Abstract
Background
A universal coverage campaign (UCC) with long-lasting insecticidal nets (LLINs) was implemented in four districts in Midwestern Uganda in 2009–2010. Entomological surveys were carried out to monitor changes in vector density, behaviour and malaria transmission following this intervention.
Methods
Anopheles mosquitoes were collected using CDC light traps quarterly and human landing catch twice a year in four sites. Collections were done at baseline before the campaign and over a three-year period following the campaign. Plasmodium falciparum circumsporozoite enzyme-linked immunosorbent assays were performed. A subset of anophelines were molecularly identified to species, and kdr L1014S frequencies were determined.
Results
The prevailing malaria vector in three sites was Anopheles gambiae s.l. (>97 %), with An. funestus s.l. being present in low numbers only. An. gambiae s.s. dominated (> 95 %) over An. arabiensis within A. gambiae s.l. In the remaining site, all three vector species were observed, although their relative densities varied among seasons and years. Vector densities were low in the year following the UCC but increased over time. Vector infectivity was 3.2 % at baseline and 1.8 % three years post-distribution (p = 0.001). The daily entomological inoculation rate (EIR) in 2012 varied between 0.0-0.98 for the different sites compared to a baseline EIR that was between 0.0-5.8 in 2009. There was no indication of a change in indoor feeding times, and both An. gambiae s.l. and An. funestus s.l. continued to feed primarily after midnight with vectors being active until the early morning. Kdr L1014S frequencies were already high at baseline (53–85 %) but increased significantly in all sites over time.
Conclusions
The entomological surveys indicate that there was a reduction in transmission intensity coinciding with an increase in use of LLINs and other antimalarial interventions in areas of high malaria transmission. There was no change in feeding behaviour, and human-vector contact occurred indoors and primarily after midnight constantly throughout the study. Although the study was not designed to evaluate the effectiveness of the intervention compared to areas with no such intervention, the reduction in transmission occurred in an area with previously stable malaria, which seems to indicate a substantial contribution of the increased LLIN coverage.
Similar content being viewed by others
Background
Sub-Saharan Africa has seen an impressive scale up in coverage of malaria prevention tools and treatment, which contributed to an overall reduction in malaria morbidity and mortality in recent years [1, 2]. Uganda is among the countries with the highest burden for malaria, and 90 % of the 37.6 M population resides in high endemicity areas [2]. The main vectors in Uganda are Anopheles gambiae s.s., An. arabiensis, and An. funestus s.s; although relative species composition varies considerably between areas. The country is scaling up a number of interventions aimed to reduce malaria prevalence and improve case management. Universal coverage (i.e., one net for every two people as defined in the context of Uganda) with long-lasting insecticidal nets (LLINs), and indoor residual spraying (IRS) in selected districts, are the main vector control interventions being implemented.
Insecticide treated nets reduce vector contact and mosquito life span, leading to reductions in both vector density and infectivity and thus transmission intensity [3], and ultimately malaria morbidity and mortality [4]. One of the methods to measure malaria transmission intensity and any changes as a result of interventions is through the entomological inoculation rate (EIR), which is the number of infective bites a person receives over a time period. Besides modifying transmission intensity, large-scale LLIN use may impact anopheline biting time and location which could undermine control efforts [5]. A number of studies reported behavioural changes as a result of insecticide treated net (ITN) introduction to early evening and morning feeding when people are not protected by nets [6–8]. In addition, changes to more outdoor feeding were observed in some studies [9, 8].
The extensive use of LLINs, coupled with agricultural use of pesticides, has led to resistance of mosquito vectors to pyrethroid insecticides in sub-Saharan Africa [10], although the impact of resistance on LLIN effectiveness is not fully understood [11]. Phenotypic resistance to pyrethroid insecticides is widespread in An. gambiae s.s. from Uganda while for An. arabiensis varying levels of resistance are observed [12–14]. The knock-down resistance (kdr) L1014S mutation has been detected in An. gambiae s.s. from Uganda from 2001 onwards [14], sometimes at high frequencies [13]. In contrast, only low kdr L1014S frequencies (<1 %) were detected in An. arabiensis [13]. The kdr L1014F mutation was rare in An. gambiae s.s and absent in An. arabiensis collected from Uganda [12, 13]. Besides kdr, evidence of metabolic resistance mechanisms were observed in An. gambiae s.s. [13, 14]. Additionally, pyrethroid resistance was observed in An. funestus s.s., mediated by metabolic resistance mechanisms [15].
