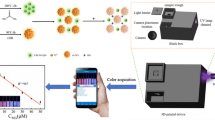
Abstract
Background
Carbon quantum dots (CQDs) are new class of carbon nanoparticles. Recently, they have been widely used as fluorescent probes due to their easy accessibility, optical properties and chemical inertness. Many available precursors are used in the synthesis of carbon quantum dots. The electrical and optical properties of CQDs could be enhanced by doping hetero atoms such as nitrogen or sulfur into their structure.
Objective
The current work presents the synthesis and characterization of water-soluble nitrogen doped carbon quantum dots (N-CQDs) and their use as fluorescent nano-sensors for the spectrofluorimetric determination of furosemide in its pharmaceutical preparations and spiked human plasma.
Methods
A domestic microwave was used to prepare the N-CQDs by heating a solution of sucrose and urea till complete charring (about ten minutes). The produced N-CQDs exhibit a strong emission band at 376 nm after excitation at 216 nm. Furosemide caused a quantitative quenching in the fluorescence intensity of the produced N-CQDs.
Results
The proposed method was validated according to ICH Guidelines. The method was found to be linear over the range of 0.1–1.0 µg/mL with LOQ of 0.087 µg/ml.
Conclusion
Ecofriendly nano fluorescent sensors (N-CQDs) were successfully synthesized. The size of N-CQDs was distributed in the range of 6.63 nm to 10.23 nm with an average of 8.2 nm. The produced N-CQDs were used as fluorescent probes for the estimation of furosemide in its pharmaceutical preparations as well as spiked human plasma samples.
Similar content being viewed by others
Introduction
Furosemide (FRS) is frequently used to treat edema associated with heart failure and hepatic or renal problems. It is a potent loop diuretic. It may be effective in patients who are not responding to thiazide diuretics [1]. It is given orally and may be given intravenously or intramuscularly as its sodium salt. Most side effects of the drug happen with high doses, such as electrolyte and fluid imbalance (hyponatremia and hypokalemia) mainly after prolonged use or large doses. The common symptoms of electrolyte imbalance are hypotension, headache, dry mouth, thirst, muscle cramps, restlessness, weakness, drowsiness, lethargy, oliguria, gastrointestinal disturbances and cardiac arrhythmias. Furosemide is fairly rapidly absorbed from GIT; bioavailability of the drug is about 60 to 70% but absorption is variable. The reported half-life time is up to about two hours although it is prolonged in patients with hepatic and renal impairment. It is mainly excreted unchanged in the urine. Non-renal elimination is increased in renal impairment. Furosemide is bound to plasma albumin up to 99% 1,2. Chemically, furosemide is: 4-Chloro-2-(furan-2-ylmethylamino)-5-sulfamoylbenzoic acid [2, 3] (Fig. 1). The reported methods for analysis of FRS in dosage forms and biological fluids include spectrophotometry [4,5,6], LC-MS [7], HPTLC [8], RP-HPLC [9,10,11] and spectrofluorimetry [12, 13]. Most of these techniques require multi-steps procedures and expensive instrumentation [7,8,9,10] for its analysis. Developing a sensitive spectrofluorimetric method for estimation of FRS in pharmaceutical preparations and human plasma samples without prior derivatization was the aim of this work. N-Doped CQDs were used for this purpose and the results were promising. The proposed method depends on synthesis of N-CQDs by domestic microwave-assisted method using mixture of sucrose and urea in water. Water serves as a highly green solvent. The method is based on formation of N-CQDs, which could be used as fluorescent nano-sensors for estimation of FRS. The drug caused quantitative quenching of the fluorescent N-CQDs. The major advantages of the proposed method are the high speed of production of N-CQDs in only about thirty minutes with high quantum yield (0.57) and the ability to detect down to nano-gram concentrations of the drug. Carbon quantum dots (CQDs) are a new generation of fluorescent nano particles characterized by being low in cost, photo-stable, safe or not toxic, very good biocompatible and highly water soluble [14,15,16,17]. In addition, the electrical and optical properties of CQDs could be boosted by doping hetero atoms such as nitrogen or sulfur into the structure of CQDs [18, 19].
