELISA:
-
Enzyme-linked immunosorbent assays
- EMBASE:
-
Excerpta Medica Database
- EU:
-
European Union
- IH:
-
Intermediate host
- IST:
-
Intestinal scraping technique
- MEDLINE:
-
MEDical Literature Analysis and Retrieval System
- NOS:
-
Newcastle-Ottawa scale
- NUTS:
-
Nomenclature of territorial units for statistics
- PP:
-
Pooled prevalence
- SciSearch:
-
Science Citation Index
- SCT:
-
Sedimentation and counting technique
- SR:
-
Systematic review
- STN:
-
Scientific and Technical Information Network International
References
Kern P, Bardonnet K, Renner E, Auer H, Pawlowski Z, Ammann RW, Vuitton DA, Kern P. European echinococcosis registry: Human alveolar echinococcosis, Europe, 1982–2000. Emerg Infect Dis. 2003;9:343–9.
Torgerson PR, Keller K, Magnotta M, Ragland N. The global burden of alveolar echinococcosis. PLoS Negl Trop Dis. 2010;4(6):e722. doi:10.1371/journal.pntd.0000722.
Moro P, Schantz PM. Echinococcosis: a review. Int J Infect Dis. 2009;13:125–33.
Gottstein B, Wang J, Boubaker G, Marinova I, Spiliotis M, Muller N, Hemphill A. Susceptibility versus resistance in alveolar echinococcosis (larval infection with Echinococcus multilocularis). Vet Parasitol. 2015;213:103–9.
Eckert J, Deplazes P. Alveolar echinococcosis in humans: the current situation in central Europe and the need for countermeasures. Parasitol Today. 1999;15:315–9.
Schweiger A, Ammann RW, Candinas D, Clavien PA, Eckert J, Gottstein B, et al. Human alveolar echinococcosis after fox population increase, Switzerland. Emerg Infect Dis. 2007;13:878–82.
Schneider R, Aspöck H, Auer H. Unexpected increase of alveolar echincoccosis, Austria, 2011. Emerg Infect Dis. 2013;19:475–7.
Said-Ali Z, Grenouillet F, Knapp J, Bresson-Hadni S, Vuitton DA, Raoul F, et al. Detecting nested clusters of human alveolar echinococcosis. Parasitology. 2013;140:1693–700.
Gottstein B, Stojkovic M, Vuitton DA, Millon L, Marcinkute A, Deplazes P. Threat of alveolar echinococcosis to public health - a challenge for Europe. Trends Parasitol. 2015;31:407–12.
Marcinkutė A, Šarkūnas M, Moks E, Saarma U, Jokelainen P, Bagrade G, et al. Echinococcus infections in the Baltic region. Vet Parasitol. 2015;213:121–31.
Davidson RK, Romig T, Jenkins E, Tryland M, Robertson LJ. The impact of globalisation on the distribution of Echinococcus multilocularis. Trends Parasitol. 2012;28:239–47.
Atkinson J-AM, Gray DJ, Clements ACA, Barnes TS, McManus DP, Yang YR. Environmental changes impacting Echinococcus transmission: research to support predictive surveillance and control. Glob Chang Biol. 2013;19:677–88.
EFSA. Scientific report on the assessment of Echinococcus multilocularis surveillance reports submitted in 2015 in the context of Commission Regulation (EU) No 1152/2011. EFSA J. 2015;13:4310.
EFSA. Panel on Animal Health and Welfare 2015. Scientific opinion on Echinococcus multilocularis infection in animals. EFSA J. 2015;13:4373.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6, e1000097.
STN. Scientific & Technical Information Network International, Fiz Karlsruhe (Fachinformationszentrum Karlsruhe). https://www.fiz-karlsruhe.de/en.html.
National Reference Laboratories for Parasites in Europe. http://w3.iss.it/site/EURLPMaps/MapsInfo.asp
Review Manager software (RevMan Version 5.2). Copenhagen. http://ims.cochrane.org/revman
Nomenclature of Territorial Units for Statistics. http://ec.europa.eu/eurostat/statisticsexplained/index.php/Glossary:Nomenclature_of_territorial_units_for_statistics_(NUTS))
Begg CB, Mazumdar M. Operating characteristics of a bank correlation test for publication bias. Biometrics. 1994;50:1088–101.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34.
Wells GA, Shea B, O’Connel D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration. 2011
EFSA. The EU summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2013. EFSA J. 2015;13:3991.
Al-Sabi MNS, Chriél M, Enemark HL. Endoparasites of the raccoon dog (Nyctereutes procyonoides) and the red fox (Vulpes vulpes) in Denmark 2009–2012 - A comparative study. Int J Parasitol Parasites Wildl. 2013;2:144–51.
Enemark HL, Al-Sabi MN, Knapp J, Staahl M, Chriél M. Detection of a high-endemic focus of Echinococcus multilocularis in red foxes in southern Denmark, January 2013. Euro Surveill. 2013;18:20420.
Saeed I, Maddox-Hyttel C, Monrad J, Kapel CMO. Helminths of red foxes (Vulpes vulpes) in Denmark. Vet Parasitol. 2006;139:168–79.
Vergles Rataj A, Bidovec A, Zele D, Vengust G. Echinococcus multilocularis in the red fox (Vulpes vulpes) in Slovenia. Eur J Wildlife Res. 2010;56:819–22.
Vergles Rataj A, Posedi J, Zele D, Vengust G. Intestinal parasites of the red fox (Vulpes vulpes) in Slovenia. Acta Vet Hung. 2013;61(4):454–62.
EFSA. The Community summary Report on Trends and Sources of Zoonoses, Zoonotic Agents, antimicrobial resistance andand Food-borne Ourbreaks in the European Union in 2006. EFSA J. 2007;130:2–352.
EFSA. The EU Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food-borne Outbreaks in 2011. EFSA J. 2013;11:3129.
Wahlström H, Isomursu M, Hallgren G, Christensson D, Cedersmyg M, Wallensten A, et al. Combining information from surveys of several species to estimate the probability of freedom from Echinococcus multilocularis in Sweden, Finland and mainland Norway. Acta Vet Scand. 2011;53.
Isaksson M, Hagström A, Armua-Fernandez MT, Wahlström H, Ǻgren EO, Miller A, et al. A semi-automated magnetic capture probe based DNA extraction and real-time PCR method applied in the Swedish surveillance of Echinococcus multilocularis in red fox (Vulpes vulpes) faecal samples. Parasit Vectors. 2014;7:583.
Osterman Lind E, Juremalm M, Christensson D, Widgren S, Hallgren G, Ǻgren EO, et al. First detection of Echinococcus multilocularis in Sweden, February to March 2011. Eurosurveillance. 2011;16:1–3.
Wahlström H, Lindberg A, Lindh J, Wallensten A, Lindqvist R, Plym-Forshell L, et al. Investigations and actions taken during 2011 due to the first finding of Echinococcus multilocularis in Sweden. Eurosurveill. 2012;17:10–7.
Wahlström H, Botero-Kleiven S, Lind EO, Christensson D, Cedersmyg M, Ǻgren EO. Present status of Echinococcus multilocularis in Sweden. Trop Med Int Health. 2013;18:96–7.
Deutz A, Fuchs K, Lassnig H, Hinterdorfer F. Prevalence of E. multilocularis in foxes in Styria taking into consideration biometrical methods. Berl Munch Tierarztl Wochenschr. 1995;108:408–11.
Duscher G, Prosl H, Joachim A. Scraping or shaking - a comparison of methods for the quantitative determination of Echinococcus multilocularis in fox intestines. Parasitol Res. 2005;95(1):40–2.
Eckert J, Deplazes P, Ewald D, Gottstein B. Parasitological and immunological methods for the detection of Echinococcus multilocularis in foxes. Mitt Osterr Ges Tropenmed Parasitol. 1991;13:25–30.
