Abstract
Background
In this work, nickel oxide nanoparticles were prepared by polyol mediated aqueous route of sol–gel process using nickel nitrate hexahydrate as precursor, a mixture of isopropyl alcohol and water as solvent and glycerol for making polyol medium followed by calcination at various temperatures ranging from 500 to 900 °C. Characterization was carried out using X–ray diffractometry, infrared spectroscopy, differential scanning calorimetry-thermogravimetry and field emission scanning electron microscopy.
Results
The results confirmed the formation of face-cantered cubic structure of nickel oxide with its complete conversion after calcination at 900 °C; significant variation in the surface morphology was observed with the increasing calcination temperature.
Conclusions
The study revealed that the aqueous sol–gel route using polyol system followed by calcination at ambient temperatures lead to the successful synthesis of nickel oxide nanoparticles.
Similar content being viewed by others
Introduction
Nickel oxide nanoparticles are one of the most promising materials for technological applications such as magnetic materials, ion storage materials, battery electrodes, photo–electron devices, thermoelectric materials, fuel cells, gas sensors, catalysts, dye–sensitized photocathodes, electrochromic films and non–enzymatic glucose sensors etc [1]. Nickel oxide is among those metal oxides that exhibit p–type nature and possesses a wide band gap ranging from 3.6 to 4.0 eV due to which it can be employed as a transparent p–type semiconducting layer [2, 3]. In particular, nano–dimensional particles because of their large surface area, display a wide range of interesting size–dependent electro–optical, magneto–optical, chemical and mechanical properties [1]. Research attention has therefore been focused by the scientists on different routes for the synthesis of nickel oxide nanoparticles. Generally, nanoparticles can be prepared by physical, chemical and biological methods. The chemical and physical methods include co–precipitation method, sol–gel technique, microemulsion method, solvothermal synthesis, ultrasonic radiation, microwave irradiation and anodic arc plasma method. In addition, biological methods comprising the use of biological materials such as different parts of plants, fungi, algae, bacteria, and actinomycetes species have also been employed [4, 5].
Synthesis of nanostructures with controlled morphology including prevention of agglomeration and oxidation of nanoparticles keeping in view their bulk production is of extensive research interest these days. Sol–gel method is the easiest one for tuning the morphology of nanoparticles simply by regulating the process parameters [6]. Another approach for adjusting the size and shape of nanostructures is by using some organic solvent or a surfactant capable of inhibiting agglomeration and thus producing well–defined monodispersed structures [7]. According to Rakshit et al. morphology controlled process for the transformation of precursors is the most favorable method for the preparation of nickel oxide nanostructures [8]. Niasari et al. reported the synthesis of nanoparticles of NiO using nickel oxalate and oleylammine as precursors [9]. Synthesis of nanosize NiO by heat treatment of nickel octanoate has also been reported by Niasari et al. [10]. Stabilizers like polyvinylpyrrolidone (PVP) surfactant are often added for particle protection and controlled particle size [11]. Zhang et al. prepared nickel oxide fibres through PVP approach [12]. Xu et al. synthesized NiO nanorods by using thermal decomposition method followed by a surfactant nonyl phenyl ether (NP-9/5) base process and NaCl flux at 1173 K [13]. Hence, it is important and significant to synthesize nickel oxide nanoparticles with a facile pathway. During last few decades, nano size nickel oxide particles have been successfully synthesized using sol–gel method which is believed to be the simplest and the most economical technique. Teoh et al. synthesized nickel oxide nanoparticles by surfactant mediated approach of sol–gel process using poly (alkylene oxide) copolymer [14].
This work describes the synthesis of nickel oxide nanoparticles by aqueous sol–gel route using glycerol as polyol for a better control on the morphology of nanoparticles, followed by calcination at ambient temperatures. The effect of temperature variation on crystal structure, surface functional groups, thermal behavior and surface morphology of the synthesized nanoparticles is determined by using XRD, IR, DSC–TGA and FESEM.
Experimental
Reagents
Nickel nitrate hexahydrate (Thermo Scientific 99%), isopropyl alcohol (WINLAB 99.9%), glycerol (99%) and nitric acid (Merck 70%) were used.
