Abstract
Background
Diabetes mellitus is a systemic disease mainly caused by the disorder of metabolism, which has become huge threat to human health. Polysaccharides are the main active substance from Inonotus obliquus (I. obliquus) with hypoglycemic effect. This study aims to evaluate the hypoglycemic activity and investigate the molecular mechanism of I. obliquus polysaccharide (IOP) in streptozotocin (STZ)-induced diabetic mice using metabolomics based on UPLC-Q-Exactive-MS method.
Results
The results showed that the oral administration of IOP in high dose (1.2 g/kg) can significantly reduce the blood glucose with 31% reduction comparing with the diabetic model and relieve dyslipidemia in diabetic mice. By UPLC-Q-Exactive-MS method and multivariate statistical analysis, a total of 15 differential metabolites were identified, including 4 up-regulated and 11 down-regulated biomarkers, of which l-tryptophan, l-leucine, uric acid, 12-HETE, arachidonic acid, PC(20:1(11Z)/14:1(9Z)) and SM(d18:0/24:1(15Z)) were exhibited an important variation, as the potential biomarkers in diabetes. Pathway analysis indicated that phenylalanine, tyrosine and tryptophan biosynthesis and arachidonic acid metabolism were prone to interference in diabetes. Moreover, leucine and proline were reversed and phytosphingosine was further reduced in diabetic mice under the intervention of IOP.
Conclusion
IOP has predominant hyperglycemic effect on STZ-induced diabetic mice via ameliorating serum profiling.
Similar content being viewed by others
Introduction
Diabetes mellitus is rapidly becoming a global epidemic disease and brings huge threat to human health and social economy. It is estimated that the diabetic patients worldwide are up to 463 million at present [1]. Diabetes mellitus is a systemic disease mainly caused by the disorder of metabolism, which divided into type I and II. Type I diabetes occurs due to the deficiency of insulin secretion caused by the damage of pancreatic β cells or other reasons, and type II diabetes is mainly characterized by insulin resistance [2,3,4]. Hyperglycemia is the most typical symptom of diabetes, moreover, diabetes patients prone to various complication, including hyperlipidemia, hypertension, retinopathy, neuropathy, and nephropathy [5, 6]. Currently chemically synthesized drugs for diabetes mellitus have numbers of serious adverse effects, such as gastrointestinal reaction, hepatic and renal impairment, and high rates of secondary failure [7, 8], and aren’t conducive to the complications treatment. Therefore, development new hypoglycemic agents with minimal adverse effect and excellent efficacy from natural products is still a challenge to the medical system.
Inonotus obliquus, also known as “Chaga”, is black-brown plant parasitic fungus and mainly distributed in Europe, Asia, and North America [9,10,11]. The I. obliquus was traditionally used as a folk medicine to treat gastrointestinal cancer, cardiovascular disease and diabetes in Russia, China, and Korea for many years [12,13,14]. It contains a variety of bioactive substances, including polysaccharides, triterpenes, phenolic acids and flavonoids [15,16,17]. To date, it has been shown that polysaccharide from I. obliquus possessed clear hypoglycemic activity [18, 19]. Several authors have reported that I. obliquus polysaccharide (IOP) may exert its antioxidant effect in treating hyperglycemia [20,21,22], of which the dry matter of culture broth of I. obliquus in 500 and 1000 mg/kg body weight showed a significant decrease in blood glucose level in alloxan-induced diabetic mice. Moreover, Wang et al. [23] showed that the effects of IOP on restoration of insulin resistance in streptozotocin-induced type II diabetic mice might involve in the PI3K/Akt signal pathway, which showed significant effect in the dose of 900 mg/kg. Joo et al. [24] suggested that IOP activated adipogenesis of 3T3-L1 preadipocytes and increased PPARγ transcriptional activities, which PPARγ has proved as a key receptor involved in insulin resistance [25]. Despite the remarkably effective of IOP in anti-diabetes, its exact molecular mechanism of the action has not been elucidated. In addition, diabetes mellitus, as a typical metabolic disease, often involves complex metabolic changes, which leads to certain difficulties in the study of pathophysiological process and drug treatment.
Recently, metabolomics has been widely used in pharmacological action and molecule mechanism due to its high sensitivity and comprehensive characterization [26, 27]. Metabolomics focus on the variation of small molecules metabolites in organism, and these differential metabolites may serve as candidate biomarkers of pharmacological efficacy or toxicity [28, 29]. Metabolomics can visually characterize the complex metabolic changes in the development of diabetes and the multi-target actions of traditional Chinese medicine, which has the characteristics of integrity, non-targeting and dynamics [30]. Here, the Q Exactive hybrid quadrupole orbitrap (Q-Exactive) high-resolution mass spectrometry in full scan mode offers a global view of sample extracts, as virtually all ionized compounds are detected with high sensitivity [31].