EFSA. The Community summary Report on Trends and Sources of Zoonoses, Zoonotic Agents, antimicrobial resistance andand Food-borne Ourbreaks in the European Union in 2005. EFSA J. 2006;94:2–228.
Gottstein B, Deplazes P, Eckert J, Muller B, Schott E, Helle O, et al. Serological (Em2-ELISA) and parasitological examinations of fox populations for Echinococcus multilocularis infections. Zentralbl Veterinarmed B. 1991;38:161–8.
Lassnig H, Prosl H, Hinterdorfer F. Parasites of the red fox (Vulpes vulpes) in Styria. Wien Tierarztl Monatsschr. 1998;85:116–22.
Prosl H, Schmid E. Prevalence of Echinococcus multilocularis in foxes in Vorarlberg, Austria. Mitt Osterr Ges Tropenmed Parasitol. 1991;13:41–6.
Duscher G, Steineck T, Gunter P, Prosl H, Joachim A. Echinococcus multilocularis in foxes in Wien and surrounding territories. Wien Tierarztl Monatsschr. 2005;92(1):16–20.
Brochier B, Coppens P, Losson B, Aubert MFA, Bauduin B, Barrat MJ, et al. Prevalence of Echinococcus multilocularis infestation in the red fox (Vulpes vulpes) in the province of Luxembourg (Belgium) - a preliminary survey. Ann Med Vet. 1992;136:497–501.
Losson B, Mignon B, Brochier B, Bauduin B, Pastoret PP. Echinococcus multilocularis infection in the red fox (Vulpes vulpes) in the province of Luxembourg (Belgium): Results of a survey conducted between 1993–1995. Ann Med Vet. 1997;141(2):149.
Vervaeke M, Dorny P, Vercammen F, Geerts S, Brandt J, Van den Berge K, Verhagen R. Echinococcus multilocularis (Cestoda, Taeniidae) in red foxes (Vulpes vulpes) in northern Belgium. Vet Parasitol. 2003;115:257–63.
Vervaeke M, Dorny P, De Bruyn L, Vercammen F, Jordaens K, Van Den Berge K, Verhagen R. A survey of intestinal helminths of red foxes (Vulpes vulpes) in northern Belgium. Acta Parasitol. 2005;50:221–7.
Vervaeke M, van der Giessen J, Brochier B, Losson B, Jordaens K, Verhagen R, et al. Spatial spreading of Echinococcus multilocularis in red foxes (Vulpes vulpes) across nation borders in Western Europe. Prev Vet Med. 2006;76(3–4):137–50.
Brochier B, De Blander H, Hanosset R, Berkvens D, Losson B, Saegerman C. Echinococcus multilocularis and Toxocara canis in urban red foxes (Vulpes vulpes) in Brussels, Belgium. Prev Vet Med. 2007;80:65–73.
Hanosset R, Saegerman C, Adant S, Massart L, Losson B. Echinococcus multilocularis in Belgium: prevalence in red foxes (Vulpes vulpes) and in different species of potential intermediate hosts. Vet Parasitol. 2008;151(2–4):212–7.
Losson B, Kervyn T, Detry J, Pastoret PP, Mignon B, Brochier B. Prevalence of Echinococcus multilocularis in the red fox (Vulpes vulpes) in southern Belgium. Vet Parasitol. 2003;117:23–8.
Saegerman C, Blander HD, Hanosset R, Berkvens D, Losson B, Brochier B, de Blander H. Risk assessment of the presence of Echinococcus multilocularis and Toxocara canis in foxes from Brussels. Epidemiologie et de Sante Anim. 2006;50:97–104.
van der Giessen J, Vervaeke M, de Vries A, Chu M, Brochier L, Losson B, et al. Is Echinococcus multilocularis increasing in prevalence in the Western European border line? Int J Antimicrob Agents. 2007;29:S51.
Van Gucht S, Van Den Berge K, Quataert P, Verschelde P, Le Roux I. No emergence of Echinococcus multilocularis in foxes in flanders and Brussels anno 2007–2008. Zoonoses Public Health. 2010;57:E65–70.
Casulli A, Széll Z, Pozio E, Sréter T. Spatial distribution and genetic diversity of Echinococcus multilocularis in Hungary. Vet Parasitol. 2010;174:241–6.
Sréter T, Széll Z, Egyed Z, Varga I. Echinococcus multilocularis: An emerging pathogen in Hungary and Central Eastern Europe? Emerg Infect Dis. 2003;9:384–6.
Sréter T, Széll Z, Sréter-Lancz Z, Varga I. Echinococcus multilocularis in northern Hungary. Emerg Infect Dis. 2004;10:1344–6.
Tolnai Z, Széll Z, Sréter T. Environmental determinants of the spatial distribution of Echinococcus multilocularis in Hungary. Vet Parasitol. 2013;198:292–7.
Manfredi MT, Genchi C, Deplazes R, Trevisiol K, Fraquelli C. Echinococcus multilocularis infection in red foxes in Italy. Vet Rec. 2002;150(24):757.
Calderini P, Magi M, Gabrielli S, Brozzi A, Kumlien S, Grifoni G, et al. Investigation on the occurrence of Echinococcus multilocularis in Central Italy. BMC Vet Res. 2009;5.
Casulli A, Manfredi MT, La Rosa G, Di Cerbo AR, Dinkel A, Romig T, et al. Echinococcus multilocularis in red foxes (Vulpes vulpes) of the Italian Alpine region: is there a focus of autochthonous transmission? Int J Parasitol. 2005;35(10):1079–83.
Di Cerbo AR, Manfredi MT, Trevisiol K, Bregoli M, Ferrari N, Pirinesi F, Bazzoli S. Intestinal helminth communities of the red fox (Vulpes vulpes L.) in the Italian Alps. Acta Parasit. 2008;53:302–11.
Magi M, Macchioni F, Dell’Omodarme M, Prati MC, Dell’Omodarme M, Calderini P, et al. Endoparasites of red fox (Vulpes vulpes) in Central Italy. J Wildl Dis. 2009;45:881–5.
Manfredi MT, Di Cerbo AR, Trevisiol K. An updating on the epidemiological situation of Echinococcus multilocularis in Trentino Alto Adige (northern Italy). Parassitologia. 2004;46:431–3.
Manfredi MT, Casulli A, La Rosa G, Di Cerbo AR, Trevisio K, Genchi C, Pozio E. Echinococcus multilocularis in north Italy. Parassitologia. 2006;48:43–6.
Takumi K, de Vries A, Chu ML, Mulder J, Teunis P, van der Giessen J. Evidence for an increasing presence of Echinococcus multilocularis in foxes in the Netherlands. Int J Parasitol. 2008;38(5):571–8.
van der Giessen JWB, Rombout YB, Franchimont JH, Limper LP, Homan WL. Detection of Echinococcus multilocularis in foxes in the Netherlands. Vet Parasitol. 1999;82(1):49–57.
van der Giessen JWB, Rombout Y, Teunis P. Base line prevalence and spatial distribution of Echinococcus multilocularis in a newly recognized endemic area in the Netherlands. Vet Parasitol. 2004;119:27–35.
Franssen F, Nijsse R, Mulder J, Cremers H, Dam C, Takumi K, van der Giessen J. Increase in number of helminth species from Dutch red foxes over a 35-year period. Parasit Vectors. 2014;7.
Kikkert PF. Detection of Echinococcus multilocularis in red fox (Vulpes vulpes) in The Netherlands at the border with Germany. Thesis. 2011
Maas M, Dam-Deisz WDC, van Roon AM, Takumi K, van der Giessen JWB. Significant increase of Echinococcus multilocularis prevalence in foxes, but no increased predicted risk for humans. Vet Parasitol. 2014;206(3–4):167–72.
Sikó Barabasi S, Bokor E, Fekeas E, Nemes I, Murai E, Gubanyi A, Barabasi SS. Occurrence and epidemiology of Echinococcus granulosus and E. multilocularis in the Covasna County, East Carpathian Mountains, Romania. Parasitol Hung. 1995;28:43–56.