The entomological studies performed here were part of the Pioneer project, which was implemented in five districts in Midwestern Uganda from April 2009 to May 2014. Prevalence of malaria in children under five in the area was between 42.7-50.7 % in the Malaria Indicator Survey of 2009 [16]. The project’s focus was on systemic malaria control working through existing structures, and strived to increase both supply and demand for quality malaria prevention, diagnostics and treatment tools [17]. As part of this project, a LLIN universal coverage campaign (UCC) using Olyset® nets, treated with permethrin [18] was conducted between December 2009 and March 2010 in all districts with the exception of Kibaale, where the campaign was only conducted in three sub-counties in August 2010 due to a shortage of nets. Household ownership of at least one ITN was 79 % approximately 1.5 years following distribution, compared with 22 % prior to the campaign; while the proportion of children under five that slept under an ITN increased from 13.7 to 59.6 % (Malaria Consortium, unpublished data). Besides the LLIN campaign, the project implemented a range of behaviour change communication activities to increase awareness and promote health care seeking behaviour. Furthermore, the area benefitted from increased availability of artemisinin-based combination therapies through the integrated community case management program, and improved malaria diagnostics at facilities through the introduction of rapid diagnostic tests and strengthening of malaria microscopy [19].
The present study aimed to gather data on entomological variables following the implementation of LLINs and other interventions to monitor changes in vector density, biting rates and infection rates, and to determine whether vector behaviour was in line with the intended use of the interventions. In addition, trends of kdr resistance genotype levels were studied following the LLIN distribution. The study was not designed to evaluate effectiveness of the interventions compared to absence of such interventions, so the entomological monitoring was restricted to the LLIN campaign target areas.
Methods
Study sites
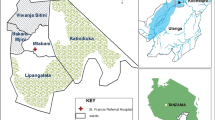
The entomological surveillance was carried out in one site each in the districts of Buliisa (N1°49' 7.78" E31°19' 29.71"), Hoima (N1° 25' 22.08" E31° 18' 24.12"), Kyankwanzi (N1° 7' 29.28" E31° 35' 53.88") and Kibaale (N1° 3' 54.72" E30° 41' 44.52") located in Midwestern Uganda (Figure 1). The elevation ranged from 621 m in Buliisa to 1281 m in Kibaale. Mosquito collections were done in the same sites for the duration of the study period except in Kibaale where the study site was changed from Rubirizi village to Muhorro (N0° 55' 0.21" E30° 45' 28.48") from the third round (May 2010) onwards as the former site did not benefit from the UCC. All four study sites experience two rainy seasons in the months of March-May, and September-November. In Kibaale district, the August 2012 collection was not performed due to an Ebola outbreak in this district resulting in restrictions on movement.
Mosquito collections & processing
Mosquito collections started in November 2009 (baseline, round 1) prior to the distribution of nets and continued until February 2013 (round 14). Mosquitoes were collected using two methods: CDC light trap collection (LTC) and human landing collection (HLC) using mouth aspirators. In each of the four sites, collections were made in six households; three houses were used for HLC while the other three were used for LTC. Houses selected represented typical constructions in the area, and the majority of houses had mud walls, sand floors, and iron roofing.
In each study site light traps were deployed quarterly in February, May, August, and November for six consecutive nights. Light traps were installed at the head end of the bed, and occupants were covered by an untreated net. Light traps were installed in bedrooms at 18:00 h and removed at 06:00 h the next morning. HLC were performed every six months (May and November) for six nights. At each house, indoor collections were done from 18:00 to 06:00 h and outdoor collections from 18:00 to midnight. Collectors worked in teams of three per house, and each collector worked a shift of three hours, to a maximum of two shifts per collector per night. Prophylaxis (doxycycline) was made available to collectors assigned to HLC collections.
Culicine females were counted and discarded. All anopheline mosquitoes were separated by sex and females identified morphologically in the field and classified into the An. gambiae complex, the An. funestus complex, and “other anophelines”. Specimens were packed individually in capsule tubes and kept in plastic bags with silica gel for further processing. Most samples from the collection in November 2010 could not be analysed due to loss of the specimens.
Ethical approval
Ethical clearance for this activity was obtained from the Uganda National Council for Science and Technology. Written informed consent was obtained from household owners while oral informed consent was obtained from collectors assigned to HLC collections.
Laboratory testing
Sporozoite assays
The majority of specimens from HLC (i.e., 82 %) were analysed to detect Plasmodium falciparum circumsporozoite protein using the sandwich enzyme-linked immunosorbent assay (ELISA) following set procedures [20]. The monoclonal antibody (MAb-Pf) and positive control were obtained from the Centers for Disease Control and Prevention (CDC) in Atlanta, USA. For each specimen, only the head and thorax were tested. Results were read visually against the positive control. Analyses were carried out at the Vector Control Division (VCD) laboratory, Ministry of Health, in Kampala.