The purpose of this study was to investigate the anti-diabetic effects of IOP in STZ-induced diabetic mice and the potential mechanism revealed by untargeted metabolomics based on ultra high performance liquid chromatography with Q Exactive hybrid quadrupole orbitrap mass spectrometer (UPLC-Q-Exactive-MS).
Material and methods
Materials and reagents
The sclerotia of I. obliquus was purchased from Hometown Products Co., Ltd (Jilin, PR China). Streptozocin (STZ) was purchased from Sigma (Saint Louis, Missouri, USA). Metformin was purchased from Sino-American Shanghai Squibb Pharmaceuticals Ltd (Shanghai, China). Blood glucose detection kit was acquired from Solarbio Science & Technology Co., Ltd (Beijing, China). ELISA kits for the determination of total cholesterol (TC), triacylglycerol (TG), low density lipoprotein-cholesterol (LDL-C), high density lipoproteincholesterol (HDL-C) were acquired from Jiancheng Bioengineering Institute (Nanjing, China). Chromatographic grade acetonitrile, methanol, ammonium formate, and formic acid were obtained from Merck (Darmstadt, Germany). Analytical grade ethanol was purchased from the China National Pharmaceutical Group Corporation (Shanghai, China). Ultrapure water (18.2 MΩ) was prepared using a Milli-Q water purification system (Millipore, Shanghai, China).
The preparation of IOP
The extraction of IOP was followed the method reported previous with the yield of 3.8% [32]. Briefly, the dried powder of I. obliquus was conduct with 90% (v/v) ethanol for 20 min at 80 °C twice by ultrasonic technology to remove the lipid soluble substances and pigment components. Then, the dried residue was extracted three times with distilled water for 30 min each at 50 °C via ultrasonic–assisted extraction. After remove the residue, the supernatant was concentrated via vacuum rotary evaporation and then the concentrated solution was precipitated by adding ethanol to final concentration of 75%. After centrifuged at 5000×g for 15 min, the IOP was obtained and then freeze-drying.
Animals and drug administration procedure
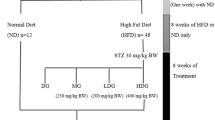
Male Kunming mice (6 weeks, 18 g ± 2 g) were obtained from Beijing Experimental Animal Center (Beijing, China) and kept in standard conditions with a normal diet under temperature of 25 ℃ ± 1 ℃ and relative humidity of 55% ± 10% for 1-week adaptive feeding, with 12:12-h light/dark cycle.
Ten healthy mice were selected as normal control group. STZ intraperitoneal injection was used to build the model of diabetes reported by Rajesh et al. [33]. Briefly, mice were injected intraperitoneally with a freshly prepared STZ solution (80 mg/kg body weight) in a citrate buffer (0.1 M, pH4.5). The development of diabetes was confirmed after a week of STZ injection, mice with blood glucose more than 11.1 mmol/L were selected as diabetic mice for further experiment. The diabetic mice were randomly divided into 5 groups of 10 mice each. Metformin group were diabetic mice treated with metformin 0.25 g/kg per day; IOP groups were set into high-, medium- and low-dose (IOPH, IOPM and IOPL groups) as follow: diabetic mice administered with 1.2, 0.8 and 0.4 g/kg per day, respectively. Diabetic model group and normal control group were administered with saline in a matched volume. The experiment was conducted continuously oral administration for 4 weeks and normal diet was given. Animals were anesthetized before eyeball enucleation with intraperitoneal injection of ketamine (80 mg/kg) and xylazine (15 mg/kg). All the serum samples were collected from the orbital sinus for biochemical analysis. Besides, the serum from control, model and IOPH groups were used for metabolomics analysis.
Oral glucose tolerance test
Oral glucose tolerance test (OGTT) was performed on the last week during experimental period. Mice were fasted for 12 h and serum samples were collected from the tip of the tail vein to measure the fasting blood glucose. Then, all the animals were received glucose (2 g/kg) orally and the blood glucose levels at 30, 60, 90 and 120 min were measured. AUC curve was calculated using GraphPad Prism 5.0 software.
Biochemical analysis
After oral administration for 4 weeks, blood was collected from the orbital sinus. After centrifugation at 5000×g for 10 min at 4 ℃, serum samples were obtained for subsequent analysis. The glucose, TC, TG, LDL-C and HDL-C levels in serum of mice were measured using reagent kits.
Metabolomics analysis
The serum samples were stored at − 80 ℃ until sample preparation. After thawing the samples on ice, 50 μL serum of each sample was spiked with 450 µL methanol solution. After vertexing for 60 s, the mixture was centrifuged at 10,000×g at 4 ℃ for 10 min. The supernatant was injected into the UPLC-Q-Exactive-MS system for metabolomics analysis. The pooled quality control (QC) samples including each serum sample were used to monitor the data acquisition performance during analysis.