Sikó Barabasi S, Deplazes P, Cozma V, Pop S, Tivadar C, Bogolin I, Popescu R. Echinococcus multilocularis confirmed in Romania. Sci Parasitol. 2010;11:89–96.
Siko Barabasi S, Fok E, Gubanyi A, Meszaros F, Cozma V. Helminth fauna of the small intestine in the European red fox, Vulpes vulpes, with notes on the morphological identification of Echinococcus multilocularis. Sci Parasitol. 2010;11:141–51.
Kharchenko VA, Kornyushin VV, Varodi EI, Malega OM. Occurrence of Echinococcus multilocularis (Cestoda, Taeniidae) in red foxes (Vulpes vulpes) from Western Ukraine. Acta Parasit. 2008;53:36–40.
Kornyushin VV, Malyshko EI, Malega AM. The helminths of wild predatory mammals of Ukraine. Cestodes. Vestn Zool. 2011;45:483–90.
Kolárová L, Pavlásek I, Chalupský J. Echinococcus multilocularis Leuckart, 1863 in the Czech Republic. Helminthologia. 1996;33:59–65.
Martínek K, Kolárová L, Cervený J, Andreas M. Echinococcus multilocularis (Cestoda: Taeniidae) in the Czech Republic: The first detection of metacestodes in a naturally infected rodent. Folia Parasitol. 1998;45(4):332–3.
Martínek K, Kolárová L, Cervený J. Echinococcus multilocularis in carnivores from the Klatovy district of the Czech Republic. J Helminthol. 2001;75:61–6.
Pavlásek I, Chalupský J, Kolárová L. Echinococcus multilocularis - a little tapeworm of foxes. Veterinarstvi. 1996;4:164–7.
Pavlásek I, Chalupský J, Kolárová L, Horyna B, Ritter J. Occurrence of Echinococcus multilocularis Leuckart, 1863, in foxes (Vulpes vulpes) in the Czech Republic. Epidemiol Mikrobiol Imunol. 1997;46:158–62.
Pavlásek I. Actual situation and occurrence of Echinococcus multilocularis in foxes both in Europe and in Czech Republic. Remedia - Klinicka Mikrobiologie. 1998;2:233–40.
Laurimaa L, Davison J, Plumer L, Süeld K, Oja R, Moks E, et al. Noninvasive detection of Echinococcus multilocularis tapeworm in urban area, Estonia. Emerg Infect Dis. 2015;21:163–4.
Moks E, Saarma U, Valdmann H. Echinococcus multilocularis in Estonia. Emerg Infect Dis. 2005;11:1973–4.
Moks E. Tapeworm parasites Echinococcus multilocularis and E. granulosus in Estonia: phylogenetic relationships and occurrence in wild carnivores and ungulates. PhD Thesis, Universitatis Tartuensis. 2008.
Aubert M, Jacquier P, Artois M. Hosts of Echinococcus multiloculairs in Lorraine and their consequences on human contamination. I. Biogeographic approach. Bull Soc Fr Parasitol. 1986;4:59–64.
Aubert M, Jacquier P, Artois M, Barrat MJ, Basile AM. Parasitism of red fox (Vulpes vulpes) by Echinococcus multilocularis in Lorraine (France) and their consequences on human contamination. Rec Med Vet Ec Alfort. 1987;163:839–43.
Baudouin MC, Aubert MFA. Echinococcus multilocularis Leuckart, 1863 in foxes (Vulpes vulpes Linnaeus, 1758) in the Vosges: a parasite dangerous to man. Rev Sci Tech OIE. 1993;12:161–3.
Coudert J, Euzeby J, Garin JP. Incidence of E. multilocularis in common fox (Vulpes vulpes) in the nord-east of France. Lyon Med. 1970;32:293–8.
Deblock S, Petavy AF, Gilot B. Intestinal helminths of the red fox Vulpes vulpes L. in the Massif Central France. Can J Zool. 1988;66:1562–9.
Pesson B, Carbiener R. Ecology of multilocular hydatidosis in Alsace. Parasitism in the red fox (Vulpes vulpes). B Ecol. 1989;20:295–301.
Petavy AF, Duriez T, Gilot B, Deblock S. Status of the focus of multilocular hydatidiosis in the Auvergne. Fourth year of study. Bull Soc Fr Parasitol. 1985;1:115–8.
Petavy AF, Deblock S, Prost C. Epidemiology of alveolar echinococcosis in France. 1. Intestinal helminths in the red fox (Vulpes vulpes L.) from Haute-Savoie. Ann Parasitolo Hum Comp. 1990;65:22–7.
Petavy AF, Deblock S, Walbaum S. Life cycles of Echinococcus multilocularis in relation to human infection. J Parasitol. 1991;77:133–7.
Raoul F, Deplazes P, Nonaka N, Piarroux R, Vuitton DA, Giraudoux P. Assessment of the epidemiological status of Echinococcus multilocularis in foxes in France using ELISA coprotests on fox faeces collected in the field. Int J Parasitol. 2001;31:1579–88.
Combes B, Comte S, Raton V, Raoul F, Boue F, Umhang G, et al. Westward spread of Echinococcus multilocularis in foxes, France, 2005–2010. Emerg Infect Dis. 2012;18:2059–62.
Goutal-Rotszyld C. Contribution to the study of internal parasitism of foxes (Vulpes vulpes) in middle Pyrenees: search for Echinococcus multilocularis. Theses University of Toulouse. 2005
Guislain M-H, Raoul F, Giraudoux P, Terrier M-E, Froment G, Ferte H, Poulle M-L. Ecological and biological factors involved in the transmission of Echinococcus multilocularis in the French Ardennes.J Helminthol. 2008;82:143–51.
Knapp J, Guislain MH, Bart JM, Raoul F, Gottstein B, Giraudoux P, Piarroux R. Genetic diversity of Echinococcus multilocularis on a local scale. Infect Genet Evol. 2008;8:367–73.
Magnaval J-F, Boucher C, Morassin B, Raoul F, Duranton C, Jacquiet P, Giraudoux P, et al. Epidemiology of alveolar echinococcosis in southern Cantal, Auvergne region, France. J Helminthol. 2004;78:237–42.
Robardet E, Giraudoux P, Caillot C, Boue F, Cliquet F, Augot D, Barrat J. Infection of foxes by Echinococcocus multilocularis in urban and suburban areas of Nancy, France: influence of feeding habits and environment. Parasite. 2008;15:77–85.
Umhang G, Woronoff-Rhen N, Combes B, Boue F. Segmental sedimentation and counting technique (SSCT): An adaptable method for qualitative diagnosis of Echinococcus multilocularis in fox intestines. Exp Parasitol. 2011;128:57–60.
Teysseyre A. Contribution to the study of internal parasitism of foxes (Vulpes vulpes). Thesis, University of Toulouse. 2005
Ballek D: Occurrence of Echinococcus multilocularis and other cestodes and nematodes in the red fox Vulpes vulpes in the administrative districts Arnsberg, Detmold and Kassel. PhD Thesis. Hannover: Tieraerztliche Hochschule Hannover; 1991.
Ballek D, Takla M, Ising-Volmer S, Stoye M. The helminth fauna of red foxes (Vulpes vulpes Linnaeus 1758) in north Hesse and east Westphalia. 1. Cestodes. Dtsch Tierarztl Wochenschr. 1992;99:362–5.
Berke O, von Keyserlingk M. Increase of the prevalence of Echinococcus multilocularis infection in red foxes in Lower Saxony. Dtsch Tierarztl Wochenschr. 2001;108:201–5.
Berke O, von Keyserlingk M, Broll S, Kreienbrock L. On the distribution of Echinococcus multilocularis in red foxes in Lower Saxony: identification of a high risk area by spatial epidemiological cluster analysis. Berl Munch Tierarztl. 2002;115:428–34.
Berke O, Romig T, von Keyserlingk M. Emergence of Echinococcus multilocularis among red foxes in northern Germany, 1991–2005. Vet Parasitol. 2008;155:319–22.