Molecular analyses
Molecular analyses on a subset of samples were performed. Samples were analysed for species identification for the An. gambiae s.l. or An. funestus s.l. complex based on original field identification. An attempt was made to obtain representative subsamples from the different sites, rounds and collection methods for An. gambiae s.l. specimens. To this end, for collections containing less than 500 samples, up to 20 samples were randomly selected; while for collections containing more than 500 samples, on average 5 % of samples were randomly taken. A random subset of An. gambiae s.s and An. arabiensis were further analysed for the kdr L1014S mutation; samples selected were representative for the different sites and collection years. A limited number of An. gambiae s.s and An. arabiensis were also analysed for the kdr L1014F mutation. Genomic DNA was extracted from body parts (leg or wings) using the Chelex method [21] and was stored at −20 °C until use. Real time polymerase chain reaction (PCR) TaqMan assays were used to distinguish between the two sibling species An. gambiae s.s and An. arabiensis [22] and to detect kdr mutations [23]. Genotyping results were analysed using MXPro software (Agilent technologies, Stratagene, USA). Some samples could not be reliably classified and were removed from the analyses. A conventional PCR was used to distinguish members of the An. funestus complex [24, 25], and samples were randomly selected from different sites and rounds. Analyses were done at PAMVERC Laboratory in Moshi, Tanzania.
Data analyses
All data were entered and validated in Epidata 3.1 (EpiData Association, Denmark). Data were analysed in STATA version 12 (Statacorps, USA).
LTC data was averaged over the number of collection nights and houses in each site to arrive at a mean anopheline density per house per night per survey. A negative binomial regression was used to compare the number of An. gambiae s.l. (all sites) and An. funestus s.l. (Buliisa site only due to low numbers collected in other sites) collected by light traps before and after the UCC for each site. Comparisons were made for the same collection month because of seasonality observed. In all sites but Kibaale, comparisons were made for the November months of 2009 and 2010. In Kibaale, the comparison was made for the month of May in 2010 and 2011 as the first two collections were done in a different site and the nets were only distributed in August of 2010. We used a negative binomial regression because it is robust to the presence of a large number of zero counts in light trap collections [26, 27].
Human biting rates (i.e., the number of bites per person per night) were calculated for each site and each survey taking into account the number of collectors working simultaneously, the number of collection nights, and the assumed night time behaviour of the local populations. It was assumed that an average villager in each of the sites spends 1 h on average outdoors between 18:00 h and 22:00 h, and all villagers are indoors after 22:00 h [28]. Sporozoite rates were calculated by dividing the number of positive mosquitoes over the total number tested. EIR figures were calculated by multiplying human biting rates with sporozoite rates.
Wilcoxon signed-rank tests were used to compare the number of anophelines collected by indoor or outdoor collection during the period of 18:00–00:00 h. For Kyankwanzi, Hoima and Kibaale samples, comparisons were made for An. gambiae s.l. only due to the low number of An. funestus s.l. observed, while in Buliisa comparisons were made separately for each species. Kdr L1014S genotype frequencies over survey years for An. gambiae s.s. were compared with Pearson chi-square tests by site.
Results
Species composition
A total number of 18,437 anopheline females and 35,133 culicine females were collected during the course of the study. The predominant malaria vector overall was An. gambiae s.l., (91 %) while An. funestus s.l. constituted 8 % of the collections (Table 1). A small number of other anophelines (1 % of the total collections) were observed but these were not identified further.
The prevailing malaria vector in each site except Buliisa was An. gambiae s.l (> 97 %), with An. funestus s.l. being present in low numbers only. In Buliisa, An. funestus s.l. made up 46 % of the collections. There was substantial variation in vector numbers among the four sites: 71 % of the An. gambiae s.l. mosquitoes were collected from the Kyankwanzi site, whereas similar numbers were observed at the other three sites.
The four collection sites represented different species compositions for the An. gambiae complex. In Kyankwanzi, Hoima and Kibaale An. gambiae s.s. was almost exclusively found, and only a small number of An. arabiensis was observed, mainly from outdoor collections (0-4 %; Table 2). An. arabiensis was more common in Buliisa, where between 36-59 % of analysed mosquitoes were An. arabiensis.
The An. funestus complex was mainly analysed from Buliisa, as in other sites few specimens were collected. An. funestus s.s. was most common and 96 % of samples in Buliisa belonged to this species (N = 161). A small number of other sibling species were observed; i.e., An. leesoni (4 specimens), An. rivulorum (1 specimen), and An. parensis (1 specimen). The majority of these were observed in the outdoor HLC collection. In Hoima all samples tested were An. funestus s.s. and came from LTC and indoor HLC (N = 18), while in Kyankwanzi the only sample positively identified was also An. funestus s.s.
Light trap collections
The average number of An. gambiae s.l. collected per house per trapping night was significantly lower compared to baseline densities in the first year after the UCC in Hoima (z = −2.70; p = 0.007), Kyankwanzi (z = −4.32; p < 0.001) and Buliisa (z = −3.88; P < 0.001; Fig. 2) when data for the month of November was compared. An. funestus s.l. densities in Buliisa declined the year after the UCC (z = −3.69; p < 0.001), yet numbers increased again in 2012. In Kibaale, vector densities remained similar throughout the collection period yet in general few anophelines were trapped. Additionally, the first two surveys were conducted in a site different from the one used in later surveys, which may explain the low densities observed during the initial two rounds.
