Abstract
Background
Ritonavir was recently combined with nirmatrelvir in a new approved co-packaged medication form for the treatment of COVID-19. Quantitative analysis based on fluorescence spectroscopy measurement was extensively used for sensitive determination of compounds exhibited unique fluorescence features.
Objective
The main objective of this work was to develop higher sensitive cost effective spectrofluorometric method for selective determination of ritonavir in the presence of nirmatrelvir in pure form, pharmaceutical tablet as well as in spiked human plasma.
Methods
Ritonavir was found to exhibit unique native emission fluorescence at 404 nm when excited at 326 nm. On the other hand, nirmatrelvir had no emission bands when excited at 326 nm. This feature allowed selective determination of ritonavir without any interference from nirmatrelvir. The variables affecting fluorescence intensity of ritonavir were optimized in terms of sensitivity parameters and principles of green analytical chemistry. Ethanol was used a green solvent which provided efficient fluorescence intensity of the cited drug.
Results
The method was validated in accordance with the ICH Q2 (R1) standards in terms of linearity, limit of detection (LOD), limit of quantification (LOQ), accuracy, precision and specificity. The described method was successfully applied for ritonavir assay over the concentration range of 2.0–20.0 ng/mL.
Conclusion
Ritonavir determination in the spiked human plasma was successfully done with satisfactory accepted results.
Similar content being viewed by others
Introduction
Fluorescence spectroscopy provides a more sensitive analytical tool for the determination of many compounds, especially those that have a unique fluorescent nature. The high sensitivity of fluorescence spectroscopy is the main advantage, as it allows the determination of compounds down to the Nano gram range [1,2,3]. In addition, the fluorescence spectroscopy instrument consumes less than 0.1 kWh of energy per sample and produces little waste, which draws attention to the principles of green chemistry [4].
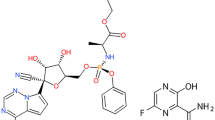
Ritonavir plus nirmatrelvir, Fig. 1, is a new FDA co-packaged drug developed for the treatment of COVID − 19. Ritonavir is a protease inhibitor and CYP3A inhibitor of human immunodeficiency virus type 1, and nirmatrelvir is a peptidomimetic inhibitor of the major protease of severe acute respiratory syndrome coronavirus 2. Ritonavir plus nirmatrelvir received the first conditional marketing authorization in the United Kingdom in December 2021 for the treatment of COVID − 19 in adults who do not require supplemental oxygen and are at high risk for progression to severe COVID − 19. Nirmatrelvir plus ritonavir was also approved in Europe and the United States for COVID-19 [5,6,7,8].
Previous reports have described various analytical methods for the determination of ritonavir in different matrices. HPLC method has been developed for the determination of ritonavir in human plasma [9] and pharmaceutical dosage forms [10]. Although the HPLC method was widely used for the determination of active pharmaceutical ingredients, it had several disadvantages, such as the high time required, the recommended sample pretreatment, and the possibility of determining active ingredients in the microgram range. Moreover, the direct UV spectrophotometric method [11] and mathematical manipulation of spectral bands including first-order derivative and area under the curve [12] were used for the determination of ritonavir in the pharmaceutical dosage form in the microgram range. Despite the simple approach and affordability of the spectrophotometric methods, they could not provide a feasible determination of ritonavir in the biological samples.
The main objective of this work was to develop a more sensitive spectrofluorometric method for the selective determination of ritonavir in the presence of nirmatrelvir in pure form, in pharmaceutical tablets, and in spiked human plasma. The method was based on the unique fluorescence properties of ritonavir, the compound of interest, and the absence of any fluorescence properties of nirmatrelvir, another compound packaged with ritonavir in a new pharmaceutical dosage form. This feature allowed direct selective determination of ritonavir without any interference from nirmatrelvir. The proposed method was efficiently used for the determination of ritonavir up to the Nano- gram range in the plasma matrix and pharmaceutical dosage forms. Moreover, the proposed method showed superiority over the previously reported spectrophotometric method in terms of sensitivity and quantification limits.
Experimental
Materials and chemicals
-
Pure reference standard of nirmatrelvir (99.36%) and ritonavir (99.62%) were kindly supplied by Pfizer, Inc, Egypt. The purity was checked by applying the reported method [13].