Bilger B, Veit P, Müller V, Merckelbach A, Kersten D, Stoppler H, Lucius R. Further-studies of Echinococcus multilocularis infection of the red fox in the district of Tubingen. Tierarztl Umsch. 1995;50:465–70.
Dinkel A, von Nickisch–Rosenegk M, Bilger B, Merli M, Lucius R, Romig T. Detection of Echinococcus multilocularis in the definitive host: Coprodiagnosis by PCR as an alternative to necropsy. J Clin Microbiol. 1998;36:1871–6.
Eskens U. On the occurrence of Echinococcus multilocularis in red fox in the areas adjoining the State Medical, Food, and Veterinary Investigation Bure in Mid-Hessen. Z Jagdwiss. 1997;43:154–65.
Ewald D. Distribution of the tapeworm Echinococcus multilocularis in the fox Vulpes vulpes and muskrat Ondatra zibethicus in the Freiburg administrative district. Mitt Bad Landesver Naturkd Naturschutz. 1990;15:81–100.
Janka S, Stoye M. Studies on Echinococcus multilocularis and Trichinella spiralis infections in the red fox in the Karlsruhe area. Tierarztl Umsch. 1998;53:221–6.
Jonas D, Hahn W. Evidence of Echinococcus multilocularis in foxes in Rheinland-Pfalz. Praktische Tierarzt. 1984;65:7–9.
Jonas D, Drager K. Investigation of Echinococcus multilocularis infection in foxes: development since 1982 and the situation in 1996/97 in Rhineland Palatinate. Tierarztl Umsch. 1998;53(214):7-21.
Koenig A, Romig T, Thoma D, Kellermann K. Drastic increase in the prevalence in Echinococcus multilocularis in foxes (Vulpes vulpes) in southern Bavaria, Germany. Eur J Wildlife Res. 2005;51:277–82.
Lucius R, Boeckeller W, Pfeiffer AS. Parasitic infestation of the domestic and wild animals of Schleswig-Holstein West Germany parasites of the inner organs of red fox Vulpes vulpes. Z Jagdwiss. 1988;34:242–55.
Manke KJ, Stoye M. Parasitological studies of red foxes (Vulpes vulpes L.) in the northern districts of Schleswig-Holstein. Tierarztl Umsch. 1998;53:207–14.
Meine K, Müller P. On the occurrence of the small fox tapeworm Echinococcus multilocularis (Leuckart 1863) in the Saarland. Z Jagdwiss. 1996;42:274–83.
Muehling A, Zeyhle E, Frank W. Epidemiological studies on Echinococcus multilocularis in southwest Germany. In: Proceedings of the second International Symposium on taeniasis/cysticercosis and echinococcosis/hydatidosis 2–7 1985
Müller B, Partridge A. The occurrence of Echinococcus multilocularis in animals in South Wurttemberg. Tierarztl Umsch. 1974;29:602–12.
Pfeiffer F, Kuschfeldt S, Stoye M. The helminth fauna of the red fox (Vulpes vulpes Linné, 1758) in the south of Saxe-Anhalt.1. Cestodes. Dtsch Tierarztl Wochenschr. 1997;104:445–8.
Romig T, Bilger B, Dinkel A, Merli M, Thoma D, Will R, Mackenstedt U, Lucius R. Impact of praziquantel baiting on intestinal helminths of foxes in southwestern Germany. Helminthologia. 2007;44:137–44.
Schelling U, Schafer E, Pfister T, Frank W. An epidemiologic-study of the prevalence of Echinococcus multilocularis in north-east Baden-Wurttemberg. Tierarztl Umsch. 1991;46:673–6.
Schelling U, Frank W, Will R, Romig T, Lucius R. Chemotherapy with praziquantel has the potential to reduce the prevalence of Echinococcus multilocularis in wild foxes (Vulpes vulpes). Ann Trop Med Parasitol. 1997;91:179–86.
Schöeffel I, Schein E, Wittstadt U, Hentsche J. Parasites of the red fox in Berlin (West). Berl Munch Tierarztl. 1991;104:153–7.
Schott E, Müller B. Prevalence of Echinococcus multilocularis in foxes in the district of Tubingen, West-Germany. Tierarztl Umsch. 1989;44:367–70.
Schott E, Müller B. Age specific prevalences of Echinococcus multilocularis infection in red foxes (Vulpes vulpes). Tierarztl Umsch. 1990;45:620–3.
Staubach C, Thulke HH, Tackmann K, Hugh-Jones M, Conraths FJ. Geographic information system-aided analysis of factors associated with the spatial distribution of Echinococcus multilocularis infections of foxes. Am J Trop Med Hygiene. 2001;65:943–8.
Staubach C, Hoffmann L, Schmid VJ, Ziller M, Tackmann K, Conraths FJ. Bayesian space-time analysis of Echinococcus multilocularis-infections in foxes. Vet Parasitol. 2011;179:77–83.
Suhrke J, Plotner J, Zemke M. Occurrence of Echinococcus multilocularis in animals in Southern Thuringia. Monatsh Veterinarmed. 1991;46(20):714–7.
Tackmann K, Beier D. The prevalence of Echinococcus multilocularis infection in wildlife carnivores in an area of Germany.1. Parasitological analysis of wild carnivores for determination of pathogen prevalence. Tierarztl Umsch. 1993;48(8):498–503.
Tackmann K, Loschner U, Mix H, Staubach C, Thulke HH, Conraths FJ. Spatial distribution patterns of Echinococcus multilocularis (Leuckart 1863) (Cestoda : Cyclophyllidea: Taeniidae) among red foxes in an endemic focus in Brandenburg, Germany. Epidemiol Infect. 1998;120:101–9.
Uhl W, Betke P, Decker J. Postmortem findings in red foxes. Praktische Tierarzt. 1993;74:1018–24.
von Keyserlingk M, Thoms B, Korfer KH, Braune S. Investigations of the occurrence of Echinococcus multilocularis in the red fox population of Lower Saxony. Tierarztl Umsch. 1998;53:202.
Vos A, Schneider L. The prevalence of Echinococcus multilocularis in red foxes (Vulpes vulpes) in Southern Bavaria. Tierarztl Umsch. 1994;49:225.
Welzel AM, Steinbach G, von Keyserlingk M, Stoye M, Von Keyserlingk M. On the helminth fauna of red foxes (Vulpes vulpes L.) in southern Lower Saxony. Part 2: cestodes. Z Jagdwiss. 1995;41(2):100–9.
Wessbecher H, Dalchow W. Stoye M. The helminth fauna of the red fox (Vulpes vulpes L., 1758) in the administrative district of Karlsruhe. 1. Cestodes. Dtsch Tierarztl Wochenschr. 1994;101:322–6.
Worbes H. The occurrence of Echinococcus granulosus and E. multilocularis in Thuringia. Angew Parasitol. 1992;33:193–204.
Zeyhle E. Echinococcus multilocularis in fox (Vulpes vulpes), fieldvoles (Microtus arvalis) and in humans in an endemic area of the Schwabische Alb. Praktische Tierarzt. 1980;61(4):360.
Zeyhle E, Abel M, Frank W. Epidemiological studies on the occurrence of Echinococcus multilocularis in definitive and intermediate hosts in Germany. Mitt Osterr Ges Tropenmed Parasitol. 1990;12:221–32.
Ahlman VP. Epidemiological studies on the occurrence of rabies and little tapeworm Echinococcus multilocularis in Saarland. PhD Thesis Freien University Berli. 1997.
Denzin N, Schliephake A, Ewert B. Echinococcus multilocularis in red foxes in Saxony-Anhalt: Identification of areas of increased risk of infestation and association of the infestation probability with the average annual maximum temperature. Berl Munch Tierarztl Wochenschr. 2005;118:404–9.