The average number (± SEM) of An. gambiae s.l. (white bars) and An. funestus s.l. (grey bars; only for Buliisa) per house per night collected by light traps. In Kibaale the first two collections were done in a different site than all subsequent rounds. No collections were done in Kibaale in Aug 12 due to an Ebola outbreak. Lines indicate when nets were introduced in the entire district, with the exception of Kibaale where nets were only distributed in three sub-counties
A marked seasonality was observed in Kyankwanzi with highest numbers of vectors collected in November and May. Vector densities in Hoima were sustained at a low level throughout 2010 and 2011 but vector density increased in 2012. In Kyankwanzi, a similar pattern was observed and densities in May of 2011 and 2012 were higher than during the baseline collection in 2009. An. gambiae s.s. was the dominant vector collected by light trap in all sites, except in Buliisa where An. arabiensis was more common (i.e., 39 %; Table 2). Vector densities in Buliisa showed an interesting pattern with An. gambiae s.l. being more prevalent than An. funestus s.l. in 2011, while the opposite trend was observed in 2012 (Fig. 2).
Human landing collections
The human biting rate (HBR), expressed as the number of bites per person per night, was highest in Kyankwanzi in November of 2009 for An. gambiae s.l. with 197.1 bites per person per night (Table 3). The HBR fell sharply in Kyankwanzi to 7.5 and 2.9 bites per person per night in May and November of 2010, respectively. In subsequent years the HBR increased, although values did not reach the baseline figure. A similar trend was observed in Hoima, although the HBR was much lower overall compared to Kyankwanzi. In Kibaale, the HBR decreased in November of 2010 following the UCC in August of 2010, but was back at similar levels in November of 2012. In Buliisa, the HBR for An. gambiae s.l. showed an increase in May 2011, similarly to the increase seen in the density collected by light trap (Fig. 2). An. funestus s.l. HBRs fell from 10.2 bites in 2009 to 1.2 and 0.1 bites in May and November of 2010, respectively, but in 2012 the HBR was similar to baseline levels (Table 3).
The sporozoite rates in An. gambiae s.l. varied between survey years and sites and were between 0.0-9.1 %, and a significant reduction over time was observed in Kyankwanzi and Buliisa (Table 4). Of the 112 An. gambiae s.l. samples positive for sporozoites, 111 were identified as An. gambiae s.s., while one was An. arabiensis. The percentage of infected An. funestus s.l. in Buliisa was 2.0 % in 2009 while in subsequent years no infected individuals were observed, yet this change was not significant. When data were combined by species and study site, a significant reduction in sporozoite rates was observed during the duration of the study (χ 2 = 17.42, p = 0.001), and sporozoite rates were 3.2 %, 3.0 %, 2.0 %, 1.8 % in 2009, 2010, 2011 and 2012, respectively.
The entomological inoculation rate (EIR), i.e., the number of infective bites per person per night, was used to measure the intensity of malaria transmission in the four study sites (Fig. 3). A decrease in the EIR was observed in all sites compared with baseline figures for An. gambiae s.l., with the exception of the Kibaale site. This reduction was most pronounced in the year following the UCC (Table 3). In Kibaale, the 2009 EIR was collected in a different site compared to following years, and the EIR in general was low in this site. In Buliisa, the EIR for An. funestus s.l. decreased after 2009.
Feeding times and location
The large majority of human-vector contact (> 80 % for almost all collections) took place indoors for all the years of the study (Table 3). Host-seeking behaviour patterns for An. gambiae s.l. showed that feeding took place throughout the night with the majority of bites occurring after midnight for most sites and years (Fig. 4). Feeding continued until the early morning hours of 04:00–06:00 h. An. funestus s.l. feeding patterns in Buliisa were similar and the majority of bites took place after midnight and activity remained high until the early morning hours, with the exception of 2011 where the peak feeding activity was between 22:00 and midnight.
The An. gambiae s.l. sample was dominated by An. gambiae s.s. in all sites except Buliisa where An. arabiensis was more prevalent and dominated outdoor collections (i.e., 59 %; Table 2). Indoors, this species was observed to be active throughout the night with a peak feeding time between 00:00–01:00 when 27 % of all bites were observed, while in outdoor collections the majority of feeding (29 %) occurred between 22:00–23:00 h.
Significantly more An. gambiae s.l. in Hoima (Z = 3.14; p = 0.002, n = 81) and Kyankwanzi (Z = 4.69; p < 0.001, n = 94) were caught indoors between the hours of six to midnight than outdoors over all collection rounds. In Kibaale, equal numbers were caught in both locations (Z = −1.01; p = 0.313, n = 49), while in Buliisa significantly more An. funestus s.l. were caught indoors during those hours (Z = 3.39; p = 0.001, n = 34). Similar numbers of An. gambiae s.l. in Buliisa were observed in indoor and outdoor HLC when considering the first half of the night (Z = 0.49; p = 0.627, n = 69).