An ultra high performance liquid chromatography (UPLC) system (Thermo Fisher Scientific, San Jose, CA, USA) was used to separate the metabolites in the serum. Five microliter aliquots of samples were loaded on a Waters 2.1 mm × 50 mm × 1.7 µm BEH C18 column with a flow rate of 0.25 mL/min. The mobile phase consisted of water containing 0.1% v/v formic acid with 2 mM ammonium formate (A) and acetonitrile (B). Column was maintained at 25 ℃ and eluted with a linear gradient as follows: 5% B at 0–1 min, 5–60% B at 1–5 min, 60–100% B at 5–8 min, 100% B at 8–11 min, 100–60% B at 11–14 min, 60–5% B at 14–15 min, 5% B at 15–18 min. A Q-Exactive mass spectrometer (Thermo Scientific, San Jose, USA) is tandem to the UPLC system equipped with an electrospray ionization (ESI) ion source. Both positive and negative ion modes were applied using full MS scan range 70–1050 m/z with a resolution of 70,000. In MS2 mode, the resolution was 35,000, samples were analyzed at 20, 30, 40 NCE (normalized collisional energy). The following parameters were as follows: Spray voltage, 3.5 kV (ESI+), 2.8 kV (ESI−); gas temperature, 350 ℃; sheath gas: 35 arbitrary units; auxiliary gas, 10 arbitrary units; capillary temperature, 320 ℃; S-lens RF, 50. Regarding the sequence of analysis, all the samples were randomly loaded in the instrument.
Data processing
Statistical analysis was performed on SPSS software version 25. All data were expressed as mean ± standard deviation (M ± SD). Independent samples t-test was used to analyze the differences between groups, with p values of less than 0.05 considered statistically significant. The LC–MS/MS raw data files were imported into thermo Scientific TraceFinder software (Thermo Scientific, San Jose, USA) for data pretreatment. The resulting data matrix were obtained with associated retention time, accurate mass, and chromatographic peak area. The data from both positive and negative ion modes were integrated and then imported to SIMCA software Version 14.1 (Umetrics, Umea, Sweden) for multivariate statistical analysis. The pathway analysis was applied in KEGG database (www.genome.jp/kegg). The potential biomarkers were chosen based on their contribution to the variation and correlation within the data set from the S-plots with the variable importance in the projection (VIP) value more than 1.0. The significance was then confirmed via Student t-test with p values less than 0.05.
Results and discussion
Oral glucose tolerance property
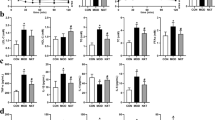
After oral administration of glucose, the blood glucose levels of all the experimental mice were measured to investigate the glucose tolerance ability, which was shown in Fig. 1a, the detailed data are shown in supplementary information files (Additional file 3: Table S1). The blood glucose levels of all mice reached the peaks at 30 min. Compared with the highest level of diabetic model group at 23.05 mmol/L, the blood glucose levels of IOP groups in high-, medium-, and low-dose were much lower (p < 0.05). Then the control group turned gradually to the normal level with 47.4% decreased after oral administration of glucose at 120 min. Meanwhile, IOP administration of mice in high-, medium-, and low-dose exhibited 24.3%, 23.8% and 19.9% reduction respectively, comparing with their peak glucose levels. However, the blood glucose levels of diabetic mice were consistently maintained in a high level throughout the whole time. The area under the curve (AUC) of mice after oral administration of glucose was shown in Fig. 1b. The model group showed the largest AUC among the six groups. The AUCs were significantly decreased in the administration of IOP (IOPH, IOPM and IOPL groups) compared to the model group (p < 0.01).
Fasting blood glucose, TC, TG, LDL-C and HDL-C levels in serum
In addition to hyperglycemia, dyslipidemia is also a prominent risk factor of the progression of diabetes, mainly in the increased in TCHO, TG and LDL-C levels and decreased in HDL-C level in serum. Table 1 shows the results of fasting blood glucose, TC, TG, LDL-C and HDL-C levels in serum of the mice. In this experiment, the fasting blood glucose levels were exhibited an extremely significant uptrend in the diabetic model group compared with the normal control group (p < 0.01). Further, the treatment of metformin and IOP in high- and medium-dose all showed a significant downtrend of fasting blood glucose levels comparing with the diabetic model group. The high-dose of IOP can decrease 31% blood glucose in diabetic mice. Moreover, the TCHO, TG and LDL-C levels were elevated and HDL-C levels were decreased in the model group, which were consisted with previous study [23]. After the treatment of IOP in high-dose, a remarkable relief of lipid profiles disorder was observed (p < 0.01), which had the same therapeutic effects of metformin.