Denzin N, Schliephake A, Wirth A. Spatiotemporal analysis of the infection of the red fox (Vulpes vulpes L.) with Echinococcus multilocularis in Saxony-Anhalt. Berl Munch Tierarztl Wochenschr. 2009;122:82–92.
Denzin N, Schliephake A, Froehlich A, Ziller M, Conraths FJ. On the move? Echinococcus multilocularis in red foxes of Saxony-Anhalt (Germany). Transbound Emerg Dis. 2014;61:239–46.
Deplazes P, Hegglin D, Gloor S, Romig T. Wilderness in the city: the urbanization of Echinococcus multilocularis. Trends Parasitol. 2004;20:77–84.
Immelt U, Thelen U, Eskens U. Investigation of Echinococcus multilocularis in red foxes and their possible relationship to human alveolar echinococcosis. Tierarztl Umsch. 2009;64:199–212.
Janko C, Linke S, Schroeder W, Koenig A, Romig T, Thoma D. Infection pressure of human alveolar echinococcosis due to village and small town foxes (Vuples vulpes) living in close proximity to residents. Eur J Wildlife Res. 2011;57:1033–42.
Koenig A, Romig T, Janko C, Hildenbrand R, Holzhofer E, Kotulski Y, et al. Integrated-baiting concept against Echinococcus multilocularis in foxes is successful in southern Bavaria, Germany. Eur J Wildlife Res. 2008;54:439–47.
Koenig A, Romig T. Fox tapeworm Echinococcus multilocularis, an underestimated threat: a model for estimating risk of contact. Wildlife Biol. 2010;16:258–66.
Bagrade G, Snabel V, Romig T, Ozolins J, Huettner M, Miterpáková M, et al. Echinococcus multilocularis is a frequent parasite of red foxes (Vulpes vulpes) in Latvia. Helminthologia. 2008;45:157–61.
Bruzinskaite R, Marcinkute A, Strupas K, Sokolovas V, Deplazes P, Mathis A, et al. Alveolar echinococcosis, Lithuania. Emerg Infect Dis. 2007;13:1618–9.
Bružinskaitė-Schmidhalter R, Šarkūnas M, Malakauskas A, Mathis A, Torgerson PR, Deplazes P. Helminths of red foxes (Vulpes vulpes) and raccoon dogs (Nyctereutes procyonoides) in Lithuania. Parasitology. 2012;139:120–7.
Malczewski A, Rocki B, Ramisz A, Eckert J. Echinococcus multilocularis (Cestoda), the causative agent of alveolar echinococcosis in humans - first record in Poland. J Parasitol. 1995;81:318–21.
Malczewski A, Ramisz A, Rocki B, Bieńko R, Balicka-Ramisz A, Eckert J. Echinococcus multilocularis in red foxes (Vulpes vulpes) in Poland: an update of the epidemiological situation. Acta Parasitol. 1999;44:68–72.
Ramisz A, Eckert J, Balicka-Ramisz A, Grupiński T, Pilarczyk B, Król-Pośpieszny A, Slowikowski P. Prevalence of Echinococcus multilocularis in foxes in the Western Poland. Med Weter. 1997;53:340–2.
Ramisz A, Eckert J, Balicka-Ramisz A, Bieńko R, Pilarczyk B. Epidemiological studies on Echinococcus multilocularis in red foxes in north-west Poland. Wiad Parazytol. 1999;45:369–73.
Ramisz A, Nicpoń J, Balicka-Ramisz A, Pilarczyk B, Pacoń J, Piekarska J. The prevalence of gastro-intestinal helminths in red foxes (Vulpes vulpes) in the south-west part of Poland. Tierarztl Umsch. 2004;59:601–4.
Rocki B, Malczewski A, Eckert J. Studies on the incidence of Echinococcus multilocularis in red foxes (Vulpes vulpes) in north-east, central and south of Poland. Wiad Parazytol. 1999;45:391–3.
Borecka A, Gawor J, Malczewska M, Malczewski A. Prevalence of Echinococcus multilocularis tapeworm in red foxes in central Poland. Medycyna Wet. 2007;63:1333–5.
Borecka A, Gawor J, Malczewska M, Malczewski A. Occurence of Echinococcus multilocularis in red foxes (Vulpes vulpes) in southern Poland. Helminthologia. 2008;45:24–7.
Borecka A, Gawor J, Malczewska M, Malczewski A. Prevalence of zoonotic helminth parasites of the small intestine in red foxes from central Poland. Med Weter. 2009;65:33–5.
Dubinský P, Malczewski A, Miterpáková M, Gawor J, Reiterová K. Echinococcus multilocularis in the red fox Vulpes vulpes from the East Carpathian region of Poland and the Slovak Republic. J Helminthol. 2006;80(3):243–7.
Karamon J, Ziomko I, Cencek T, Sroka J, Zięba P. Prevalence of Echinococcus multilocularis in red foxes in the Lublin voivodeship, Poland: preliminary study. Med Weter. 2008;64:1237–9.
Karamon J, Sroka J, Cencek T, Michalski MM, Zięba P, Karwacki J. Prevalence of Echinococcus multilocularis in red foxes in two eastern provinces of Poland. Bull Vet Inst Pulawy. 2011;55:429–33.
Karamon J, Kochanowski M, Sroka J, Cencek T, Różycki M, Chmurzyńska E, Bilska-Zając E. The prevalence of Echinococcus multilocularis in red foxes in Poland - current results (2009–2013). Parasitol Res. 2014;113:317–22.
Machnicka-Rowińska B, Rocki B, Dziemian E, Kolodziej-Sobocinska M. Raccoon dog (Nyctereutes procyonoides) the new host of Echinococcus multilocularis in Poland. Wiad Parazytol. 2002;48:65–8.
Machnicka B, Dziemian E, Rocki B, Kołodziej–Sobocińska M. Detection of Echinococcus multilocularis antigens in faeces by ELISA. Parasitol Res. 2003;91:491–6.
Malczewski A, Gawor J, Malczewska M. Infection of red foxes (Vulpes vulpes) with Echinococcus multilocularis during the years 2001–2004 in Poland. Parasitol Res. 2008;103:501–5.
Pacon J, Sołtysiak Z, Nicpoń J, Janczak M. Prevalence of internal helminths in red foxes (Vulpes vulpes) in selected regions of Lower Silesia. Med Weter. 2006;62:67–9.
Antolová D, Miterpáková M, Reiterová K, Dubinský P. Influence of anthelmintic baits on the occurrence of causative agents of helminthozoonoses in red foxes (Vulpes vulpes). Helminthologia. 2006;43:226–31.
Antolová D, Miterpáková M, Radoňak J, Hudačkova D, Szilagyiova M, Začek M. Alveolar echinococcosis in a highly endemic area of Northern Slovakia between 2000 and 2013. Euro Surveill. 2014;19(34).
Dubinský P, Várady M, Reiterová K, Miterpáková M, Turčeková L. Prevalence of Echinococcus multilocularis in red foxes in the Slovak Republic. Helminthologia. 2001;38(4):215–9.
Hurníková Z, Miterpáková M, Chovancová B. The important zoonoses in the protected areas of the Tatra National Park (TANAP). Wiad Parazytol. 2009;55:395–8.
Letková V, Lazăr P, Čurlík J, Goldová M, Kočišová A, Kosuthová L, Mojžišová J. The red fox (Vulpes vulpes L.) as a source of zoonoses. Veterinarski Arhiv. 2006;76:73–81.
Letková V, Lazăr P, Soroka J, Goldová M, Čurlík J. Epizootiology of game cervid cysticercosis. Natura Croatica. 2008;17:311–8.
Miterpáková M, Várady M, Reiterová K, Turčeková L, Dubinský P. Present state of the occurrence of Echinococcus multilocularis in red foxes in Slovakia. Helminthologia. 2001;38:182.
Miterpáková M, Dubinský P, Reiterová K, Machková N, Várady M, Šnábel V. Spatial and temporal analysis of the Echinococcus multilocularis occurrence in the Slovak Republic. Helminthologia. 2003;40(4):217–26.