Synthesis of nickel oxide nanoparticles
Nickel oxide nanoparticles were synthesized by aqueous sol–gel route in acidic medium. 19.45 g nickel nitrate hexahydrate precursor was added to a mixture of solvents i.e. isopropyl alcohol and water in 1:8 ratio. 10 mL of glycerol were added and pH of the solution was maintained at 1 using nitric acid. The mixture was stirred constantly for 2 h at 70 °C. The sol thus formed was dried at 80 °C for several hours in order to dry it completely to obtain gel which was ground well. The powdered sample obtained was divided into five portions that were calcined for 2 h at 500, 600, 700, 800 and 900 °C and were labeled as NN–1, NN–2, NN–3, NN–4 and NN–5 respectively. The graphical representation of the process is shown in Fig. 1.
Characterization
The crystalline nature of nickel oxide nanoparticles was investigated by X–ray diffraction technique. The XRD patterns of the prepared samples were recorded with the help of Bruker D2 PHASER X–ray diffractometer using monochromatised CuKα1 radiation at a wavelength of 1.54060 Å. IR spectra were recorded with Thermo Nicolet IR 200 (USA). Thermal behavior was studied using differential scanning calorimeter Universal V4.5A, TA instruments USA, varying the temperature from room temperature to 1000 °C at a rate of 10 °C/min in nitrogen atmosphere. Structural studies of the samples were done by FESEM FEI Nova 450 NanoSEM.
Results and discussion
Synthesis of nickel oxide nanoparticles was carried out using glycerol for making polyol medium because of its lower cost and nontoxicity. The polyol medium significantly controls the formation of hard agglomerates during the synthesis of metal oxide nanoparticles obtained through aqueous sol–gel routes; therefore the use of high boiling polyols is more appropriate. Glycerol has also been reported to be used for the reduction of metal salt precursors for the preparation of various metal nanoparticles like silver, tantalum, palladium, platinum, ruthenium and gold. Moreover, manganese hydroxide, manganese carbonate and vanadium oxide have been synthesized in the presence of glycerol as a reducing agent [15]. Hence, glycerol added to a mixture of isopropanol and water acts as a solvent as well as a mild reducing agent leading to slow transformation of nickel alkoxide intermediate to nickel oxide nanoparticles.
X-ray diffraction analysis
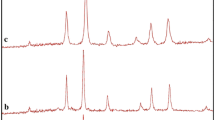
The phase composition of synthesized nanoparticles NN–1, NN–2, NN–3, NN–4 and NN–5 was studied using X–ray diffraction analysis and the diffraction patterns are presented in Fig. 2a–e. The diffraction peaks for NN–1 at 2θ degrees of 45.37, 52.76 and 77.08° correspond to the (111), (200), and (220) crystal faces of Ni as compared with the standard JCPDS data reported in JCPDS card No. 04–0850 [16]. The sharp peaks showed the formation of nickel alkoxide intermediate at 500 °C. The pattern for NN–2 exhibited additional distinct peaks at 37.68, 43.70, 63.22, 75.72 and 79.61° relative to (111), (200), (220), (311), and (222) crystal planes of NiO respectively compared with the reported standard JCPDS data for NiO (JCPDS card No: 78–0429) which revealed partial conversion of the intermediate into nickel oxide at 600 °C [17, 18]. NN–3 and NN–4 displayed diffraction patterns similar to NN–2 with slight variation in intensities of peaks indicating the gradual increase in conversion of intermediate to nickel oxide at 700 and 800 °C leading to its complete transformation to well–crystallized single phase face centered cubic geometry with lattice constants, a = b = c = 4·1771 Å at 900 °C.