Experimental
Materials and reagents
-
Methanol (HPLC grade) was obtained from Fisher (Belgium).
-
Orthophosphoric acid 85% and boric acid: (Sigma-Aldrich, Switzerland).
-
Glacial acetic acid 99% :(Alfa Chemical Group, Cairo, Egypt).
-
Britton Robinson Buffer (BRB)was prepared by mixing each of 0.04 M boric acid, 0.04 M phosphoric acid and 0.04 M acetic acid, then the pH was adjusted using 0.2 M sodium hydroxide over the range from 2.1 to 12.
-
Furosemide was kindly supplied by NODCAR, Cairo, Egypt, its purity was 99.9%.
-
Lasix® tablets: 40 mg/tablet (Batch No. BEG009) and Lasix® ampoules 20 mg/2mL (Batch No. BEG010). Products of Sanofi Pharmaceutical Company, Cairo, Egypt. Both were purchased from local Pharmacy.
-
Deionized water was used throughout the work.
-
Human plasma samples were kindly supplied by Mansoura University Hospital, Mansoura, Egypt. Samples were kept frozen at − 80 °C until use after gentle thawing.
Instrumentation
-
Shimadzu RF-6000 series Fluorescence Spectrophotometer with 150 W Xenon lamp was used for Fluorescence measurements. High sensitivity mode was used throughout the work.
-
Shimadzu UV-Visible Spectrophotometer, Model 1900i (Japan), was used for the spectrophotometric measurements.
-
The FT-IR spectrum was recorded using Thermo Fisher Scientific Nicolet - iS10 FT-IR Spectrometer (168 Third Avenue Waltham, MA, USA).
-
The produced N-CQDs were examined for their morphology using JEM-2100 high resolution transmission electron microscope (HRTEM) (JEOL, Tokyo). The instrument was operated at 200 KV.
-
Vortex mixer, ZX3 (Velp. Scientific) Italy.
-
Ultrasonic bath, Elma Sonic S 100(H), Germany.
-
Centrifuge, model sigma 2-16P, Germany.
-
Jenway3510 pH-Meter, UK.
-
Domestic LG microwave Model No: MH7043BARS (Power Input 230 V AC /50Hz, output of 900 W and frequency of 2,450 MHz.
Standard solutions
Stock solution (1000.0 µg/mL) of FRS was prepared by weighing out 10.0 mg of FRS, placing it in 10 mL volumetric flask and dissolve it in methanol. Different concentrations were prepared by diluting the stock solution as appropriate to obtain working solutions.
Preparation of N-CQDs
The fluorescent N-CQDs were synthesized as previously reported [19] using 15 g of sucrose and 3 g of urea dissolved in 30 mL of deionized water then heated in microwave for 10 min to ensure complete charring. The resulting N-CQDs were left to cool, diluted with water and centrifuged at 6000 rpm for 10 min. Then, 0.22 μm Syringe filters were used to remove the suspended impurities. Finally, the volume of N-CQDs was adjusted with deionized water to final volume of 100 mL to get stock solution.
General Procedure
For raw material
Aliquots of 400 µL of N-CQDs were transferred into a series of 10 mL measuring flasks. Various volumes from working standard solution (10.0 µg/mL) of FRS were added to each flask. 1 mL of BRB of pH 6.0 was also added. The solutions were completed to the mark with deionized water. The values of fluorescence quenching (ΔF) were estimated at 376 nm using an excitation wavelength of 216 nm). The calibration curve was plotted between ΔF versus final drug concentration in (µg/mL). Consequently, the linear regression equation was derived.
Pharmaceutical preparations
Lasix® tablets
Ten of Lasix® tablets were weighed individually, transferred to a mortar and powdered. A weight equivalent to 10.0 mg of FRS was quantitatively transferred into a small flask followed by 5 ml of methanol. The flask was sonicated for 10 min, then filtered into 10 ml measuring flask. Methanol was used to adjust the volume to give a solution of (1000.0 µg/mL). Methanol was used to dilute 0.1mL aliquot to give solution of 10.0 µg/mL in a 10.0 volumetric flask. The described procedure above (For Raw Material) was then applied. % Recoveries were calculated adopting the corresponding regression equation.