Miterpáková M, Dubinský P, Reiterová K, Stanko M. Climate and environmental factors influencing Echinococcus multilocularis occurrence in the Slovak Republic. Ann Agric Environ Med. 2006;13(2):235–42.
Miterpáková M, Hurníková Z, Antolová D, Dubinský P. Endoparasites of red fox (Vulpes vulpes) in the Slovak Republic with the emphasis on zoonotic species Echinococcus multilocularis and Trichinella spp. Helminthologia. 2009;46(2):73–9.
Miterpáková M, Dubinský P. Fox tapeworm (Echinococcus multilocularis) in Slovakia - summarizing the long-term monitoring. Helminthologia. 2011;48(3):155–61.
Reiterová K, Miterpáková M, Turčeková U, Antolová D, Dubinský P. Field evaluation of an intravital diagnostic test of Echinococcus multilocularis infection in red foxes. Vet Parasitol. 2005;128(1–2):65–71.
Reiterová K, Dziemian E, Miterpáková M, Antolová D, Kołodziej-Sobocińska M, Machnicka B, Dubinský P. Occurrence of Echinococcus multilocularis in red foxes from the Carpathian regions of Slovakia and Poland. Acta Parasitol. 2006;51(2):107–10.
Anonymous. Alveolar echinococcosis. Prevalence of Echinococcus multilocularis in foxes. Wkly Epidemiol Rec. 1993;68:165–8.
Brossard M, Andreutti C, Siegenthaler M. Infection of red foxes with Echinococcus multilocularis in western Switzerland. J Helminthol. 2007;81:369–76.
Deplazes P, Gloor S, Stieger C, Hegglin D. Urban transmission of Echinococcus multilocularis. In: Craig P, Pawlowski Z, editors. Cestode Zoonoses: Echinococcosis and Cysticercosis: An Emergent and Global Problem. 2002;341:287–97.
Ewald D, Eckert J. Distribution and frequency of Echinococcus multilocularis among red fox in north, South and East Switzerland as well as in the principality of Liechtenstein. 1993;39:171–80.
Gottstein B, Saucy F, Wyss C, Siegenthaler M, Jacquier P, Schmitt M, et al. Investigations on a Swiss area highly endemic for Echinococcus multilocularis. Appl Parasitol. 1996;37(2):129–36.
Hofer S, Gloor S, Bontadina F, Mathis A, Hegglin D, Mueller U, et al. Life cycle of Echinococcus multilocularis in the City of Zurich: A new risk? Schweiz Med Wochenschr. 1999;129:1125.
Hofer S, Gloor S, Muller U, Mathis A, Hegglin D, Deplazes P. High prevalence of Echinococcus multilocularis in urban red foxes (Vulpes vulpes) and voles (Arvicola terrestris) in the city of Zurich, Switzerland. Parasitology. 2000;120:135–42.
Reperant LA, Weber JM, Hegglin D, Deplazes P. Echinococcus multilocularis infections of rural, residential and urban foxes (Vulpes vulpes) in the canton of Geneva, Switzerland. Parasite. 2005;12:339–46.
Guerra D, Hegglin D, Bacciarini L, Schnyder M, Deplazes P. Stability of the southern European border of Echinococcus multilocularis in the Alps: evidence that Microtus arvalis is a limiting factor. Parasitology. 2014;141(12):1593–602.
Hegglin D, Ward PI, Deplazes P. Anthelmintic baiting of foxes against urban contamination with Echinococcus multilocularis. Emerg Infect Dis. 2003;9:1266–72.
Nagy A, Ziadinov I, Schweiger A, Schnyder M, Deplazes P. Hair coat contamination with zoonotic helminth eggs of farm and pet dogs and foxes. Berliner Munchener Tierarztl Wochenschr. 2011;124:503–11.
Reperant LA, Hegglin D, Fischer C, Kohler L, Weber J-M, Deplazes P. Influence of urbanization on the epidemiology of intestinal helminths of the red fox (Vulpes vulpes) in Geneva, Switzerland. Parasitol Res. 2007;101:605–11.
Smith GC, Gangadharan B, Taylor Z, Laurenson MK, Bradshaw H, Hide G, et al. Prevalence of zoonotic important parasites in the red fox (Vulpes vulpes) in Great Britain. Vet Parasitol. 2003;118:133–42.
Tanner F, Hegglin D, Thoma R, Brosi G, Deplazes P. Echinococcus multilocularis in Grisons: distribution in foxes and presence of potential intermediate hosts. Schweiz Arch Tierheilkd. 2006;148:501–10.
EFSA. Assessment of Echinococcus multilocularis surveillance reports submitted 2013 in the context of Commission Regulation (EU) No 1152/2011. EFSA J. 2013;11:3465.
EFSA. Assessment of Echinococcus multilocularis surveillance reports submitted in 2014 in the context of Commission Regulation (EU) No 1152/2011. EFSA J. 2014;12:3875.
Murphy TM, Wahlstrom H, Dold C, Keegan JD, McCann A, Melville J, Murphy D, McAteer W. Freedom from Echinococcus multilocularis: An Irish perspective. Vet Parasitol. 2012;190:196–203.
Learmount J, Zimmer IA, Conyers C, Boughtflower VD, Morgan CP, Smith GC. A diagnostic study of Echinococcus multilocularis in red foxes (Vulpes vulpes) from Great Britain. Vet Parasitol. 2012;190:447–53.
Davidson RK, Oines O, Madslien K, Mathis A. Echinococcus multilocularis - adaptation of a worm egg isolation procedure coupled with a multiplex PCR assay to carry out large-scale screening of red foxes (Vulpes vulpes) in Norway. Parasitol Res. 2009;104:509–14.
Davidson R, Oines O, Albin-Amiot C, Hopp P, Madslien K, Hagström A, Isaksson M. Ghost- hunting - is Echinococcus multilocularis really absent from mainland Norway? Trop Med Int Health. 2013;18:97.
EFSA. Assessment of Echinococcus multilocularis surveillance data 2012–2013 submitted by Norway in the context of Commission Regulation (EU) No 1152/2011. EFSA J. 2015;13:4035.
Madslien K, Davidson R, Handeland K, Oines O, Urdahl AM, Hopp P. The surveillance and control programme for Echinococcus multilocularis in red foxes (Vulpes vulpes) in Norway. Annual Report 2011. 2011
Henttonen H, Fuglei E, Gower CN, Haukisalmi V, Ims RA, Niemimaa J, Yoccoz NG. Echinococcus multilocularis on Svalbard: introduction of an intermediate host has enabled the local life-cycle. Parasitology. 2001;123:547–52.
Fuglei E, Stien A, Yoccoz NG, Ims RA, Eide NE, Prestrud P, Deplazes P, Oksanen A. Spatial distribution of Echinococcus multilocularis, Svalbard, Norway. Emerg Infect Dis. 2008;14:73–5.
Stien A, Voutilainen L, Haukisalmi V, Fuglei E, Mørk T, Yoccoz NG, et al. Intestinal parasites of the Arctic fox in relation to the abundance and distribution of intermediate hosts. Parasitology. 2010;137:149–57.
Laurimaa L, Süeld K, Moks E, Valdmann H, Umhang G, Knapp J, Saarma U. First report of the zoonotic tapeworm Echinococcus multilocularis in raccoon dogs in Estonia, and comparisons with other countries in Europe. Vet Parasitol. 2015;212:200–5.
Schwarz S, Sutor A, Staubach C, Mattis R, Tackmann K, Conraths FJ. Estimated prevalence of Echinococcus multilocularis in raccoon dogs Nyctereutes procyonoides in northern Brandenburg, Germany. Curr Zool. 2011;57:655–61.
Thiess A, Schuster R, Nöckler K, Mix H. Helminth findings in indigenous raccoon dogs Nyctereutes procyonoides (Gray, 1843). Berliner Munchener Tierarztl Wochenschr. 2001;114:273–6.