Infrared spectroscopy
Figure. 3a–e exhibit the infrared spectra of NN–1, NN–2, NN–3, NN–4 and NN–5 recorded in the range of 4000–400 cm−1. The figures illustrate similar spectra for all the prepared samples comprising various significant absorption bands. The sharp absorption bands in the region between 3800 and 3000 cm−1 are attributed to the O–H stretching vibrations of hydroxyl groups. The appearance of such bands represents the adsorption of water molecules on the external surface of nanoparticles in addition to hydrogen bonded hydroxyl groups. The weak bands between 3000 and 2800 cm−1 correspond to the stretching vibrations of alkyl groups of organic impurities entangled by the nanocrystals during their formation [19]. Another weak band at 1587.30 cm−1 is assigned to H–O–H bending vibrational modes [20].
The prominent absorption band in the region below 1000 cm−1 is ascribed to the stretching modes of metal–oxygen bonds that substantiate the presence of Ni–O. The broadness of this band indicates the nanosize of nickel oxide nanoparticles [21]. Similar strong absorption bands below 1000 cm−1 have been reported for nickel oxide nanoparticles by the scientists in the past [22,23,24].
Thermal analysis
Thermal behavior of synthesized NN samples was studied by DSC–TGA and curves are illustrated in Fig. 4a–e. The curves exhibit negligible weight gain and weight loss ranging from 0.1 to 0.34% from room temperature to 1000 °C. The heat flow curves depict all the weight changes accompanied with their corresponding endothermic and exothermic peaks shown by the NN samples. These heat changes can be attributed to the removal of adsorbed moisture, organic residues entrapped between the crystals during their synthesis as well as the heat transfer between sample and crucible. According to previous researchers the endothermic reactions are attributed to the decomposition of water, whereas the exothermic reactions occur due to the oxidation of the decomposed product [25]. Most of the studies have reported negligible weight loss for nickel oxide nanoparticles above 400 °C indicating the formation of thermally stable product [26].
Field emission scanning electron microscopy
The surface morphology of nickel oxide nanoparticles was investigated using field emission scanning electron microscopy and the images recorded at 50,000 × magnification are presented in Fig. 5a–c. The as–prepared nanoparticles show aggregated structures of irregular shape having size in the range of several nanometers; the aggregation rate of the nanoparticles is believed to be the structure and morphology determining factor for the nanoparticles. The results show that NN–1 demonstrates higher aggregation that decreases with the increasing calcination temperature in case of NN–3 and NN–5 due to intensified nucleation rate of nanoparticles. The rate of nucleation is greatly influenced by high temperature due to accelerated core formation after attaining super–saturation of the product. The particle size on the other hand, increases due to quick particle growth at higher calcination temperatures resulting in the formation of flakes and hence, NN–5 bares flake–like morphology with reduced aggregation.
Figure. 5b clearly depicts that NN–3 exhibits quite different morphology with elongated structures combined to form a network due to aggregation of particles. According to Lim et al. cylindrical rod–like structures of nickel oxide go through a two–step process, a first step comprising nucleation of atoms formed by metal salt reduction and another step of crystal growth from nuclei through atomic growth [27]. The other reason for aggregation of nickel oxide nanocrystals often noticed by the former scientists is their high surface area and high surface energy attained during sol–gel synthesis [14]. Moreover, nanosize nickel oxide has greater tendency to aggregate due to the fact that it is antiferromagnetic in nature; this aggregation resulting in increased diameter leads to enhanced magnetic moment of nickel oxide nanoparticles [17]. Bani–Fawaz et al. obtained a three–dimensional network of randomly oriented structures of nickel oxide aggregated without significant spacing between individual nanoparticles [28]. Uddin et al. also confirmed highly agglomerated structures of synthesized nickel oxide nanoparticles [29]. Further comparison of morphology of nickel oxide nanoparticles prepared by different routes is given in Table 1.
Conclusion
Nickel oxide nanoparticles were successfully synthesized by aqueous sol–gel route adopting glycerol for making polyol system. The method employed for the synthesis of nanoparticles is quite simple, economical and operates under mild conditions making the process suitable for large scale production. Characterization of the as–prepared nickel oxide nanoparticles revealed the formation of face–cantered cubic structure of nickel oxide with its complete conversion after calcination at 900 °C. However, high aggregation of particles was observed that decreased with the rise in calcination temperature resulting in the formation of flakes of irregular shape and different sizes in several nanometers range.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available in order to avoid their misuse but are available from the corresponding author on reasonable request.