-
Paxlovid tablets, pink oval nirmatrelvir film-coated tablet (150 mg) co-packaged with white ritonavir film-coated tablet (100 mg), (B. NO: A1324, manufactured by Pfizer Company), were kindly supplied by Pfizer, Inc., Egypt. The recommended dose is two tablets of nirmatrelvir plus one tablet of ritonavir.
-
Acetonitrile, chloroform, ethanol and methanol (Sigma-Aldrich, Germany).
-
Tween 80, Sodium dodecyl sulphate “SDS”, El-Nasr Company, Egypt. Cetyl trimethyl ammonium bromide “CTAB” (Win lab, UK). β-cyclodextrin “β-CD”, Sigma-Aldrich, Germany.
-
Hydrochloric acid and Sodium hydroxide, El-Nasr Company, Egypt. 0.1 N aqueous solutions were prepared.
-
Boric acid, glacial acetic acid, potassium chloride acid and sodium acetate trihydrate, El-Nasr Company, Egypt.
-
Acetate buffer solutions in the pH range 4 to 6 and alkaline borate buffer in the pH range 8 to 10.
-
Drug-free human plasma of healthy volunteers was supplied by Blood Bank, Al-Azhar University Hospital, Damietta, Egypt.
Instrumentation
Spectrofluorometric apparatus, Jasco FP-6200, Tokyo, Japan.
Standard solutions
Prepared stock solutions of ritonavir and nirmatrelvir [100 µg/mL] were prepared by dissolving 10 mg of ritonavir or nirmatrelvir in 50 mL of ethanol and adding ethanol to a 100-mL volumetric flask. Definitive working solutions were prepared by dilution with ethanol.
Procedures
Calibration graph construction
Specific volumes of ritonavir stock solutions were added to a series of 10-mL volumetric flasks to produce a series of solutions with accurate concentrations ranging from 20.0to 20.0 ng/mL. The solutions were excited at 326 nm and the intensity of fluorescence emission was measured at 404 nm. The values of the evaluated emission intensity were plotted against the corresponding ritonavir concentrations and the regression equations were derived.
Laboratory prepared mixture analysis
Laboratory mixtures of ritonavir and nirmatrelvir were prepared in various ratios, assuming that the recommended dose consisted of two tablets of nirmatrelvir and one tablet of ritonavir, corresponding to a nirmatrelvir to ritonavir ratio of 3:1. The mixtures were tested using the procedures described, and the drug concentration was determined using the regression equation. In general, all procedures described were performed in accordance with the relevant guidelines.
Procedures for tablets
Two units of ritonavir tablets were scratched, weighed, and finely powdered. In a 100-mL volumetric flask, the drug powder equivalent to one tablet was dissolved in 30 mL ethanol and sonicated for 20 min. The volume was made up to 100 mL with ethanol to obtain a solution containing 1 mg/mL ritonavir. Finally, five concentrations of ritonavir were prepared and evaluated as previously described.
Procedure for spiked human plasma
Various spiked human plasma samples were prepared by placing aliquots of different concentrations of ritonavir together with 1 mL of human plasma and 3 mL of acetonitrile in a series of 10-mL centrifugation tubes. The tubes were shaken in a vortex mixer for 1 min and centrifuged for 30 min. The resulting supernatant was evaporated to dryness, and the residues were dissolved in a fixed volume of ethanol. In 10-mL volumetric flasks, 3 mL of acetate buffer pH 4 was added and made up with ethanol. Samples were evaluated as previously described.
Results and discussion
Many efforts have been made to improve the sensitivity of quantitative analysis of drugs. Spectrophotometry and spectrofluorometry are considered the most commonly used analytical techniques in pharmaceutical analysis because they are simple and inexpensive [14,15,16,17,18,19,20,21,22,23,24]. In addition, spectrofluorometry is a preferred technique for compounds with unique native fluorescent features in terms of higher sensitivity metrics and lower detection limits [2, 3].
In this work, a spectrofluorometric method for the efficient determination of ritonavir in the presence of nirmatrelvir in pure form, in pharmaceutical tablets, and in spiked human plasma was developed, adopted, and applied. The described method was based on the fluorescence properties of ritonavir, whereas the coexisting drug nirmatrelvir showed no fluorescence behavior. This property allowed selective determination of ritonavir without any interference from nirmatrelvir.