Lasix® Ampoules
The contents of ten ampoules of Lasix® were transferred to a beaker. 1.0 mL from the mixed solutions was transferred into 10 mL volumetric flask followed by 5 ml of methanol. The same solvent was used to adjust the volume to give a solution of 1000.0 µg/mL. Then 0.1mL aliquots were diluted to 10.0 mL with methanol to give solution of 10.0 µg/mL. The described procedure under section (For Raw Material) was adopted. Then the regression equation was used to calculate % recoveries.
Spiked human plasma
Aliquots of FRS standard working solution were quantitatively transferred into a series of 15 mL centrifuge tubes each containing 1 mL of human plasma then vortexed for 30 s. Acetonitrile up to 5 mL was added to each tube. Centrifugation at 3600 rpm was carried out for 30 min. Aliquots of 1.0 ml of the produced supernatant were transferred into 10.0 mL volumetric flasks, completed to the mark, and the described procedure (For Raw Material) was applied. The percentage recoveries were calculated adopting the regression equation.
Results and discussion
The present study reports a rapid, simple, cost-effective and sensitive spectrofluorometric method for estimation of FRS. The method of synthesis of N-CQDs is green and rapid using simple procedure and available starting materials compared to other reported methods [18, 19]. The method of synthesis takes only 30 min to produce highly fluorescent probe which could be used for its determination.
Characterization of N-CQDs
Water-soluble N-CQDs were synthesized using sucrose as a carbon source and urea as a nitrogen source using microwave assisted method. An aqueous solution of orange color indicating the production of N-CQDs (Fig. 2). TEM image of N-CQDs is presented in (Fig. 3) showings spherical N-CQDs with diameters in the range of 6.63 nm to 10.23 with an average of 8.2 nm. (FT-IR) analysis was also carried out to identify N-CQDs functional groups (Fig. 4). The broad band in the range of 3600–3100 cm− 1 is characteristic for N-H and O-H groups, the peak at 1638 cm − 1indicates the presence of C = C/C = O. The vibrations at 1265 cm− 1 represents the stretching modes of C-O-C band [20]. The high fluorescence intensity of the produced N-CQDs is proved by the emission and excitation spectra of fluorescent N-CQDs in deionized water as presented in (Fig. 5). FRS has an obvious quenching effect on N-CQDs as presented in (Fig. 6). The UV-VIS absorption spectrum of the formed quantum dots is presented in (Fig. 7).
Quantum Yield
In the present work, the N-CQDs are characterized by high quantum yield (0.57). Equation 1 was used to calculate their quantum yield by the single point method adopting the following formula [21]:
In which:
Φ refers to the quantum yield, (x) and (st) subscripts refer to the unknown sample and the standard solutions. F is the integrated measured intensity of emission, A is the absorbance and η indicates the refractive index of the solvent. In aqueous solutions, ηx/ηst is equal to 1. Quinine sulfate in 0.1 M sulfuric acid was used as the standard fluorescent substance (QY: 0.54) [21].
Fluorescence response mechanism of N-CQDs to FRS
The process of fluorescence quenching can be resolved into different types, dynamic, static quenching and inner filter effect. For better understanding of the mechanism of quenching, Stern Volmer’s Eq. (2) was used to investigate the fluorescence emission intensity of the N-CQDs FRS system [22].