References
Hong SJ, Mun HJ, Kim BJ, Kim YS. Characterization of nickel oxide nanoparticles synthesized under low temperature. Micromachines. 2021;12:1168–77.
Li X, Xhang X, Li Z, Qian Y. Synthesis and characteristics of NiO nanoparticles by thermal decomposition of nickel dimethylglyoximate rods. Solid State Commun. 2006;137:581–4.
Shajudheen VPM, Sivakumar M, Kumar SS. Synthesis and characterization of NiO nanoparticles by thermal oxidation of nickel sulfide nanoparticles. Mater Today Proc. 2016;3:2450–6.
Kalam A, Al-Sehemi AG, Al-Shihri AS, Du G, Ahmad T. Synthesis and characterization of NiO nanoparticles by thermal decomposition of nickel linoleate and their optical properties. Mater Charact. 2012;68:77–81.
Gebretinsae HG, Tsegay MG, Nuru ZY. Biosynthesis of nickel oxide (NiO) nanoparticles from cactus plant extract. Mater Today Proc. 2021;36(2):566–70.
Zahra S, Mazhar S, Zahra S, Idrees H, Hussnain A. Synthesis and characterization of magnesium doped titania for photocatalytic degradation of methyl red. Rev Mater. 2022;27(1):1–14.
Kumar A, Saxena A, De A, Shankar R, Mozumdar S. Controlled synthesis of size-tunable nickel and nickel oxide nanoparticles using water-in-oil microemulsions. Adv Nat Sci Nanosci Nanotechnol. 2013;4:025009–0250017.
Rakshit S, Chall S, Mati SS, Roychowdhury A, Moulik SP, Bhattacharya SC. Morphology control of nickel oxalate by soft chemistry and conversion to nickel oxide for application in photocatalysis. RSC Adv. 2013. https://doi.org/10.1039/c3ra21978j.
Niasari MS, Mir N, Davar F. Synthesis and characterization of NiO nanoclusters via thermal decomposition. Polyhedron. 2009;28:1111–4.
Niasari MS, Davar F, Fereshteh Z. Synthesis of nickel and nickel oxide nanoparticles via heat-treatment of simple octanoate precursor. J Alloys Compd. 2010;494:410–4.
Tientong J, Garcia S, Thurber CR, Golden TD. Synthesis of nickel and nickel hydroxide nanopowders by simplified chemical reduction. J Nanotechnol. 2014;2014:1–6.
Zhang CF, Zhan J, Wu JH, Li CJ. Preparation and characterization of fibrous NiO particles by thermal decomposition of nickelous complex precursors. Trans Nonferrous Met Soc China. 2004;14:713–7.
Xu CK, Xu GD, Wang GH. Preparation and characterization of NiO nanorods by thermal decomposition of NiC2O4 precursor. J Mater Sci. 2002;38:779–82.
Teoh LG, Li KD. Synthesis and characterization of NiO nanoparticles by sol–gel method. Mater Trans. 2012;53(12):2135–40.
Álvarez AED, Cadierno V. Glycerol: a promising green solvent and reducing agent for metal-catalyzed transfer hydrogenation reactions and nanoparticles formation. Appl Sci. 2013;3:55–69.
Li J, Li P, Li J, Tian Z, Yu F. Highly-dispersed Ni-NiO nanoparticles anchored on an SiO2 support for an enhanced CO methanation performance. Catalysts. 2019;9:506–17.
Zahra S, Naz N, Rehman MZ, Irfan M, Sheikh A, Izhar S. Characterization of sol-gel prepared silica supported NiO-CuO composites. J Chem Soc Pak. 2020;42(2):164–70.
Bonomo M. Synthesis and characterization of NiO nanostructures: a review. J Nanopart Res. 2018;20:222–43.
Zahra S, Deeba F, Irshad M, Sheikh A, Zahra S, Khan AM. Synthesis and characterization of ultra-fine titania powders for wastewater treatment. Dig J Nanomater Biostructures. 2021;16(4):1595–605.