Fluorescence characteristics
Ritonavir showed a unique native emission spectrum at 404 nm when excited at 326 nm (see Fig. 2). (a) Nirmatrelvir, on the other hand, had no emission bands when excited at 326 nm (see Fig. 2). (b) The obtained emission bands of ritonavir allowed a more sensitive and selective approach for its determination in the pharmaceutical dosage form as well as in spiked human plasma without any interference from nirmatrelvir.
Factors affecting the fluorescence properties
Diluting solvent effect
The effect of different diluents such as water, acetonitrile, methanol, ethanol, chloroform, 0.1 N HCl, and 0.1 N NaOH on the fluorescence efficiency of ritonavir was tested. Complete quenching of fluorescence was observed when 0.1 N NaOH was used. Water did not result in quantitative feasibility of the cited drug. On the other hand, chloroform and ethanol were found to be the best dilution solvents as they exhibited higher fluorescence intensity with lower blank values. Since chloroform had undesirable environmental and health effects, ethanol was selected due to its environmental green superiority [25]. The effect of the different diluents is shown in Fig. 3.
Surfactant effect
The effect of adding surfactants including SDS, CTAB, Tween 80 and β-CD on the ritonavir fluorescence efficiency were tested. Adding of these surfactants resulted in fluorescence quenching of ritonavir. So there was no need for adding any surfactant, Fig. 4.
pH effect
Various types of buffer at several pH values were tested. The fluorescence intensity of ritonavir was decreased by adding any type of buffer solution. So there was no need for adding any surfactant, Fig. 5.
Method validation
The method was validated in accordance to ICH guidelines [26]. The linearity was tested by analyzing five concentration sets of ritonavir. The fluorescence intensity was plotted against drug concentration and the linearity was obtained over a concentration range of 2.0–20.0 ng/mL. The regression parameters were assessed and the obtained results revealed acceptable values as listed in Table 1. Limit of quantitation [LOQ] was defined as the lowest concentration of compound of interest which can be accurately analyzed. While the limit of detection [LOD] was defined as the lowest concentration of compound of interest which can be detected. Values were calculated and listed in Table 1. Accuracy, expressed as mean percent recovery [%R], was determined by applying the proposed procedure for triplicate determinations of [5, 10,15 ng/mL] ritonavir. The %R was calculated and presented in Table 2. Precision, as percent relative standard deviation [%RSD], was determined by determining [5, 10,15 ng/mL] ritonavir. For repeatability, it was performed within one day and for mean precision, it was performed on three consecutive days. The lower values of %RSD prove the higher precision of the described method, as shown in Table 1.
Selective determination of ritonavir in a laboratory prepared mixture with nirmatrelvir
The described method was selectively applied to the determination of ritonavir in a laboratory prepared mixture with nirmatrelvir, assuming that the recommended dose consisted of two tablets of nirmatrelvir and one tablet of ritonavir, corresponding to a nirmatrelvir to ritonavir ratio of 3: 1. The results were in good agreement with the label claim as listed in Table 2.
Method application for ritonavir determination in the pharmaceutical dosage form and spiked human plasma
The method described was applied to determine ritonavir in the pharmaceutical dosage form, Table 3. The results were in good agreement with statistical accepted results in comparison to the reported spectrophotometric method [11]. Furthermore, the method was applied for the determination of ritonavir in spiked human plasma regarding to the high sensitivity detection limits which allowed the determination of ritonavir in the plasma matrix. The data revealed selective determination of ritonavir without interference of endogenous components of the plasma matrix as listed in Table 4.
Comparative evaluation of the described method with previously reported spectrophotometric method
In general, the spectrofluorometric procedures described represented the first analytical method to selectively determine ritonavir in the presence of nirmatrelvir in its recently FDA-approved co-packaged form and spiked human plasma. The current results show that the nano- level determination of ritonavir has higher sensitivity compared to previously reported analytical methods. In particular, in terms of sensitivity, detection limits and quantification, the proposed method was compared with the previously used spectrophotometric method [11]. The proposed method allowed the determination of ritonavir in a concentration range of 2–20 ng/mL with a lower limit of detection up to 0.146 ng/mL and a limit of quantification up to 0.444 ng/mL, which confirmed its higher sensitivity compared to the spectrophotometric method (see Table 5). In addition, ethanol was chosen as the solvent in the described method compared to methanol, which was used as the solvent in the reported method. Ethanol is less toxic, has lower vapor pressure and evaporation, resulting in lower inhaled amount with lower disposal cost and high environmental friendliness [21, 27]. This advantage shows the environmental friendliness of the described method compared to previous methods.