Multivariate statistical analysis and identification of differential metabolites
All data matrix acquired from the LC–MS analysis platform were pre-processing by TraceFinder Software. Ion peaks presenting in at least 50% of any group were retained for subsequent analysis. Thus, a total of 508 compounds were detected and identified in both positive and negative ion modes in the mass spectrometric data by comparing to the database. The total ion chromatograms (TICs) of the QC samples showed good reproducibility in intensity and retention time of ion peaks in both positive and negative ion mode (shown in Additional file 1: Fig. S1 and S2). Multivariate statistical analyses are widely applied in the metabolomics analysis process, of which Principal Component Analysis (PCA) is an unsupervised pattern recognition method which used to analyze a general overview of the main discriminants among these samples. In the PCA analysis of three groups (shown in Fig. 2), the control group (C group) showed a good distinction with others, while there was a blurred boundary among the areas occupied by the diabetic model group (M group) and IOPH group (H group). Therefore, a supervised pattern recognition method, OPLS-DA was used to discover the differences between M group and C/H group. The OPLS-DA model of control and model groups showed clearly distinguishable (Fig. 3). sevenfold cross-validation was conducted to ensure the robustness of the model with the parameters of R2X = 0.252, R2Y = 0.981, and Q2 = 0.949. Then, 15 significant differential metabolites were screened by comparing the model with control group. Heat maps of these differential metabolites are shown in Fig. 4. Among them, 4 metabolites were upregulated while 11 metabolites were downregulated in the diabetic mice. Moreover, Table 2 shows the detailed results of differential metabolites in the C and M groups. A volcano plot of differential metabolites was obtained via log 2 (fold change of the metabolite) as abscissa and the -log10 (p value of the metabolite) as ordinate. As seen from Figs. 5, 7 marked metabolites, l-tryptophan, l-leucine, uric acid, 12-HETE, arachidonic acid, PC(20:1(11Z)/14:1(9Z)) and SM(d18:0/24:1(15Z)) were those exhibited an important variation between C and M groups with more than twofold change (|log2 FC|> 1) and p < 0.01 (−Log10 p > 2). In the following analysis of OPLS-DA model between M and H group (shown in Additional file 2: Fig. S3), 12 significant differential metabolites were screened and the heat maps was displayed in Fig. 6. Comparing with the above 15 significant differential metabolites selected in diabetic mice, there were three metabolites significantly altered, of which leucine and proline were reversed and phytosphingosine was further reduced in diabetic mice under the intervention of IOP. A set of boxcharts of the three metabolites visualize the contents variations in C, M and H groups, which was shown in Fig. 7. Pathway analysis indicated that phenylalanine, tyrosine and tryptophan biosynthesis and arachidonic acid metabolism were prone to interference in diabetes (shown in Fig. 8). To further explore the pathological processes of diabetes and the intervention of IOP, a network of the metabolism involved in amino acid metabolism, lipid metabolism and fatty acid metabolism was drawn based on the KEGG database (shown in Fig. 9).
Interpretation of differential metabolites
In this experiment, the serum profiling revealed the metabolism disorders in diabetic mice. In the amino acid metabolism, the six amino acids l-leucine, l-valine, l-tryptophan, l-phenylalanine, l-proline and l-alanine were decreased in diabetic mice with the negative correlations with blood glucose, which verified the previous demonstrations [34, 35]. Moreover, it is considered that branched chain amino acid leucine, valine, and isoleucine can improve glucose homeostasis and have the synergism effect on glucose homeostasis [36, 37]. l-Phenylalanine is the precursor of tyrosine and can improve glucose tolerance in rats by reducing plasma ghrelin and stimulating insulin release [38]. Mirsky et al. [39] suggested that l-tryptophan can significantly decrease the blood sugar concentration of normal rats through oral administration. The tryptophan disordered metabolite xanthurenic acid is concerned with the hyperglycemia due to the ability to bind insulin [40, 41]. Researches showed that l-proline had the hypoglycemic effect in experimental diabetic rats by improving the nerve growth factor and brain-derived neurotrophic factor [42]. l-Alanine is an important participant as well as a regulator of glucose metabolism and it was said that the mechanism of hypoglycemic action of biguanides may be a reduction in the Na+/l-alanine transport system [43]. Moreover, l-alanine is important in immune competence. After the administration of IOP, the contents of l-leucine and l-proline were reversed comparing with those in M group. Some evidence indicates that leucine stimulates protein synthesis in pancreatic β cell, which as a potentially important leucine-target tissue [44, 45]. Additionally, it was also reported that leucine supplementation could improve glucose tolerance in mice [46, 47]. This may explain the reversion of leucine in H group.