Thiess A: Studies on the helminth fauna and the occurrence of Trichinella species of the raccoon dog (Nyctereutes procyonoides) in the Federal State Brandenburg. PhD Thesis. Mensch & Buch Verlag, Berlin 2004.
Széll Z, Marucci G, Pozio E, Sréter T. Echinococcus multilocularis and Trichinella spiralis in golden jackals (Canis aureus) of Hungary. Vet Parasitol. 2013;197(1–2):393–6.
Bagrade G, Kirjušina M, Vismanis K, Ozoliņš J. Helminth parasites of the wolf Canis lupus from Latvia. J Helminthol. 2009;83:63–8.
Dyachenko V, Pantchev N, Gawlowska S, Vrhovec MG, Bauer C. Echinococcus multilocularis infections in domestic dogs and cats from Germany and other European countries. Vet Parasitol. 2008;157(3–4):244–53.
Umhang G, Raton V, Comte S, Hormaz V, Boucher JM, Combes B, Boué F. Echinococcus multilocularis in dogs from two French endemic areas: No evidence of infection but hazardous deworming practices. Vet Parasitol. 2012;188(3–4):301–5.
Umhang G, Comte S, Raton V, Hormaz V, Boucher JM, Favier S, Combes B, Boué F. Echinococcus multilocularis infections in dogs from urban and peri-urban areas in France. Parasitol Res. 2014;113(6):2219–22.
Tackmann K, Löschner U, Mix H, Staubach C, Thulke HH, Ziller M, Conraths FJ. A field study to control Echinococcus multilocularis infections of the red fox (Vulpes vulpes) in an endemic focus. Epidemiol Infect. 2001;127:577–87.
Bruzinskaite R, Sarkūnas M, Torgerson PR, Mathis A, Deplazes P. Echinococcosis in pigs and intestinal infection with Echinococcus spp. in dogs in southwestern Lithuania. Vet Parasitol. 2009;160(3–4):237–41.
Antolová D, Reiterová K, Miterpaková M, Dinkel A, Dubinský P. The first finding of Echinococcus multilocularis in dogs in Slovakia: an emerging risk for spreading of infection. Zoonoses Publ Hlth. 2009;56(2):53–8.
Szabova E, Juris P, Miterpakova M, Antolova D, Papajova I, Sefcikova H. Prevalence of important zoonotic parasites in dog populations from the Slovak Republic. Helminthologia. 2007;44:170–6.
Gottstein B, Saucy F, Deplazes P, Reichen J, Demierre G, Busato A, Zuercher C, Pugin P. Is high prevalence of Echinococcus multilocularis in wild and domestic animals associated with disease incidence in humans? Emerg Infect Dis. 2001;7(3):408–12.
Stieger C, Hegglin D, Schwarzenbach G, Mathis A, Deplazes P. Spatial and temporal aspects of urban transmission of Echinococcus multilocularis. Parasitology. 2002;124:631–40.
Sager H, Moret CS, Grimm F, Deplazes P, Doherr MG, Gottstein B. Coprological study on intestinal helminths in Swiss dogs: temporal aspects of anthelminthic treatment. Parasitol Res. 2006;98(4):333–8.
Petavy AF, Tenora F, Deblock S, Sergent V. Echinococcus multilocularis in domestic cats in France - A potential risk factor for alveolar hydatid disease contamination in humans. Vet Parasitol. 2000;87(2–3):151–6.
Fesseler M, Schott E, Mueller B. Occurrence of Echinococcus multilocularis among cats in the Tuebingen region of the Federal Republic of Germany. Tierarztl Umsch. 1989;44:766–75.
Meyer H, Svilenov D. Finding of Echinococcus multilocularis in stray domestic cats in South Germany. Zentralblatt Fur Veterinarmedizin Reihe B. 1985;32(10):785–6.
Rehmann P, Gröne A, Gottstein B, Sager H, Müller N, Völlm J, Bacciarini LN. Alveolar echinococcosis in the zoological garden Basle. Schweiz Arch Tierheilkd. 2005;147(11):498–502.
Cada F, Martinek K, Kolarova L. Cats (Felis catus f. dom.) as definitive host of Echinococcus multilocularis. Veterinarstvi. 1999;49:6–7.
Hartel KS, Spittler H, Doering H, Winkelmann J, Hoerauf A, Reiter-Owona I. The function of wild nutria (Myocastor coypus) as intermediate hosts for Echinococcus multilocularis in comparison to wild muskrats (Ondatra zibethicus). Int J Med Microbiol. 2004;293:62–3.
Mathy A, Hanosset R, Adant S, Losson B. The carriage of larval Echinococcus multilocularis and other cestodes by the muskrat (Ondatra zibethicus) along the ourthe river and its tributaries (Belgium). J Wildl Dis. 2009;45(2):279–87.
Umhang G, Richomme C, Boucher J-M, Guedon G, Boué F. Nutrias and muskrats as bioindicators for the presence of Echinococcus multilocularis in new endemic areas. Vet Parasitol. 2013;197(1–2):283–7.
Boussinesq M, Bresson S, Liance M, Houin R. A new natural intermediate host of Echinococcus multilocularis in France - the muskrat (Ondatra zibethicus). Ann Parasitol Hum Comp. 1986;61(4):431–4.
Baumeister S, Pohlmeyer K, Kuschfeldt S, Stoye M. On the prevalence of Echinococcus multilocularis and other metacestodes and cestodes in the muskrat (Ondatra zibethicus Link, 1795) in Lower Saxony. Dtsch Tierarztl Wochenschr. 1997;104(10):448–52.
Borgsteede FHM, Tibben JH, van der Giessen JWB. The musk rat (Ondatra zibethicus) as intermediate host of cestodes in the Netherlands. Vet Parasitol. 2003;117:29–36.
Friedland T, Steiner B, Boeckeler W. Prevalence of cysticercosis in muskrats Ondatra zibethica in Schleswig-Holstein west Germany. Z Jagdwiss. 1985;31:134–9.
Nicodemus S. Echinococcus multilocularis and other Cestoda larvae in muskrat (Ondarta zibethicus) in Luxembourg. PhD Thesis. Universitat Hohenheim2012.
Schichowski HD. Investigations on the occurrence of finned stadia of Echinococcus multilocularis in muskrats (Ondatra zibethicus) in the district of Arnsberg North-Rhine Westfalia. Z Jagdwiss. 2002;48(2):119–24.
Frank B, Zeyhle E. Echinococcus and other tapeworm larvae in muskrat (Ondatra zibethicus). Nachr Dtsch Pflanzenschutzd. 1981;33:166–70.
Seegers G, Baumeister S, Pohlmeyer K, Stoye M. Echinococcus multilocularis -metacestodes in muskrats (Ondatra zibethicus) in Lower Saxony. Dtsch Tierarztl Wochenschr. 1995;102:256.
Bonnin JL, Artois M, Aubert M. Incidence and distribution of larval cestode infections in rodents in Lorraine. Rev Med Vet. 1989;140:589–97.
Houin R, Deniau M, Liance M. Arvicola terrestris (L) 1758 1st rodent found naturally infested with Echinococcus multilocularis Leuckart, 1863, in France. C R Hebd Seances Acad Sci. 1980;290(19):1269–71.
Petavy AF, Tenora F, Deblock S. Contributions to knowledge on the helminths parasitizing several Arvicolidae (Rodentia) in Auvergne (France). Helminthologia. 1996;33:51–8.
Pétavy AF, Deblock S. The Auvergnan focus of alveolar echinococcosis. Research on the intermediate host, description of the lesions. Ann Parasitol Hum Comp. 1983;58:439–53.
Pétavy AF, Tenora F, Deblock S. Co-occurrence of metacestodes of Echinococcus multilocularis and Taenia taeniaeformis (Cestoda) in Arvicola terrestris (Rodentia) in France. Folia Parasitol. 2003;50:157–8.