Insecticide resistance markers
A subset of An. gambiae s.l. specimens (N = 1086) were screened for the kdr L1014S mutation. In 2009, kdr L1014S frequencies were high in most sites in An. gambiae s.s. and varied between 70.8-85.4 % (Table 5). In Kibaale a lower frequency of 52.7 % was observed. In all sites, kdr L1014S frequencies increased significantly over the years and approached fixation in all sites in 2012 with the exception of Buliisa where the frequency was 86.0 % in 2012 down from 96.2 % in 2011. The kdr L1014S mutation was not observed in An. arabiensis with the exception of one heterozygous individual in Kyankwanzi in 2012. A much smaller number of samples were subsequently screened for the kdr L1014F mutation (47 for An. gambiae s.s. and 19 for An. arabiensis across study sites and years). Some kdr L1014F homozygous and heterozygous individuals were observed, and for all sites and years combined kdr L1014F genotype frequencies were 24.5 % for An. gambiae s.s. and 31.6 % for An. arabiensis.
Discussion
The entomological surveys indicate that there was a reduction in malaria transmission intensity, measured by the EIR, in four high burden districts in Midwestern Uganda coinciding with a large scale increase in both coverage and use of LLINs and other antimalarial interventions. This decline was the result of both a decreased vector density and reduced infectivity of the vectors. The study was not designed to evaluate the effectiveness of the intervention in comparison with absence of such intervention. As no similar entomological data were collected in other areas, the potential contributions of other factors such as temporal variations in climate could not be assessed.
The decline in the EIR was most pronounced in the year following the LLIN UCC. As the area had high perennial malaria transmission, the campaign appears to have contributed to the reduction. Reductions in the EIR following deployment of vector control interventions were observed in a number of other settings [3, 29]. In subsequent years, an increase was observed in mosquito densities and the EIR, although values remained below the baseline figures. Ownership and use rates of nets in the project area declined due to a loss of nets as there was no additional campaign and only a limited number of LLINs were available through antenatal clinics. At the end of the project (approximately three years after the UCC), 64 % of households owned at least one ITN, and only 38 % of residents had slept under an ITN the previous night (Malaria Consortium, unpublished data). The decline in LLIN ownership due to attrition and deterioration of the nets in the years following the campaign may have contributed to the increased densities of vectors observed in the area. This highlights the importance of maintaining high levels of LLIN ownership and use following campaigns. Coverage with LLINs has recently been restored, as districts received nets as part of Uganda’s national universal coverage campaign in 2013.
The main vector species observed in all sites was An. gambiae s.l. Within this species complex, An. gambiae s.s. was identified as the dominant species in Kibaale, Hoima and Kyankwanzi, with An. arabiensis only observed at low frequencies in the latter two sites. In contrast, in Buliisa An. arabiensis was common, and in outdoor human landing catch collections this vector dominated, in line with the more exophagic nature of this species [30]. An. funestus s.l. was also primarily observed in Buliisa, with low numbers seen in Hoima and Kyankwanzi. An. funestus s.s. was the main species observed in the complex, but a small number of other species were also identified, i.e., An. leesoni, An. parensis and An. rivulorum. These species have all been previously identified from Uganda [31–34]. The Buliisa site was characterized by a complex species composition, and An. arabiensis, An. gambiae s.s., and An. funestus s.s. were all observed. This site is situated on the shores of Lake Albert, and suitable larval habitats are found along the fringes of the lake, as well as inland for more temporary habitats. The relative species composition in this site varied across the study years, potentially due to the availability of suitable larval habitats and other factors. Vector densities were much higher in Kyankwanzi compared to the other study sites. Reasons for these high densities are not clear.
Vector densities declined in the light trap and human landing catch collections in the year following the UCC. Densities largely recovered in subsequent years, with seasonal peaks in the months of May and November following the rains, similarly to findings by Kilama et al. [35]. Although two different collection methods were used, the non-rotation of study houses between these methods did not allow for a direct comparison of trap efficiency. The daily EIR varied between 0–5.8 in 2009 to 0–0.98 in 2012 in this study. Prior to any scale up of interventions, the annual EIR in Uganda was determined in seven sites in 2001–2002 and varied between 4–1,568 infective bites per person with the highest EIR recorded in the Northern part of the country [36]. In a more recent study performed in 2011–2012, the estimated annual EIR varied between 4–125 depending on study site, and strong seasonal variation was observed [35]. Annual EIRs were not calculated here due to a lack of monthly data and the large seasonal fluctuations observed.
Extensive net use can result in behavioural changes of the vectors to earlier or outdoor feeding [6–9]. Before the scale up of LLINs in Uganda, An. gambiae s.l. was observed to mainly feed indoors after 11 pm in the majority of study locations [36]. Here, we did not observe any shifts in feeding behaviour to earlier times following the large scale introduction of LLINs. Throughout the study period, vectors were most active from midnight till 6 am when indoor collections ceased. The early morning feeding behaviour (i.e., 4–6 am) of both An. gambiae s.l. and An. funestus s.l. in most study sites was striking. In another recent study from the eastern region in Uganda, both An. funestus and An. gambiae were similarly active throughout the night until 6 am, although feeding rates remained more constant after midnight [37]. Further work is required to understand if feeding continues throughout the morning, as was recently demonstrated in An. funestus s.s. from Senegal [6]. Outdoor collections in this study were performed until midnight only. Although villagers are likely to be indoors after midnight and any outdoor biting preference after that time would have little relevance for malaria transmission, outdoor biting behaviour before dawn when inhabitants might be exposed due to early rising should be investigated further by including whole night biting collections outdoors.