In the lipid metabolism, our data displayed decreased levels of phytosphingosine and sphinganine in diabetic mice, which were consistent with previous results [48]. According to the reports, sphinganine were identified to be significantly associated with diabetes mellitus and cardiovascular complications [49, 50]. As to phytosphingosine, its content was further reduced in the administration of IOP comparing with the model group. Lysophosphatidylcholine (LysoPC), the important intermediate products in the lipid metabolism, is referred to be concerned with the formations of cholesterol and low-density lipoprotein [51] and considered as bioactive medium in the process inflammation and increase oxidative stress [52, 53]. In general, phosphatidylcholine (PC) can transform into LysoPC via lecithin-cholesterol acyltransferase in plasma and participate in the production of arachidonic acid [54]. In this study, the decreased levels of PC(20:1(11Z)/14:1(9Z)) and LysoPC(20:4(8Z,11Z,14Z,17Z)) and the increased levels of SM(d18:0/24:1(15Z)) and LysoPC(16:1(9Z)) were also observed in diabetic mice. The result of up-regulation in LysoPC(16:1(9Z)) was similar to that in previous report [55]. Meanwhile, the lipid metabolism disturbance was proved to be involved in the pathogenesis of diabetic retinopathy [56].
Arachidonic acid is a polyunsaturated, essential fatty acid that mediates inflammation in organism. Arachidonate lipoxygenase enzymes metabolize arachidonic acid to generate potent inflammatory mediators and play an important role in inflammation-associated diseases [57]. Studies showed that arachidonic acid can reverse the decrease of prostaglandin E2 content in the incubation media of diabetic retinas [58]. It is frequently reported that arachidonic acid and its metabolite 12-hydroxyeicosatetraenoic acid (12-HETE) are closely related to the diseases of inflammation and oxidative stress [57, 59]. It was reported that 12-HETE participated in macrophage recruitment in islets and pro-inflammatory cytokine mediated islet damage in type 1 diabetic mouse models [60, 61], showing an inhibition of insulin secretion and metabolic activity [62]. Same to previous researches [63, 64], an increase of 12-HETE and a decrease of arachidonic acid were observed in hyperglycemia in this experiment, which may show the metabolic disorders associated with inflammation.
Conclusions
In this study, a metabolomics technique based on UPLC-Q-Exactive-MS method was used to illustrate the underlying mechanism of pathophysiological process in diabetes and therapeutic effect of IOP. A total of 15 differential metabolites were identified by comparing the normal control group with diabetic model group, including 4 up-regulated and 11 down-regulated biomarkers, of which l-tryptophan, l-leucine, uric acid, 12-HETE, arachidonic acid, PC(20:1(11Z)/14:1(9Z)) and SM(d18:0/24:1(15Z)) were exhibited an important variation and can be seen as the potential biomarkers in diabetes. Pathway analysis indicated that phenylalanine, tyrosine and tryptophan biosynthesis and arachidonic acid metabolism were prone to interference in diabetes. After oral administration of IOP, the blood glucose was declined and the lipid metabolism disorder was alleviative. Moreover, the differential metabolites leucine and proline were reversed and phytosphingosine was further reduced in diabetic mice under the intervention of IOP in the dose of 1.2 g/kg. The metabolomics approach in this study would lead to a further understanding on the pathophysiological process in diabetes and thus facilitate target screening for therapeutic intervention. Additionally, clarifying the metabolic regulation effect of IOP at molecular level could provide scientific evidence for hypoglycemic effect.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files.
Abbreviations
- IOP:
-
Inonotus obliquus polysaccharide
- UPLC-Q-Exactive-MS:
-
Ultra high performance liquid chromatography with Q Exactive hybrid quadrupole orbitrap mass spectrometer
- TC:
-
Total cholesterol
- TG:
-
Triacylglycerol
- LDL-C:
-
Low density lipoprotein-cholesterol
- HDL-C:
-
High density lipoprotein-cholesterol
- STZ:
-
Streptozotocin
- LysoPC:
-
Lysophosphatidylcholine
- PC:
-
Phosphatidylcholine
- SM:
-
Sphingomyelin
- 12-HETE:
-
12-Hydroxyeicosatetraenoic acid
References
International Diabetes Federation. The 9th Edition of the IDF Diabetes Atlas. 2019
Defronzo RA. Pathogenesis of type 2 diabetes. Drugs. 1999;58:29–30.
Pang SM. Health literacy complication awareness and diabetic control in patients with type 2 diabetes mellitus. J Adv Nurs. 2010;62(1):74–83.
Wang HY, Kan WC, Cheng TJ, Yu SH, Chang LH, Chuu JJ. Differential anti-diabetic effects and mechanism of action of charantin-rich extract of Taiwanese Momordica charantia between type 1 and type 2 diabetic mice. Food Chem Toxicol. 2014;69:347–56.
Mazzone T, Chait A, Plutzky J. Cardiovascular disease risk in type 2 diabetes mellitus: insights from mechanistic studies. Lancet. 2008;371(9626):1800–9.
Kokil GR, Veedu RN, Ramm GA, Prins JB, Parekh HS. Type 2 diabetes mellitus: limitations of conventional therapies and intervention with nucleic acid-based therapeutics. Chem Rev. 2015;115(11):4719–43.