Conclusion
In the described work, a more sensitive spectrofluorometric method for the selective determination of ritonavir in the presence of nirmatrelvir in pure form, in pharmaceutical tablets as well as in spiked human plasma was presented. The method was fully optimized, validated, and compared with the previously reported spectrophotometric method. In terms of sensitivity metrics, the described method was superior to published analytical methods in terms of Nano-level determination of ritonavir in various matrices.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Nahata A. Spectrofluorimetry as an analytical tool. Pharm Anal Acta. 2011;2(8):1–2.
Ramzy S, Abdelazim AH, Osman A, Hasan MA. Spectrofluorimetric quantitative analysis of favipiravir, remdesivir and hydroxychloroquine in spiked human plasma. Spectrochim Acta Part A Mol Biomol Spectrosc. 2022:121625.
Ramzy S, Abdelazim AH, Hasan MA. Application of green first derivative synchronous spectrofluorometric method for quantitative analysis of fexofenadine hydrochloride and pseudoephedrine hydrochloride in pharmaceutical preparation and spiked human plasma. BMC Chem. 2022;16(1):1–11.
Hicks MB, Farrell W, Aurigemma C, Lehmann L, Weisel L, Nadeau K, et al. Making the move towards modernized greener separations: introduction of the analytical method greenness score (AMGS) calculator. Green Chem. 2019;21(7):1816–26.
Hiremath S, McGuinty M, Argyropoulos C, Brimble KS, Brown PA, Chagla Z, et al. Prescribing nirmatrelvir/ritonavir for COVID-19 in advanced CKD. Clin J Am Soc Nephrol. 2022;17(8):1247–50.
Hung Y-P, Lee J-C, Chiu C-W, Lee C-C, Tsai P-J, Hsu I-L, et al. Oral Nirmatrelvir/Ritonavir therapy for COVID-19: the Dawn in the Dark? Antibiotics. 2022;11(2):220.
Lamb YN. Nirmatrelvir plus Ritonavir: first approval. Drugs. 2022:1–7.
Reina J, Iglesias C. Nirmatrelvir plus ritonavir (Paxlovid) a potent SARS-CoV-2 3CLpro protease inhibitor combination. Revista Espanola de Quimioterapia: Publicacion Oficial de la Sociedad Espanola de Quimioterapia; 2022.
Marsh KC, Eiden E, McDonald E. Determination of ritonavir, a new HIV protease inhibitor, in biological samples using reversed-phase high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl. 1997;704(1–2):307–13.
Birajdar A, Tegeli V, Ingale S, Nangare G. Development and validation of RP-HPLC method for the estimation of Ritonavir in API and tablet Formulation. Res J Pharm Technol. 2021;14(10):5457–60.
Seetaramaiah K, Smith AA, Ramyateja K, Alagumanivasagam G, Manavalan R. Spectrophotometric determination of ritonavir in bulk and pharmaceutical formulation. Sci Reviews Chem Commun. 2012;2:1–6.
Behera A, Moitra S, Si S, Meher A, Sankar AG. Method development, validation and stability study of ritonavir in bulk and pharmaceutical dosage form by spectrophotometric method. Chronicles of Young Scientists. 2011;2(3):161.
Martens-Lobenhoffer J, Böger CR, Kielstein J, Bode-Böger SM. Simultaneous quantification of nirmatrelvir and ritonavir by LC-MS/MS in patients treated for COVID-19. J Chromatogr B. 2022:123510.
Abdelazim AH, Ramzy S. Spectrophotometric quantitative analysis of lesinurad using extractive acid dye reaction based on greener selective computational approach. Spectrochim Acta Part A Mol Biomol Spectrosc. 2022:121292.