In this equation:
[Q] is the quencher molarity, F and F0 are the fluorescence intensities in presence and absence of quencher, respectively. τ 0 is the average lifetime of fluorescence (10− 8 s). Ksv and Kq represent the Stern-Volmer quenching constant and the quenching rate constant. The quenching experiments were done at ambient temperature. Quenching values were applied to the Stern Volmer`s equation resulting in Kq value of 3.96 × 1012 which was larger than (2.0 × 1010 L.mol− 1.s− 1) the maximum diffusion rate constant [23]. The Kq values at 303 k and 313 k were 3.31 × 1012 and 2.02 × 1012. Consequently, the mechanism is assumed to be static quenching. In this study, UV-VIS absorption spectrum of FRS overlapped with the excitation spectrum of NCQDs (Fig. 8). Thus, Inner Filter Effect (IFE) might take place. The correction of N-CQDs fluorescence intensity for possible IFE was studied upon adding increased concentrations of FRS, the corrected fluorescence intensity was expressed using Eq. (3) [24]:
In which:
Fcorr is the corrected fluorescence intensity after excluding inner filter effect from Fobs, Fobs represents the observed fluorescence intensity, Aem and Aex are the absorbance of the drug at the emission wavelength and excitation wavelength of N-CQDs. The suppressed efficiency (%E) was calculated for observed and corrected fluorescence intensities using Eq. 4:
%E of both corrected and observed fluorescence intensities of N-CQDs against drug concentration plot (Fig. 9) revealed that, IFE has a significant role in the quenching of N-CQDs fluorescence intensity by the cited drug.
Method optimization and development
Effect of pH
The effect of pH on quenching of fluorescence intensity by FRS was studied using BRB solution over pH range from 2.1 to 12. Maximum ΔF value was obtained at pH 6.
Effect of volume of the buffer
To investigate the effect of volume of buffer, increasing volumes of BRB were used. The maximum quenching effect on the fluorescence of N-CQDs was attained using 1 mL of BRB buffer of pH 6.
Effect of volume of N-CQDs
Upon adding different volumes of N-CQDs, it was found that the optimum volume of N-CQDs is 400 µL. As it gave maximum fluorescence quenching with the drug.
Effect of temperature
The influence of temperature on the relative fluorescence intensities was studied. The fluorescence intensities were recorded at 376 nm at 298, 303, 313 K. As temperature increased, the degree of quenching decreased (Fig. 10). As a result, the study was carried out at ambient temperature.
Effect of incubation time
After adding the studied drug to N-CQDs, the fluorescence emission spectra at various time intervals were recorded starting from 1 to 40 min. The reaction was found to be instantaneous and 1 min was enough to complete the reaction. The fluorescence signals were stable for 30 min after which the quenching effect decreased significantly.
Method validation
ICHQ2(R1) guidelines [25] were used to check the validity of the proposed method. The plot of concentrations in µg/mL of the drug against relative quenched fluorescence intensity was used to assess the linearity of the proposed method. The linear range was found to be 0.1 to 1.0 µg/mL. Equation 5 represents the linear regression:
where F is the fluorescence intensity of N-CQDs in the presence of studied drug, F0 is the fluorescence intensity of N-CQDs in the absence of FRS, C is the drug concentration in µg/mL.
The values of LOD and LOQ proved that the suggested method is sufficiently sensitive as summarized in Table 1 The accuracy of the proposed method was confirmed by calculating the mean % recoveries of raw material of FRS. The comparison method [4] depends on measuring the absorbance of FRS at 277 nm in methanol. Variance F-test and Student’s t-test were used to compare the results obtained from the comparison and proposed method as illustrated in (Table 2) [26]. There was no significant difference between the two methods regarding accuracy and precision.
The precision of the method was evaluated by investigating the inter-day and intraday precisions. The %RSD values were calculated using three replicates of three different concentrations in three successive days and in the same day. The developed method exhibited a relatively lower values of % RSD than 2% which is acceptable (Table 3). Changing the temperature by ± 2 C and the pH by ± 0.2 gave no change on the study results. Those minor and small changes may arise during everyday work. As a result, the robustness of the method was confirmed. The % recoveries of FRS in its dosage forms were calculated to ensure the selectivity of the proposed method as illustrated in (Table 4). From the obtained results, it was obvious that the common tablet excipients didn`t interfere with the results of the method. Thus, the method could be used for the estimation of FRS in its pharmaceutical preparations. The selectivity of the method was confirmed by studying the interference from some co-administered drugs, such as valsartan and spironolactone. The tolerance limit for valsartan was 1.0 µg/mL, and for spironolactone, it was 0.5 µg/ml.