Nisbet JC, Sturtevant JM, Prins JB. Metformin and serious adverse effects. Med J Aust. 2004;180(2):53–4.
Mukai J, Tada H, Watanabe Y, Miura M, Katsuyama S, Shoji T, et al. Lipids behavior and adverse effects for oral antidiabetic agents in patients with type 2 diabetes treated with sulfonylureas alone based on systematic review. Yakugaku Zasshi. 2007;127(10):1747.
Cui Y, Kim DS, Park KC. Antioxidant effect of Inonotus obliquus. J Ethnopharmacol. 2005;96(1–2):79–85.
Dai YC. Polypore diversity in China with an annotated checklist of Chinese polypores. Mycoscience. 2012;53(1):49–80.
Du WJ, Qi W. A review of the distribution and pharmacological activity of Inonotus obliquus. J Fungal Res. 2013;11(1):49–56.
Hwang YJ, Noh GW, Kim SH. Effect of Inonotus obliquus extracts on proliferation and caspase-3 activity in human gastro-intestinal cancer cell lines. Korean J Nutr. 2003;36(1):18–23.
Kahlos K, Kangas L, Hiltunen R. Antitumour activity of some compounds and fractions from an n-hexane extract of Inonotus obliquus. Acta Pharmaceutica Fennica. 1987;96(1):33–40.
Park YK, Kim JS, Jeon EJ, Kang MH. The improvement of chaga mushroom (Inonotus obliquus) extract supplementation on the blood glucose and cellular dna damage in streptozotocin-induced diabetic rats. Korean J Nutr. 2009;42(1):5–13.
Hu T, Xie LX, Xu L, Lu CT, Liu P. Extraction the polysaccharides and triterpenes from Inonotus obliquus by ultrasonic wave. Food Sci Tech. 2012;37(2):213–7.
Liang L, Zhang Z, Wang H. Antioxidant activities of extracts and subfractions from Inonotus obliquus. Int J Food Sci Nutr. 2009;60(2):175–84.
Ju HK, Chung HW, Hong SS, Park JH, Lee J, Kwon SW. Effect of steam treatment on soluble phenolic content and antioxidant activity of the chaga mushroom (Inonotus obliquus). Food Chem. 2010;119(2):619–25.
Ping L, Jiao X, Tong S, Dong W, Wu P. Chemical characterization and hypoglycaemic activities in vitro of two polysaccharides from Inonotus obliquus by submerged culture. Molecules. 2018;23(12):3261.
Wang M, Zhao Z, Xia Z, Zhou X, Hu J, Xue J, Liu X, et al. Simultaneous use of stimulatory agents to enhance the production and hypoglycaemic activity of polysaccharides from Inonotus obliquus by submerged fermentation. Molecules. 2019;24(23):4400.
Hu Y, Sheng Y, Yu M, Li K, Ren G, Xu X, et al. Antioxidant activity of Inonotus obliquus polysaccharide and its amelioration for chronic pancreatitis in mice. Int J Biol Macromol. 2016;87:348–56.
Diao B-Z, Jin W-R, Yu X-J. Protective effect of polysaccharides from Inonotus obliquus on streptozotocin-induced diabetic symptoms and their potential mechanisms in rats. Evid-Based Compl Alt. 2014;2014:1–5.
Sun JE, Ao ZH, Lu ZM, Xu HY, Zhang XM, Dou WF, et al. Antihyperglycemic and antilipidperoxidative effects of dry matter of culture broth of Inonotus obliquus in submerged culture on normal and alloxan-diabetes mice. J Ethnopharmacol. 2008;118(1):7–13.
Wang J, Wang C, Li S, Li W, Yuan G, Pan Y, et al. Anti-diabetic effects of Inonotus obliquus polysaccharides in streptozotocin-induced type 2 diabetic mice and potential mechanism via pi3k-akt signal pathway. Biomed Pharmacother. 2017;95(11):1669–77.
Joo JI, Dong HK, Yun JW. Extract of chaga mushroom (Inonotus obliquus) stimulates 3t3-l1 adipocyte differentiation. Phytother Res. 2010;24(11):1592–9.
Olefsky JM. Treatment of insulin resistance with peroxisome proliferator–activated receptor γ agonists. J Clin Invest. 2000;106(4):467–72.
Li Y, Wang Y, Su L, Li L, Zhang Y. Exploring potential chemical markers by metabolomics method for studying the processing mechanism of traditional Chinese medicine using RPLC-Q-TOF/MS: a case study of Radix aconiti. Chem Cent J. 2013;7(1):36–44.
Xu T, Lin Z, Shi Y, Liu L, Zuo L, Jia Q, et al. Metabolomics approach in lung tissue of septic rats and the interventional effects of Xuebijing injection using UHPLC-Q-Orbitrap-HRMS. J Biochem. 2018;164(6):427–35.