Abdelazim AH, Ramzy S, Abdel-Monem AH, Almrasy AA, Abdel-Fattah A, Shahin M. Quantitative spectrophotometric analysis of Celecoxib and Tramadol in their Multimodal Analgesia Combination Tablets. Journal of AOAC International; 2022.
Ramzy S, Abdelazim AH, Shahin M. Quantitative analysis of two pharmaceutical combinations containing amlodipine with either bisoprolol or candesartan using different UV spectrophotometric methods. J AOAC Int. 2022.
Abdelazim AH, Abourehab MA, Abd Elhalim LM, Almrasy AA, Ramzy S. Different spectrophotometric methods for simultaneous determination of lesinurad and allopurinol in the new FDA approved pharmaceutical preparation; additional greenness evaluation. Spectrochim Acta Part A Mol Biomol Spectrosc. 2022:121868.
Abdelazim AH, Ramzy S, Abdelzaher AM, Shahin M. Comparative evaluation of different mathematical models for simultaneous UV spectrophotometric quantitative analysis of velpatasvir and sofosbuvir. Spectrochim Acta Part A Mol Biomol Spectrosc. 2022;267:120536.
Ramzy S, Abdelazim AH. Application of different spectrophotometric methods for quantitative analysis of direct acting antiviral drugs simeprevir and sofosbuvir. Spectrochim Acta Part A Mol Biomol Spectrosc. 2022;272:121012.
Abdelazim AH, Ramzy S. Application of different quantitative analytical techniques for estimation of aspirin and omeprazole in pharmaceutical preparation. BMC Chem. 2022;16(1):1–8.
Abdelazim AH, Abourehab MA, Abd Elhalim LM, Almrasy AA, Ramzy S. Green adherent spectrophotometric determination of molnupiravir based on computational calculations; application to a recently FDA-approved pharmaceutical dosage form. Spectrochim Acta Part A Mol Biomol Spectrosc. 2023;285:121911.
Abourehab M, Shahin M, Sheikh R, Ellateif A, Fawzi S, Gouda A, editors. Utilization of N-bromosuccinimide for the sensitive spectrophotometric determination of pipazethate HCl as antitussive drug in pure and dosage forms. Annales Pharmaceutiques Françaises; 2021.
Kassem MA, Guesmi NE. Sensitive kinetic spectrophotometric determination of Cyclobenzaprine Hcl in pure form and pharmaceutical formulations. Anal Chem Lett. 2016;6(5):657–68.
velopment. and Validation of spectrophotometric methods for estimation of antimigraine drug eletriptan hydrobromide in pure form and pharmaceutical formulations. Annales Pharmaceutiques Françaises; 2021.
Pacheco-Fernández I, Pino V. Green solvents in analytical chemistry. Curr Opin Green Sustainable Chem. 2019;18:42–50.
Branch SK. Guidelines from the international conference on harmonisation (ICH). J Pharm Biomed Anal. 2005;38(5):798–805.
Hameed AM. An eco-friendly ultrasound-assisted deep eutectic solvent-based liquid–phase microextraction method for enrichment and quantification of nickel in environmental samples. J Umm Al-Qura Univ Appl Sci. 2022:1–12.
Acknowledgements
The authors would like to thank the Deanship of Scientific Research at Shaqra University for supporting this work.
Funding
This work was funded by the Deanship of Scientific Research at Shaqra University for supporting this work.
Author information
Authors and Affiliations
Contributions
MSI: Review and editing. AHA: Investigation, methodology and writing-original draft. SR: Methodology and data curation. AAA: Data analysis.MG: Review and editing. ASB: Review and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This work was approved by the Committee of Research Ethics in the Faculty of Pharmacy, Al-Azhar University, Cairo, Egypt. All participants signed informed consent statement before participation in the study. All described procedures were performed in accordance with relevant guidelines and regulations.
Consent for publication
Written informed consents were obtained from the participants for publication of this report.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Imam, M.S., Abdelazim, A.H., Ramzy, S. et al. Higher sensitive selective spectrofluorometric determination of ritonavir in the presence of nirmatrelvir: application to new FDA approved co-packaged COVID-19 pharmaceutical dosage and spiked human plasma. BMC Chemistry 17, 120 (2023). https://doi.org/10.1186/s13065-023-01030-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13065-023-01030-0