Rahal HT, Awad R, Gaber AMA, Bakeer DES. Synthesis, characterization, and magnetic properties of pure and EDTA-capped NiO nanosized particles. J Nanomater. 2017;2017:1–9.
Ghazal S, Akbari A, Hosseini HA, Sabouri Z, Forouzanfar F, Khatami M, Darroudi M. Sol-gel biosynthesis of nickel oxide nanoparticles using Cydonia oblonga extract and evaluation of their cytotoxicity and photocatalytic activities. J Mol Struct. 2020;1217:128378–85.
Khalaji AD. Preparation and characterization of NiO nanoparticles via solid-state thermal decomposition of nickel (II) schiff base complexes [Ni (salophen)] and [Ni (Me-salophen)]. J Clust Sci. 2013;24(1):209–15.
Kayani ZN, Butt MZ, Riaz S, Naseem S. Synthesis of NiO nanoparticles by sol-gel technique. Mater Sci Pol. 2018;36(4):547–52.
Kumar PV, Ahamed AJ, Karthikeyan M. Synthesis and characterization of NiO nanoparticles by chemical as well as green routes and their comparisons with respect to cytotoxic effect and toxicity studies in microbial and MCF-7 cancer cell models. SN Appl Sci. 2019;1:1083–97.
Zorkipli NNM, Kaus NHM, Mohamad AA. Synthesis of NiO nanoparticles through sol-gel method. Procedia Chem. 2016;19:626–31.
El-Kemary M, Nagy N, El-Mehasseb I. Nickel oxide nanoparticles: synthesis and spectral studies of interactions with glucose. Mater Sci Semicond. 2013;16:1747–52.
Lim HH, Horri BA, Salamatinia B. Synthesis and characterizations of nickel (II) oxide submicro rods via co-precipitation methods. IOP Conf Ser Mater Sci Eng. 2018;398:012033–42.
Bani-Fwaz MZ, El-Zahhar AA, Abd-Rabboh HSM, Hamdy MS, Shkir M. Synthesis of NiO nanoparticles by thermal routes for adsorptive removal of crystal violet dye from aqueous solutions. Int J Environ Anal Chem. 2019;101(8):1126–44.
Uddin S, Safdar LB, Anwar S, Iqbal J, Laila S, Abbasi BA, Saif MS, Ali M, Rehman A, Basit A, Wang Y, Quraishi UM. Green synthesis of nickel oxide nanoparticles from Berberis balochistanica stem for investigating bioactivities. Molecules. 2021;26:1548–61.
Ananda GT, Nithiyavathia R, Ramesha R, Sundarama SJ, Kaviyarasu K. Structural and optical properties of nickel oxide nanoparticles: investigation of antimicrobial applications. Surf Interfaces. 2020;18:100460–6.
Ezhilarasi AA, Vijaya JJ, Kaviyarasu K, Zhang X, Kennedy LJ. Green synthesis of nickel oxide nanoparticles using solanum trilobatum extract for cytotoxicity, antibacterial and photocatalytic studies. Surf Interfaces. 2020;20:100553–62.
Sagadevan S, Podder J. Investigations on structural, optical, morphological and electrical properties of nickel oxide nanoparticles. Int J Nanoparticles. 2015;8(3/4):289–301.
Rifaya MN, Theivasanthi T, Alagar M. Chemical capping synthesis of nickel oxide nanoparticles and their characterizations studies. Nanosci Nanotechnol. 2012;2(5):134–8.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Conceptualization: SZ; Methodology: SZ; Validation: SZ; Investigation: WS; Resources: SZ; Data Curation: WS; Writing—Original Draft: BK; Writing—Review and Editing: SZ; Visualization: SZ; Supervision: CAA; Project Administration: CAA. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zahra, S., Shahid, W., Amin, C.A. et al. Polyol–mediated synthesis of nickel oxide nanoparticles through aqueous sol–gel route. BMC Chemistry 16, 105 (2022). https://doi.org/10.1186/s13065-022-00898-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13065-022-00898-8