The impact of LLINs on pyrethroid resistance was investigated by assessing kdr frequencies over time. The kdr L1014S mutation was observed at high frequencies in An. gambiae s.s. prior to the universal distribution of LLINs. The project areas had received ITNs as part of small targeted campaigns prior to 2010; additionally exposure to agricultural pesticides could have primed vectors [10]. Kdr L1014S frequencies increased in all sites and reached fixation in three sites, while in Buliisa frequencies increased to 96.2 % in 2011 but reduced to 86.0 % in 2012. Similar high kdr L1014S frequencies in An. gambiae s.l. were observed in another study from Uganda in 2011 [13]. Low kdr L1014S frequencies were observed in An. arabiensis (0–3.1 %), similarly to results from another study in Uganda [13]. The kdr L1014F mutation has not been observed at high frequencies in Uganda [13, 14, 12]. In this study, only a limited number of samples were screened for the kdr L1014F mutation. Some homozygous resistant An. gambiae s.s. and An. arabiensis were observed, but sample sizes were too small to deduce reliable estimates of frequencies per study site or assess trends over the years, and further studies are required.
No phenotypic resistance assays were undertaken as part of this study which would have allowed for a better understanding of the impact of kdr on resistance, but data collected in 2013 from the Kyankwanzi site showed phenotypic resistance to deltamethrin, permethrin, and DDT in An. gambiae s.s. in WHO tube assays (Malaria Consortium, unpublished data). Metabolic resistance mechanisms were not studied here, yet these could have also mediated pyrethroid resistance in these populations [14, 13].
Limitations of the study
The aim of this study was to gather data on entomological variables to monitor changes in vector densities, behaviour and transmission intensity following the implementation of LLINs and other interventions. The study was not designed to investigate the effectiveness of these interventions in relation to a control situation where these interventions were not used. Therefore, the study is unable to assess the potential contributions of other factors that might affect transmission. Another limitation of the study was the lack of phenotypic resistance data in three of the four sites, as well as a more thorough assessment of other resistance mechanisms such as metabolic resistance.
Conclusions
In conclusion, the entomological monitoring showed that malaria transmission intensity declined in the year following a large scale increase in coverage and use of LLINs and other antimalarial interventions in a high transmission area in Midwestern Uganda, but thereafter transmission started to increase again, although not to levels observed prior to the interventions. The increase could be due to decreased ownership and use of LLINs among others factors including vector resistance, temporal variations in climatic conditions, or a combination of these and other factors. The study showed that the distribution of LLINs did not result in changes in biting behaviour of the main vectors. Entomological surveillance of vector populations informs intervention performance and should be incorporated in routine monitoring of interventions.
References
Murray CJ, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, Haring D, et al. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet. 2012;379(9814):413–31. doi:10.1016/S0140-6736(12)60034-8.
WHO. Global Malaria Program. World Malaria Report. 2014. http://www.who.int/malaria/publications/world_malaria_report_2014/en/. Accessed 10.02 2015.
Shaukat AM, Breman JG, McKenzie FE. Using the entomological inoculation rate to assess the impact of vector control on malaria parasite transmission and elimination. Malar J. 2010;9:122. doi:10.1186/1475-2875-9-122.
Lengeler C. Insecticide-treated bed nets and curtains for preventing malaria. Cochrane Database Syst Rev. 2004;2, CD000363. doi:10.1002/14651858.CD000363.pub2.
Russell TL, Beebe NW, Cooper RD, Lobo NF, Burkot TR. Successful malaria elimination strategies require interventions that target changing vector behaviours. Malar J. 2013;12:56. doi:10.1186/1475-2875-12-56.
Sougoufara S, Diedhiou SM, Doucoure S, Diagne N, Sembene PM, Harry M, et al. Biting by Anopheles funestus in broad daylight after use of long-lasting insecticidal nets: a new challenge to malaria elimination. Malar J. 2014;13:125. doi:10.1186/1475-2875-13-125.
Braimaha N, Drakeley C, Kweka E, Mosha A, Helinski ME, Pates H, et al. Tests of bednet traps (Mbita traps) for monitoring mosquito populations and time of biting in Tanzania and possible impact of prolonged insecticide treated net use. Int J Trop Insect Sci. 2005;25(3):208–13.
Moiroux N, Gomez MB, Pennetier C, Elanga E, Djenontin A, Chandre F, et al. Changes in Anopheles funestus biting behavior following universal coverage of long-lasting insecticidal nets in Benin. J Infect Dis. 2012;206(10):1622–9. doi:10.1093/infdis/jis565.