Applications
Drug analysis in its Pharmaceutical Preparations
FRS was successfully estimated in its pharmaceutical preparations by the suggested method. The numerical results in (Table 4) show a good agreement and conformity with those obtained from the comparison method [4]. Variance ratio F-test and Student’s t-test [26] were used to statistically analyze the results obtained by both methods. No significant difference was observed between the calculated and tabulated values.
Analysis of Spiked Human plasma
After oral dosing of 40 mg FRS, the peak plasma concentration is 1.163 µg/mL [27], as a result the method could be used in the estimation of the drug in plasma. After preparing the samples, they were analyzed as described before. The method proved its suitability to estimate FRS in the studied matrix. The range of the average recovery values was from 89.16 to 114.33% as shown in Table 5 and Fig. 11.
Conclusion
Ecofriendly nano fluorescent sensors (N-CQDs) were successfully synthesized and applied to the estimation of FRS in pharmaceutical preparations as well as spiked human plasma. The water-soluble N–CQDs were prepared using sucrose and urea, the size of N-CQDs was distributed in the range of 6.63 to 10.23 nm with an average of 8.2 nm. They are highly stable and have strong fluorescence intensity. The proposed method is characterized by being highly sensitive, selective, simple and low cost.
Data Availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- N-CQDS:
-
Nitrogen doped carbon quantum dots
- FRS:
-
Furosemide
- BRB:
-
Britton Robinson Buffer
- CQDS:
-
Carbon quantum dots
- IFE:
-
Inner filter effect
References
Harvey RA. Lippincott`s Illustrated Reviews. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2012. pp. 284–5.
Brayfield A, Martindale. The complete drug reference. 38th ed. London: The Pharmaceutical Press; 2014.
The British Pharmacopoeia. British Pharmacopoeia Commission. London: The Stationary Office; 2018.
Gahandule M, Banerjee SK. Development of UV spectrophotometric methods and validation for estimation of furosemide in bulk and tablet dosage form by absorbance maxima and area under the curve method. Int J Adv Pharm. 2016;5:160–70. https://doi.org/10.7439/ijap.v5i6.3791.
Hassouna MEM, Issa YM, Zayed AG. Spectrophotometric determination of furosemide drug in different formulations using Schiff ’s bases. Forensic Res Criminol Int J. 2015;1:214–21. https://doi.org/10.15406/frcij.2015.01.00036.
Naveed S, Qamar F, Zainab S. Simple UV spectrophotometric assay of furosemide. J Innov Pharm Biol Sci. 2014;3:97–101.
Romantsik O, Barco S, Bruschettini M, Tripodi G, Ley D, Cangemi G. A LC-MS/MS method for the quantification of caffeine, betamethasone, clonidine and furosemide in cerebrospinal fluid of preterm infants. J Pharm Biomed Anal. 2020;197:112996. https://doi.org/10.1016/j.jpba.2019.112996.
Chen F, Fang B, Wang S. A fast and validated HPLC method for simultaneous determination of dopamine, dobutamine, phentolamine, furosemide, and aminophylline in infusion samples and injection formulations. J Anal Method Chem. 2021; 2021:8821126. https://doi.org/10.1155/2021/8821126
Maulik MB, Dadhania K, Faldu S. Development and validation of RP-HPLC method for simultaneous estimation of furosemide and spironolactone in their combined tablet dosage form. J Pharm Sci Bio-Sci Res. 2012;2:144–7.
Semaan FS, Neto AJ, Lanças FM, Cavalheiro ÉT. Rapid HPLC- DAD determination of furosemide in tablets using a short home‐ made column. Anal Lett. 2005;38:1651–8. https://doi.org/10.1081/AL-200065813.
Naguib IA, Abdelaleem EA, Emam AA, Ali NW, Abdallah FF. Development and validation of HPTLC and green HPLC methods for determination of furosemide, spironolactone 15 and canrenone, in pure forms, tablets and spiked human plasma. Biomed Chromatogr. 2018;32:e4304. https://doi.org/10.1002/bmc.4304.
Liu Y, Wang H, Wang J, Li YF. A simple and sensitive spectrofluorimetric method for the determination of furosemide using zinc (II)–1,4-bis(imidazol-1-ylmethyl) benzene complexes. Luminescence. 2013;28:882–7. https://doi.org/10.1002/bio.2451.