Deblock S, Pétavy AF. Hepatic larvae of Cestode parasites of the vole rat Arvicola terrestris in Auvergne (France). Ann Parasitol Hum Comp. 1983;58:423–37.
Houin R, Deniau M, Liance M, Puel F. Arvicola terrestris an intermediate host of Echinococcus multilocularis in France - epidemiological consequences. Int J Parasitol. 1982;12(6):593–600.
Delattre P, Giraudoux P, Quere JP. Epidemiologic consequences of the receptivity of a new intermediate host of Echinococcus multilocularis, and of the space-time localization of the infected rodents. C R Acad Sci III-Vie. 1990;310(8):339–44.
Loos-Frank B. Larval cestodes in Southwest German rodents. Zeitschrift fuer Angewandte Zoologie. 1987;74:97–106.
Malczewski A, Borecka A, Malczewska M, Gawor J. An attempt to determine intermediate hosts of the tapeworm Echinococcus multilocularis in Poland. Wiad Parazytol. 2008;54(2):137–41.
Burlet P, Deplazes P, Hegglin D. Age, season and spatio-temporal factors affecting the prevalence of Echinococcus multilocularis and Taenia taeniaeformis in Arvicola terrestris. Parasit Vectors. 2011;4:6.
Reperant LA, Hegglin D, Tanner I, Fischer C, Deplazes P. Rodents as shared indicators for zoonotic parasites of carnivores in urban environments. Parasitology. 2009;136:329–37.
Schmitt M, Saucy F, Wyborn S, Gottstein B. Infestation of water voles (Arvicola terrestris) with metacestodes of Echinococcus multilocularis in the canton of Freiburg (Switzerland). Schweiz Arch Tierheilkd. 1997;139(2):84–93.
Delattre P, Pascal M, Damange JP. Towards a strategy for the epidemiological study of alveolar echinococcosis. Apropos of cases of infestation seen in Microtus arvalis P. in the Doubs (France). Ann Parasitol Hum Comp. 1985;60(4):389–405.
Delattre P, Pascal M, Lepesteur MH, Giraudoux P, Damange JP. Ecological and epidemiological characteristics of Echinococcus multilocularis during a complete population-cycle in a secondary host (Microtus arvalis). Can J Zool. 1988;66(12):2740–50.
Pétavy AF, Deblock S, Gilot B. First occurrence of the larval stage of Echinococcus multilocularis in two voles, Microtus arvalis and Clethrionomys glareolus, from a focus of alveolar echinococcosis in the massif central (France). C R Acad Sci III-Vie. 1984;299(18):735–7.
Sydler T, Mathis A, Deplazes P. Echinococcus multilocularis lesions in the livers of pigs kept outdoors in Switzerland. Eur J Vet Pathol. 1998;4(1):43–6.
Remde I. Investigations on the occurrence of Echinococcus multilocularis and Trichinella spp. in wild boars (Sus scrofa scrofa) in the Wartburg region. PhD Thesis. Freie Universitaet Berlin, Berlin: Freie Universitaet Berlin; 2008.
Dubinsky P, Svobodova V, Turcekova L, Literak I, Martinek K, Reiterova K, et al. Echinococcus multilocularis in Slovak Republic: The first record in red foxes (Vulpes vulpes). Helminthologia. 1999;36(2):105–10.
Kapel CMO, Torgerson PR, Thompson RCA, Deplazes P. Reproductive potential of Echinococcus multilocularis in experimentally infected foxes, dogs, raccoon dogs and cats. Int J Parasitol. 2006;36:79–86.
Siko Barabasi S, Marosfoi L, Siko Barabasi Z, Cozma V. Natural alveolar echinococcosis with Echinococcus multilocularis in wild rodents. Sci Parasitol. 2011;12(1):11–21.
Pfeiffer AS, Boeckeler W, Lucius R. Parasites of the domestic and wild animals of Schleswig-Holstein West Germany parasites of the inner organs of the Beech Marten (Martes foina). Z Jagdwiss. 1989;35(2):100–12.
Andreyanov ON. Alveolar echinococcosis in fur animals from Ryazan district. Russian Parasitol J. 2011;3:7–11.
Abuladze KI. Fundamental Cestodology. Taenia tapeworms in animals and man and the diseases they cause. Academy of sciences of the USSR. 1964;4:130pp. Available: http://lekmed.ru/info/arhivy/teniaty-lentochnye-gelminty.html
Machulskii SN. Helminths of the transbaikalian fox in Buryat-Mongol Autonomous Soviet Socialist Republic. Works of the Buryat-Mongol Zoovet Institute. 1949;5
Tranbenkova NA. The ecology of Echinococcus multilocularis (Leukart, 1863) and E. granulosus (Batsch 1786) on the Kamchatka Peninsular. Med Parazitol (Mosk). 1992;1:45–7.
Mamdov MM. Questions on the natural foci of alveolar echinococcosis in the Turukhansk district of the Krasnoyarsk Region. Medical Parasitology and Parasitic Diseases. 1960:2
Kokolova L M. Epizootiology and epidemiology of helminths of carnivores in Yakutia. Skrjabin Helminthology Institute, Yakutia Scientific Institute of Agricultural Research. PhD Thesis, Moscow 2007.
Kabardiev SS, Bittirov AM, Gazimagomedov MG, Musayev ZG, Bittirova AA. Character of the distribution of alveococcosis in the foothill zone of the north Caucasus in different species of murine. Successes contemporary science. 2014;12:541–2. http://cyberleninka.ru/article/n/harakter-rasprostraneniya-alveokokkoza-v-predgornoy-zone-severnogo-kavkaza-u-raznyh-vidov-myshinyh.
Kokolova LM, Sofronov VM, Platonov TA, Zakharov ES, Verkhovtseva LA, Gavrilyeva LY. Epizootological situation on zoonosis and parasitic diseases of animalsand fish in Yakutia. Annals of the M.K. Ammosov North Western Federal University. 2012;9(3):86–90.
Acknowledgements
The authors would like to thank Relja Beck for the provision of raw data on red foxes from Croatia. We are grateful to EFSA staff in the persons of Andrea Gervelmeyer, Frank Verdonck and Gabriele Zancanaro for supporting this project. We are also grateful to Rosaria Rosanna Cammarano, Information Specialist (Documentation Centre, Istituto Superiore di Sanità, Rome) for performing the literature search.
Funding
This research received funding from the European Food Safety Authority (EFSA; http://www.efsa.europa.eu/) under the grant agreement GP/EFSA/AHAW/2012/01 (Project: Echinococcus multilocularis infection in animals). This work was also supported by the DG SANCO of the European Commission (2015). The funding bodies had no involvement in the conception, preparation and writing of the manuscript, in the viewpoints expressed, nor in the decision to submit this article.
Availability of data and material
The datasets supporting the conclusions of this article are included within the article and its additional files.
Authors’ contributions
AC conceived and designed the research. AP, AM, DM and GLT supported the systematic review approach. AO, MSL, AP and JK extracted the data. AM, DM and GLT analyzed the data. AO, MSL and JK wrote the first draft of the manuscript. AC, AP and BB finalized the manuscript. All authors contributed to the manuscript, read and approved the final version.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Additional files
Additional file 1: Text S1.
Questionnaire. (PDF 65 kb)
Additional file 2: Text S2.
Grey literature searching. (DOC 29 kb)
Additional file 3: Text S3.
List of the studies included in meta-analyses. (DOCX 47 kb)
Additional file 4: Text S4.
List of excluded studies. (DOCX 45 kb)
Additional file 5: Table S1.
Sampling strategy for red foxes (and Arctic foxes in Svalbard Islands, Norway). (DOC 66 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Oksanen, A., Siles-Lucas, M., Karamon, J. et al. The geographical distribution and prevalence of Echinococcus multilocularis in animals in the European Union and adjacent countries: a systematic review and meta-analysis. Parasites Vectors 9, 519 (2016). https://doi.org/10.1186/s13071-016-1746-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-016-1746-4