Russell TL, Govella NJ, Azizi S, Drakeley CJ, Kachur SP, Killeen GF. Increased proportions of outdoor feeding among residual malaria vector populations following increased use of insecticide-treated nets in rural Tanzania. Malar J. 2011;10:80. doi:10.1186/1475-2875-10-80.
Ranson H, N'Guessan R, Lines J, Moiroux N, Nkuni Z, Corbel V. Pyrethroid resistance in African anopheline mosquitoes: what are the implications for malaria control? Trends Parasitol. 2011;27(2):91–8. doi:10.1016/j.pt.2010.08.004.
Strode C, Donegan S, Garner P, Enayati AA, Hemingway J. The impact of pyrethroid resistance on the efficacy of insecticide-treated bed nets against African anopheline mosquitoes: systematic review and meta-analysis. PLoS Med. 2014;11(3):e1001619. doi:10.1371/journal.pmed.1001619.
Ramphul U, Boase T, Bass C, Okedi LM, Donnelly MJ, Muller P. Insecticide resistance and its association with target-site mutations in natural populations of Anopheles gambiae from eastern Uganda. Trans R Soc Trop Med Hyg. 2009;103(11):1121–6. doi:10.1016/j.trstmh.2009.02.014.
Mawejje HD, Wilding CS, Rippon EJ, Hughes A, Weetman D, Donnelly MJ. Insecticide resistance monitoring of field-collected Anopheles gambiae s.l. populations from Jinja, eastern Uganda, identifies high levels of pyrethroid resistance. Med Vet Entomol. 2013;27(3):276–83. doi:10.1111/j.1365-2915.2012.01055.x.
Verhaeghen K, Bortel WV, Roelants P, Okello PE, Talisuna A, Coosemans M. Spatio-temporal patterns in kdr frequency in permethrin and DDT resistant Anopheles gambiae s.s. from Uganda. Am J Trop Med Hyg. 2010;82(4):566–73. doi:10.4269/ajtmh.2010.08-0668.
Morgan JC, Irving H, Okedi LM, Steven A, Wondji CS. Pyrethroid resistance in an Anopheles funestus population from Uganda. PLoS One. 2010;5(7):e11872. doi:10.1371/journal.pone.0011872.
Uganda Bureau of Statistics (UBOS) and ICF Macro. 2010. Uganda Malaria Indicator Survey 2009. http://dhsprogram.com/pubs/pdf/MIS6/MIS6.pdf. Accessed 07.09.2015.
MalariaConsortium. Pioneer project. 2015. http://www.malariaconsortium.org/pioneer/. Accessed 10.02 2015.
Tami A, Mubyazi G, Talbert A, Mshinda H, Duchon S, Lengeler C. Evaluation of Olyset insecticide-treated nets distributed seven years previously in Tanzania. Malar J. 2004;3:19. doi:10.1186/1475-2875-3-19.
MalariaConsortium. Pioneer project, a holistic systems strenghtening approach towards malaria control in mid-western Uganda-successes and lessons. 2014. http://www.malariaconsortium.org/pioneer/downloads/0544-pioneer-project-2009-2014%2D-a-holistic-systems-strengthening-approach-towards-malaria-control-in-mid-western-uganda.pdf. Accessed 10.02 2015.
Wirtz R, Avery M, Benedict M. 3.3 Plasmodium Sporozoite ELISA. In: Specific Anopheles Techniques. Malaria Research and Reference Reagent Resource Center. MR4. 2007. https://www.mr4.org/Portals/3/MR4_Publications/Methods%20in%20Anopheles%20Research%202014/2014MethodsinAnophelesResearchManualFullVersionv2tso.pdf. accessed 07.09.2015.
Grimberg J. A simple and efficient non-organic procedure for the isolation of genomic DNA from blood. Nucleic Acids Res. 1989;17:8390.
Bass C, Williamson MS, Field LM. Development of a multiplex real-time PCR assay for identification of members of the Anopheles gambiae species complex. Acta Trop. 2008;107(1):50–3. doi:10.1016/j.actatropica.2008.04.009.
Bass C, Nikou D, Donnelly MJ, Williamson MS, Ranson H, Ball A, et al. Detection of knockdown resistance (kdr) mutations in Anopheles gambiae: a comparison of two new high-throughput assays with existing methods. Malar J. 2007;6:111. doi:10.1186/1475-2875-6-111.
Cohuet A, Simard F, Toto JC, Kengne P, Coetzee M, Fontenille D. Species identification within the Anopheles funestus group of malaria vectors in Cameroon and evidence for a new species. Am J Trop Med Hyg. 2003;69(2):200–5.
Koekemoer LL, Kamau L, Hunt RH, Coetzee M. A cocktail polymerase chain reaction assay to identify members of the Anopheles funestus (Diptera: Culicidae) group. Am J Trop Med Hyg. 2002;66(6):804–11.