Wishart DS. Emerging applications of metabolomics in drug discovery and precision medicine. Nat Rev Drug Discov. 2016;15(7):473–84.
Baker M. Metabolomics: from small molecules to big ideas. Nat Methods. 2011;8(2):117–21.
Jin D. Progress in the application research of metabonomics in diabetes mellitus. China Pharmacist. 2021;24(3):542–7.
Liu X, Wang J, Hu B, Yan P, Jia S, Du Z, et al. Qualitative distribution of endogenous sphingolipids in plasma of human and rodent species by UPLC-Q-Exactive-MS. J Chromatogr B. 2021;1173(5): 122684. https://doi.org/10.1016/j.jchromb.2021.122684.
Xu T, Lv C, Wang X, Du X, Wang T. Optimization of extraction of polysaccharides from Inonotus obliquus by response surface methodology and antioxidant activity. Food Res Dev. 2021;42(4):143–8.
Rajesh V, Perumal P. In vivo assessment of antidiabetic and antioxidant activities of methanol extract of smilax zeylanica leaves in wistar rats. Oriental Pharm Exp M. 2014;14(2):127–44.
Alena S, Mete C, Niyas KS, Pasi S, Antti JK, Henna C, et al. Hyperglycemia and a common variant of GCKR are associated with the levels of eight amino acids in 9,369 finnish men. Diabetes. 2012;61(7):1895–902.
Allison T, Bruttig S, Crass MF, Eliot R, Shipp J. Reduced high-energy phosphate levels in rat hearts. I. Effects of alloxan diabetes. Am J Physiol. 1976;230(6):1744–50.
Pedroso JAB, Zampieri TT, Donato J. Reviewing the effects of l-leucine supplementation in the regulation of food intake, energy balance, and glucose homeostasis. Nutrients. 2015;7(5):3914–37.
Khoo NK, Iravani A, Arjmand M, Vahabi F, Lajevardi M, Akrami SM, et al. A metabolomic study on the effect of intravascular laser blood irradiation on type 2 diabetic patients. Laser Med Sci. 2013;28(6):1527–32.
Alamshah A, Spreckley E, Norton M, Kinsey-Jones JS, Amin A, Ramgulam A, et al. L-phenylalanine modulates gut hormone release and glucose tolerance, and suppresses food intake through the calcium-sensing receptor in rodents. Int J Obesity. 2017;41(11):1693–701.
Mirsky IA, Perisutti G, Jinks R, Kaufman M. The hypoglycemic action of metabolic derivatives of L-tryptophan by mouth. Endocrinology. 1957;60(2):318–24.
Hattori M, Kotake Y. Studies on the urinary excretion of xanthurenic acid in diabetics. Acta Vitaminol Enzymol. 1984;6(3):221–8.
Wolf H. Studies on tryptophan metabolism in man: the effect of hormones and vitamin B6 on urinary excretion of metabolites of the kynurenine pathway: part 1. Scand J Clin Lab Invest. 1974;33(36):11–87.
Ostrovskaya RU, Antipova TA, Nikolaev SV, Kruglov SV, Ozerova IV, Gudasheva TA, et al. Deficit of neurotrophins in experimental diabetes—correction with a proline-containing dipeptide. Neurosci Behav Physi. 2019;49(1):809–15.
Komori T, Hotta N, Kobayashi M, Sakakibara F, Sakamoto N. Biguanides may produce hypoglycemic action in isolated rat hepatocytes through their effects on l-alanine transport. Diabetes Res Clin Pr. 1993;22(1):11–7.
Xu G, Kwon G, Marshall CA, Lin TA, Lawrence JC, Mcdaniel ML. Branched-chain amino acids are essential in the regulation of PHAS-I and p70 S6 kinase by pancreatic β-cells: a possible role in protein translation and mitogenic signaling. J Biol Chem. 1998;273(43):28178–84.
Sans MD, Tashiro M, Vogel NL, Kimball SR, Al E. Leucine activates pancreatic translational machinery in rats and mice through mTOR independently of CCK and insulin. J Nutr. 2006;136(7):1792–9.
Freudenberg A, Petzke KJ, Klaus S. Comparison of high-protein diets and leucine supplementation in the prevention of metabolic syndrome and related disorders in mice. J Nutr Biochem. 2012;23(11):1524–30.
Zhang Y, Guo K, Leblanc RE, Loh D, Schwartz GJ, Yu YH. Increasing dietary leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in mice via multimechanisms. Diabetes. 2007;56(6):1647–54.
Zhang J, Yan LJ, Lin L, Chen WG, Song XY, Yan XM, et al. Metabonomics research of diabetes mellitus and diabetic nephropathy based on UPLC-oaTOF-MS system. Chem J Chinese U. 2008;29(11):2171–3.
Sui J, He M, Wang Y, Zhao X, He Y, Shi B. Sphingolipid metabolism in type 2 diabetes and associated cardiovascular complications. Exp Ther Med. 2019;18(5):3603–14.