El-Wasseef DR, El-Sherbiny DT, Abu-El-Enein MA, El-Ashry SM. Simultaneous determination of labetalol and furosemide by first-derivative synchronous spectrofluorimetry. J Fluorescence. 2009;19:817–28. https://doi.org/10.1007/s10895-009-0479-6.
Kurian M, Paul A. Recent trends in the use of green sources for carbon dot synthesis–A short review. Carbon Trends. 2021;3:100032. https://doi.org/10.1016/j.cartre.2021.100032.
Molaei MJ. Principles, mechanisms, and application of carbon quantum dots in sensors: a review. Anal Methods. 2020;12:1266–87. https://doi.org/10.1039/C9AY02696G.
Molaei MJ. Carbon quantum dots and their biomedical and therapeutic applications: a review. RSC Adv. 2019;9:6460–81. https://doi.org/10.1039/C8RA08088G.
Wang R, Lu KQ, Tang ZR, Xu YJ. Recent progress in carbon quantum dots: synthesis, properties and applications in photocatalysis. J Mater Chem A. 2017;5:3717–34.
Shaikh SA, Sengupta S, Varma RS, Gawande MB, Goswami A. Syntheses of N-doped carbon quantum dots (NCQDs) from bioderived precursors: a timely update. ACS Sustain Chem Eng. 2021;9:3–49. https://doi.org/10.1021/acssuschemeng.0c04727.
Liu X, Liu J, Zheng B, Yan L, Dai J, Zhuang Z, Du J, Guo Y, Xiao D. N-Doped carbon dots: green and efficient synthesis on a large-scale and their application in fluorescent pH sensing. New J Chem. 2017;4:10607–12. https://doi.org/10.1039/C7NJ01889D.
Pavia DL, Lampman GM, Kriz GS, Vyvyan JR. Introduction to Spectroscopy. 5th ed. USA: Cengage learning; 2015. pp. 16–01.
Rurack K. Fluorescence Quantum Yields: Methods of Determination and Standards. Standardization and Quality Assurance in Fluorescence Measurements. Berlin:Springer; 2008.pp. 101–145.
Lakowicz JR. Fluorescence Sensing. Principles of Fluorescence Spectroscopy. Berlin:Springer; 1999.pp. 531–572.
Albani JR. Principles and applications of fluorescence spectroscopy. London: John Wiley &Sons; 2008.
Chen S, Yu YL, Wang JH. Inner filter effect-based fluorescent sensing systems: a review. Anal Chim Acta. 2018;999:13–26.
Guideline ICH. Validation of analytical procedures: Text and methodology. Q2 (R1) 1(20),05(2005). https://www.ich.org/page/quality-guidelines [accessed 24May 2022].
Miller JC, Miller JN. Statistics and Chemometrics for Analytical Chemistry. 5th ed. England: Pearson Education; 2018.
Najib N, Idkaidek N, Beshtawi M, Bader M, Admour I, Alam SM, Zaman Q, Dham R. Bioequivalence evaluation of two brands of furosemide 40 mg tablets (salurin and lasix) in healthy human volunteers. Biopharm Drug Dispos. 2003;24:245–9.
Acknowledgements
No acknowledgement.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
MA Methodology, Investigation, Validation, Writing original draft. NE Conceptualization, Writing-review & editing, Supervision. AM Validation, Writing-review& editing. GH Conceptualization, Writing-review & editing, Supervision. FB Conceptualization, Methodology, Resources, Writing-review and editing, Supervision.
All authors approved the manuscript for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
There are no human subjects in this research and informed consent is not applicable. The used plasma is pooled plasma from the blood bank of Mansoura university hospital.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Abo Zaid, M.H., El-Enany, N., Mostafa, A.E. et al. Use of green fluorescent nano-sensors for the determination of furosemide in biological samples and pharmaceutical preparations. BMC Chemistry 17, 25 (2023). https://doi.org/10.1186/s13065-023-00937-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13065-023-00937-y