Mathenge EM, Omweri GO, Irungu LW, Ndegwa PN, Walczak E, Smith TA, et al. Comparative field evaluation of the Mbita trap, the Centers for Disease Control light trap, and the human landing catch for sampling of malaria vectors in western Kenya. Am J Trop Med Hyg. 2004;70(1):33–7.
Fornadel CM, Norris LC, Norris DE. Centers for Disease Control light traps for monitoring Anopheles arabiensis human biting rates in an area with low vector density and high insecticide-treated bed net use. Am J Trop Med Hyg. 2010;83(4):838–42. doi:10.4269/ajtmh.2010.10-0088.
WHO. Malaria entomology and vector control – Guide for participants. 2013. http://apps.who.int/iris/bitstream/10665/85890/1/9789241505819_eng.pdf. Accessed 07.09.2010
Ouattara AF, Dagnogo M, Constant EA, Kone M, Raso G, Tanner M, et al. Transmission of malaria in relation to distribution and coverage of long-lasting insecticidal nets in central Cote d'Ivoire. Malar J. 2014;13:109. doi:10.1186/1475-2875-13-109.
White G. The Anopheles gambiae complex and malaria transmission around Kisumu, Kenya. Trans R Soc Trop Med Hyg. 1972;66:572–81.
Mulamba C, Irving H, Riveron JM, Mukwaya LG, Birungi J, Wondji CS. Contrasting Plasmodium infection rates and insecticide susceptibility profiles between the sympatric sibling species Anopheles parensis and Anopheles funestus s.s: a potential challenge for malaria vector control in Uganda. Parasit Vectors. 2014;7:71. doi:10.1186/1756-3305-7-71.
Echodu R, Okello-Onen J, Lutwama J, Enyaru J, Ocan R, Asaba R, et al. Heterogeneity of Anopheles Mosquitoes in Nyabushozi County, Kiruhura district, Uganda. Journal of Parasitology and Vector Biology. 2010;2(3):28–34.
Mutebi JP, Crabtree MB, Kading RC, Powers AM, Lutwama JJ, Miller BR. Mosquitoes of western Uganda. J Med Entomol. 2012;49(6):1289–306.
Gillies M, Coetzee M. A supplement to the Anophelinae of Africa south of the Sahara (Afrotropical region). Johannesburg: The South African Institute for Medical Research; 1987.
Kilama M, Smith DL, Hutchinson R, Kigozi R, Yeka A, Lavoy G, et al. Estimating the annual entomological inoculation rate for Plasmodium falciparum transmitted by Anopheles gambiae s.l. using three sampling methods in three sites in Uganda. Malar J. 2014;13(1):111. doi:10.1186/1475-2875-13-111.
Okello PE, Van Bortel W, Byaruhanga AM, Correwyn A, Roelants P, Talisuna A, et al. Variation in malaria transmission intensity in seven sites throughout Uganda. Am J Trop Med Hyg. 2006;75(2):219–25.
Kabbale FG, Akol AM, Kaddu JB, Onapa AW. Biting patterns and seasonality of Anopheles gambiae sensu lato and Anopheles funestus mosquitoes in Kamuli District. Uganda Parasit Vectors. 2013;6:340. doi:10.1186/1756-3305-6-340.
Acknowledgement
The entomological collections were led by the vector control officers Godfrey Kaija, Charles Ntege, Morris Okumu and Thomson Isingoma and were supervised by David Odong Salandini and Nicholas Vuciri. Data entry and cleaning was supervised by Charles Kapuru, John-Baptist Bwanika and Andrew Anguko. We would like to thank the entomology assistants in all the study sites, the Vector Control Division in Kampala (Ministry of Health of Uganda) for providing the laboratory facilities and staff for ELISA analyses, and Alex Wright and Enock Kessy from PAMVERC Tanzania for the molecular analyses of samples. The authors would like to acknowledge the supervisory support of Robin Altaras at the start of the project and the data analyses support provided by Geoffrey Namara. We are grateful to the District Health Management Teams of Buliisa, Hoima, Kibaale, Kiboga and Kyankwanzi Districts for facilitating the studies. A special thanks to all residents of the study households and all community residents in the study sites for their cooperation and support. The funding for this study was provided by Comic Relief, UK as part of the Pioneer Project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
MH provided technical oversight, performed the data analyses and wrote the manuscript. AN provided technical and managerial oversight and contributed to the writing of the manuscript. NP designed the study, provided technical oversight and contributed to the writing of the manuscript. MF designed the study and provided technical and managerial oversight. SM provided technical and managerial oversight. PO participated in field collections and morphological identifications and performed the ELISAs. DO performed the ELISAs. TA provided technical oversight and contributed to data analyses and the writing of the manuscript. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Helinski, M., Nuwa, A., Protopopoff, N. et al. Entomological surveillance following a long-lasting insecticidal net universal coverage campaign in Midwestern Uganda. Parasites Vectors 8, 458 (2015). https://doi.org/10.1186/s13071-015-1060-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-015-1060-6