Dong Y, Chen Y-T, Yang Y-X, Shou D, Li C-Y. Urinary metabolomic profiling in zucker diabetic fatty rats with type 2 diabetes mellitus treated with glimepiride, metformin, and their combination. Molecules. 2016;21(11):1446.
Vuong TD, Stroes ESG, Willekes-Koolschijn N, Rabelink TJ, Joles JA. Hypoalbuminemia increases lysophosphatidylcholine in low-density lipoprotein of normocholesterolemic subjects. Kidney Int. 1999;55(3):1005–10.
Aiyar N, Disa J, Ao Z, Ju H, Nerurkar S, Willette RN, et al. Lysophosphatidylcholine induces inflammatory activation of human coronary artery smooth muscle cells. Mol Cell Biochem. 2007;295(1–2):113–20.
Zou Y, Kim DH, Jung KJ, Heo HS, Kim CH, Baik HS, et al. Lysophosphatidylcholine enhances oxidative stress via the 5-lipoxygenase pathway in rat aorta during aging. Rejuv Res. 2009;12(1):15–24.
Pihlajamäki J, Valve R, Karjalainen L, Karhapää P, Laakso M. The hormone sensitive lipase gene in familial combined hyperlipidemia and insulin resistance. Eur J Clin Invest. 2015;31(4):302–8.
Gooding J, Cao L, Whitaker C, Mwiza JM, Fernander M, Ahmed F, et al. Meprin β metalloproteases associated with differential metabolite profiles in the plasma and urine of mice with type 1 diabetes and diabetic nephropathy. BMC Nephrol. 2019;20(1):141.
Zhang CY. The Effects of lipid and high glucose on diabetic retinopathy and the expressions of NO, NOS and PDGF-B of retinal endothelial cells. Changsha: Central South University; 2007.
Moscatelli D. Membrane and matrix localization of proteinases: a common theme in tumor cell invasion and angiogenesis. BBA - Rev Cancer. 1988;948(1):67–85.
Naveh-Floman N, Weissman C, Belkin M. Arachidonic acid metabolism by retinas of rats with streptozotocin-induced diabetes. Curr Eye Res. 1984;3(9):1135–9.
Cheng Q, Tian L, Liang H, Luo Y. Research progress of 12-hete in the inflammation and oxidative stress. Chin Crit Care Med. 2019;31(12):1555–8.
Mcduffie M, Maybee NA, Keller SR, Stevens BK, Garmey JC, Morris MA, et al. Nonobese diabetic (NOD) mice congenic for a targeted deletion of 12/15-lipoxygenase are protected from autoimmune diabetes. Diabetes. 2008;57(1):199–208.
Bleich D, Chen S, Zipser B, Sun D, Nadler JL. Resistance to type 1 diabetes induction in 12-lipoxygenase knockout mice. J Clin Invest. 1999;103(10):1431–6.
Ma K, Nunemaker CS, Wu R, Chakrabarti SK, Taylor-Fishwick DA, Nadler JL. 12-lipoxygenase products reduce insulin secretion and β-cell viability in human islets. J Clin Endocrino Metab. 2010;95(2):887–93.
Hennessy E, Tisdall AR, Murphy N, Carroll A, Gorman DO, Breen L, et al. Elevated 12-hydroxyeicosatetraenoic acid (12-HETE) levels in serum of individuals with newly diagnosed type 1 diabetes. Diabet Med. 2017;34(2):292–4.
Hu Q, Ishii E, Nakagawa Y. Differential changes in relative levels of arachidonic acid in major phospholipids from rat tissues during the progression of diabetes. J Biochem. 1994;115(3):405–8.
Acknowledgements
Not applicable.
Funding
This work was supported and funded scheme by Post-Doctoral Research Center, the 967th Hospital of PLA Joint Logistics Support Force.
Author information
Authors and Affiliations
Contributions
TX designed the work, wrote and edited the manuscript; TX, GL and YT performed the experiments and analyzed the data; XW supervised the project. CL extracted the polysaccharides. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All experiments were approved by the Ethics Committee of the 967th Hospital of PLA Joint Logistics Support Force and carried out in accordance with relevant guidelines and regulations. This study was conducted in accordance with the ARRIVE guidelines (https://arriveguidelines.org).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
The total ion chromatogram (TIC) of six QC samples in both positive and negative ion modes.
Additional file 2.
OPLS-DA score plot of serum samples from in M (model group) and H (IOPH group).
Additional file 3.
The OGTT detailed results of mice.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Xu, T., Li, G., Wang, X. et al. Inonotus obliquus polysaccharide ameliorates serum profiling in STZ-induced diabetic mice model. BMC Chemistry 15, 64 (2021). https://doi.org/10.1186/s13065-021-00789-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13065-021-00789-4